C57bl/6 Mice Show Equivalent Taste Preferences toward Ruminant and Industrial Trans Fatty Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanovesicle Preparation
2.3. Animal Study
2.4. Statistical Analyses
3. Results
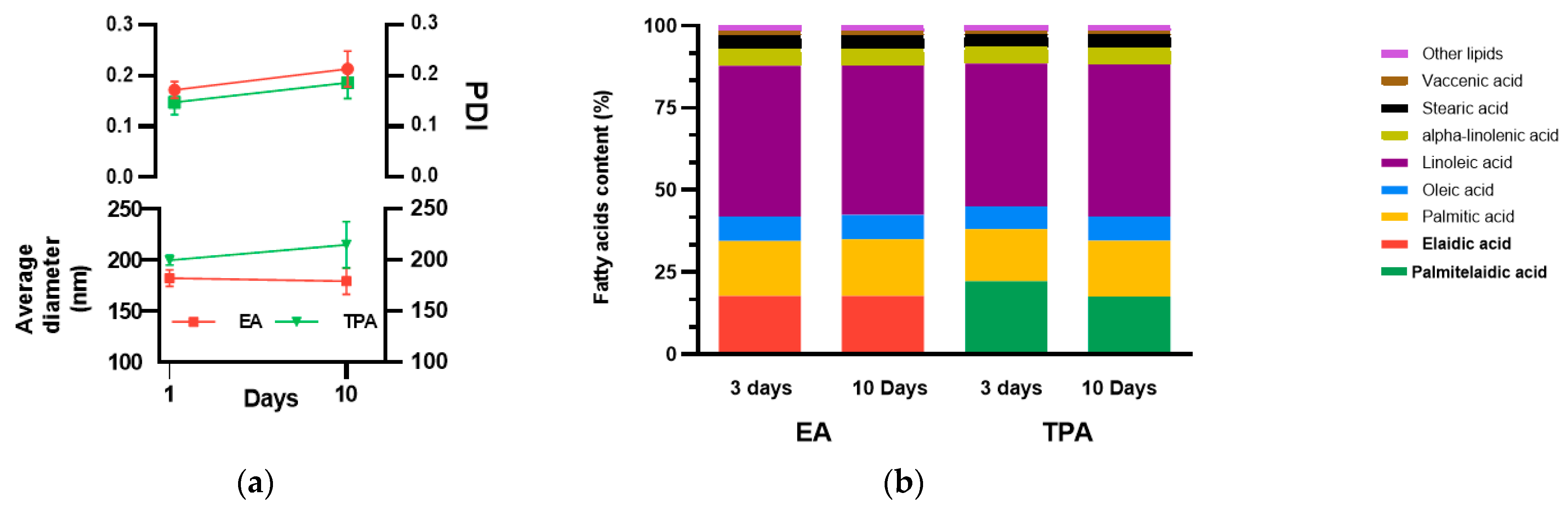
3.1. Vesicles Contain Similar Amounts of TFA and Remain Physicaly and Chemically Stable over a Period of 10 Days
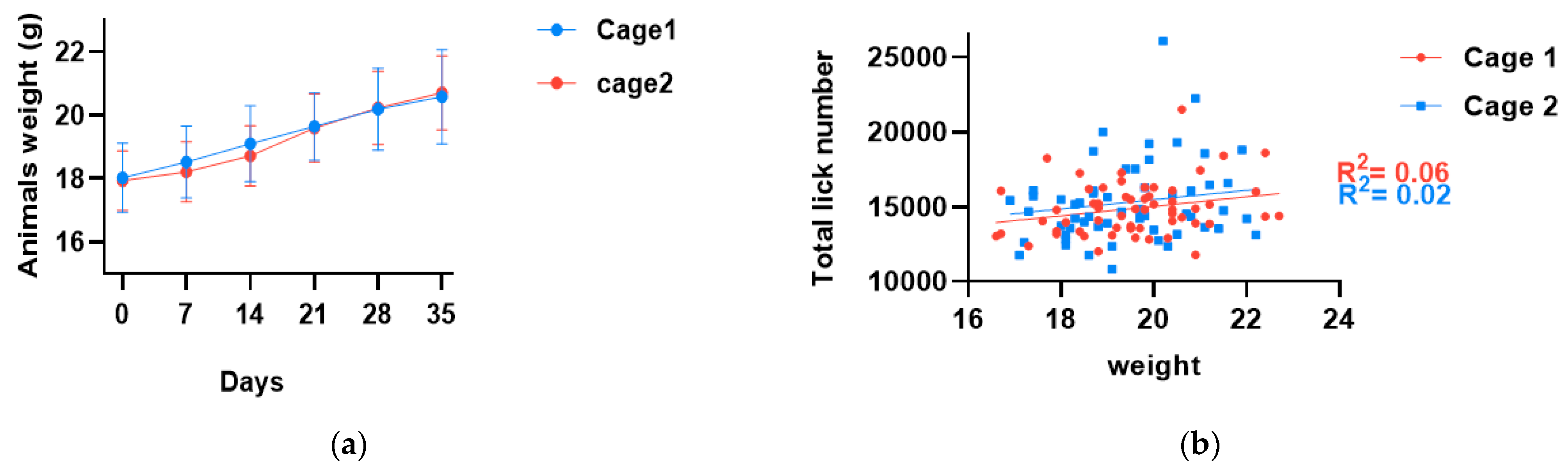
3.2. Animals Gained Weight Consistently during the Study, and Weight Gain Was Comparable in Both Cages
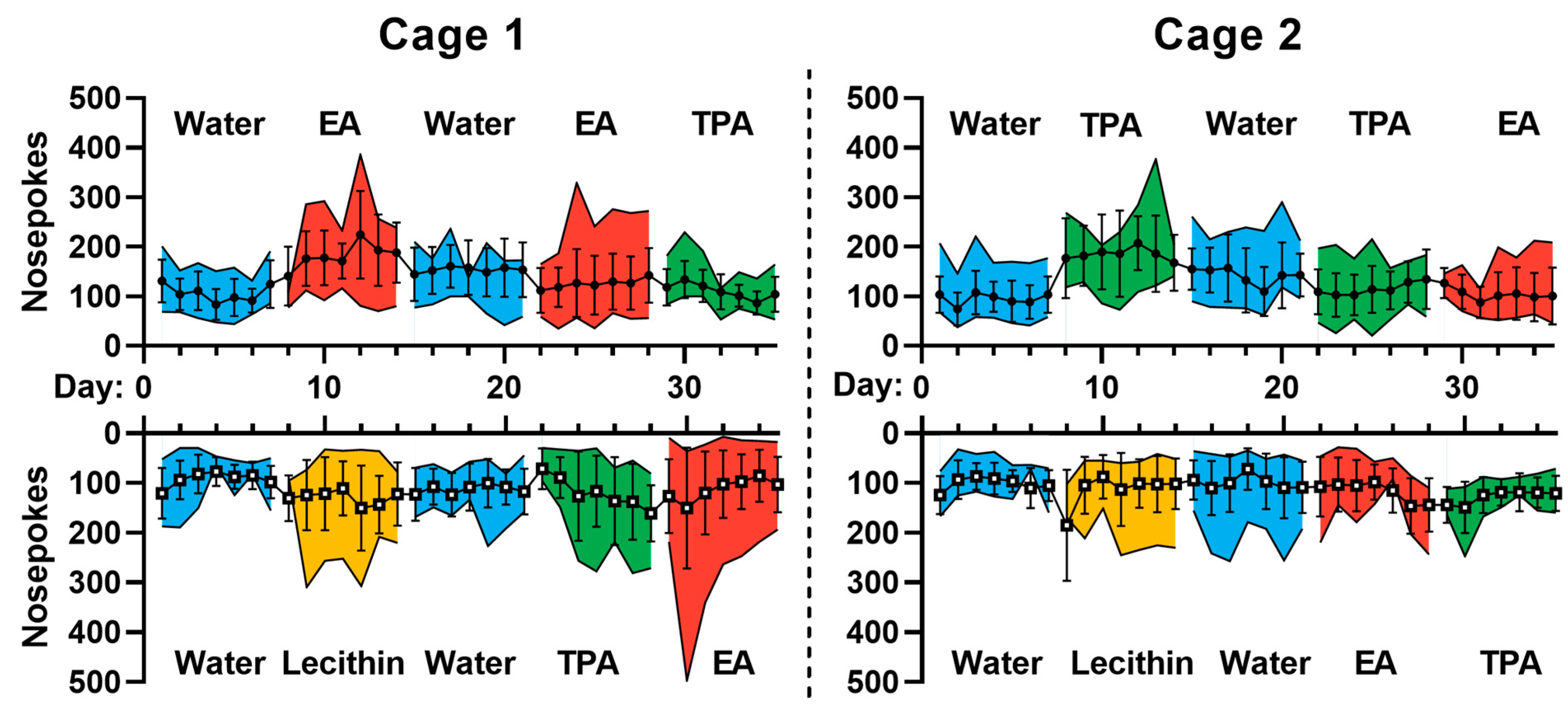
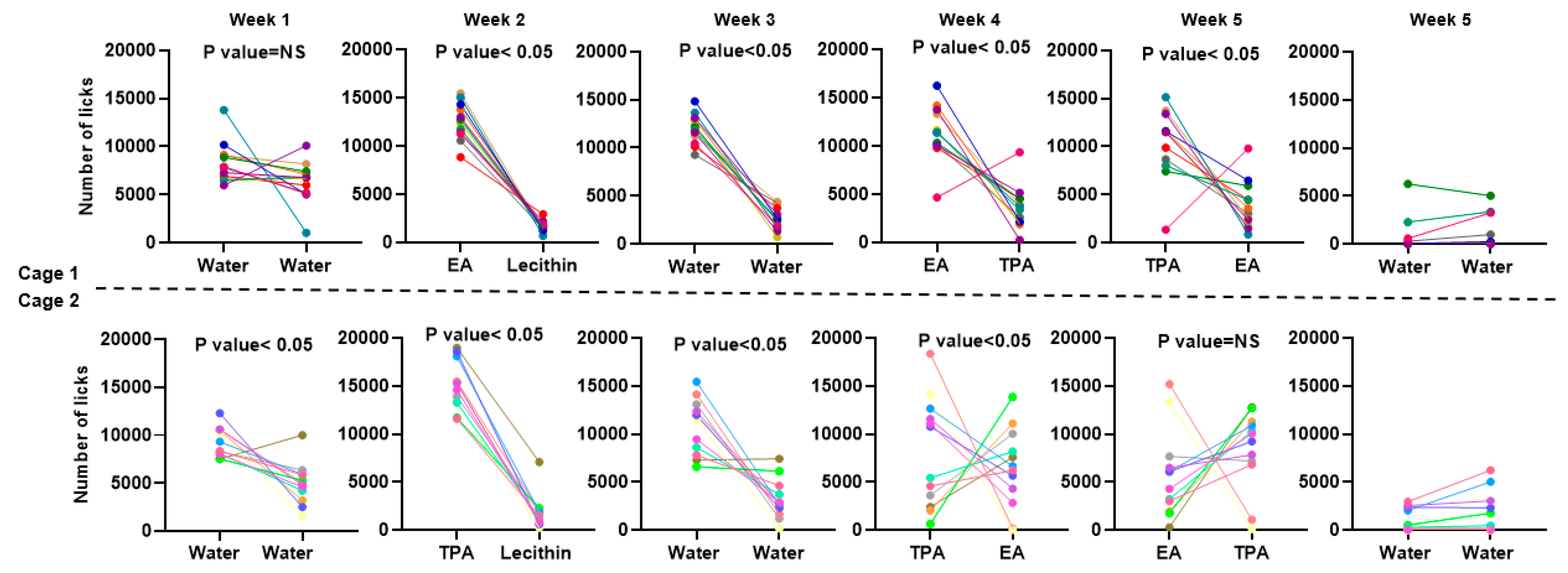
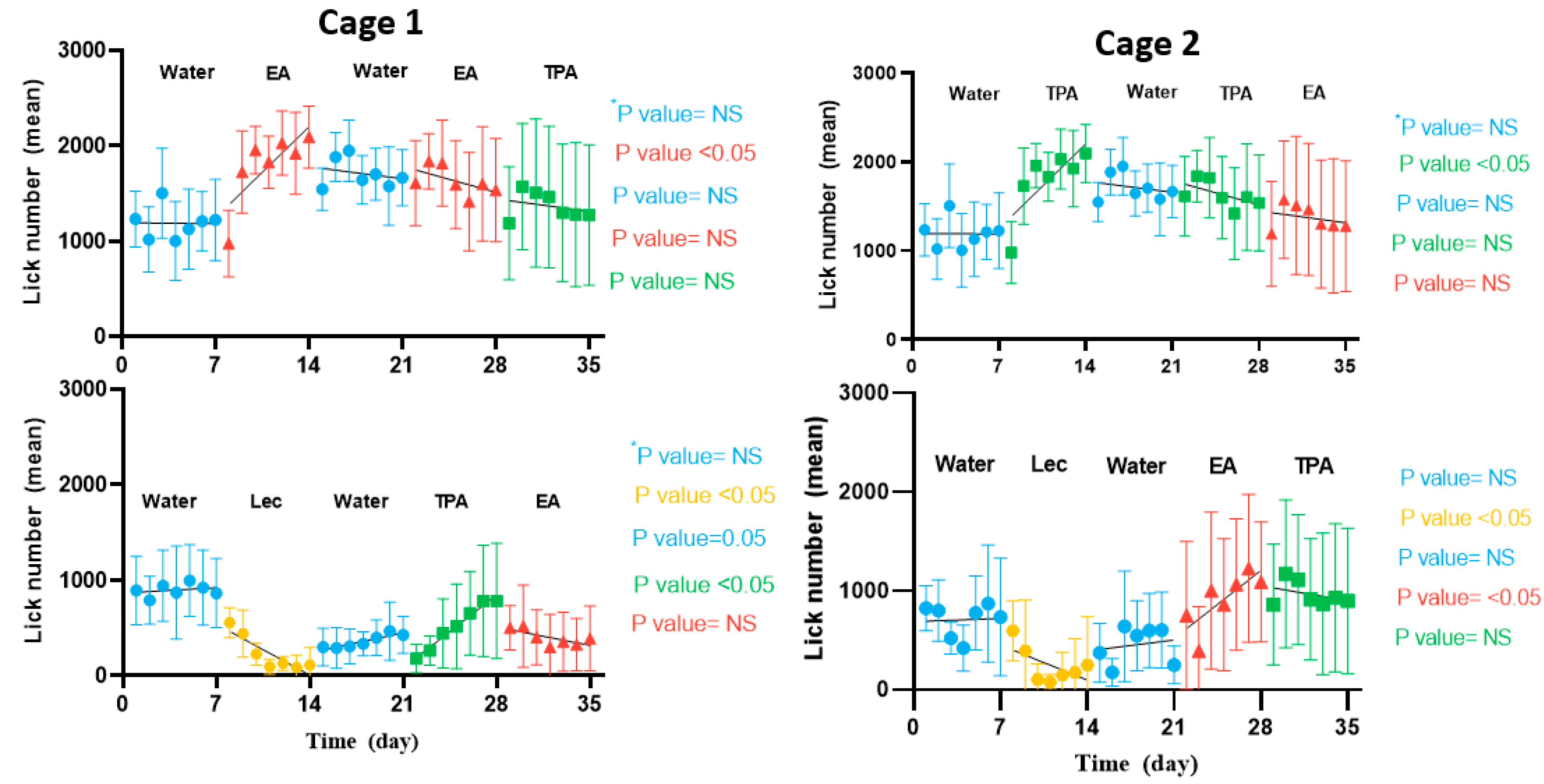
3.3. IntelliCage® Allow to Document the Preference of Mice for TFA-Containing Vesicles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucas, F.; Ackroff, K.; Sclafani, A. High-fat diet preference and overeating mediated by postingestive factors in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 275, R1511–R1522. [Google Scholar] [CrossRef]
- Drewnowski, A.; Almiron-Roig, E. 11. Human perceptions and preferences for fat-rich foods. In Fat Detection: Taste, Texture, and Post Ingestive Effects; CRC Press: Boca Raton, FL, USA, 2010; Volume 23, p. 265. [Google Scholar]
- Drewnowski, A. Taste preferences and food intake. Annu. Rev. Nutr. 1997, 17, 237. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L. High-carbohydrate high-fat diet–induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [CrossRef] [PubMed]
- Tierney, A.C.; McMonagle, J.; Shaw, D.; Gulseth, H.; Helal, O.; Saris, W.; Paniagua, J.; Gołąbek-Leszczyñska, I.; Defoort, C.; Williams, C.M. Effects of dietary fat modification on insulin sensitivity and on other risk factors of the metabolic syndrome—LIPGENE: A European randomized dietary intervention study. Int. J. Obes. 2011, 35, 800–809. [Google Scholar] [CrossRef]
- Canada, S. Table 13-10-0769-01 Percentage of Total Energy Intake from Fat, by Dietary Reference Intake Age-Sex Group, Household Population Aged 1 and over, Canadian Community Health Survey (CCHS)—Nutrition, Canada and Provinces; Canada Statistics: Ottawa, ON, Canada, 2015. [Google Scholar] [CrossRef]
- Harcombe, Z. US dietary guidelines: Is saturated fat a nutrient of concern? Br. J. Sport. Med. 2019, 53, 1393–1396. [Google Scholar] [CrossRef]
- Fukuwatari, T.; Shibata, K.; Iguchi, K.; Saeki, T.; Iwata, A.; Tani, K.; Sugimoto, E.; Fushiki, T. Role of gustation in the recognition of oleate and triolein in anosmic rats. Physiol. Behav. 2003, 78, 579–583. [Google Scholar] [CrossRef]
- Oteng, A.-B.; Kersten, S. Mechanisms of action of trans fatty acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef]
- Bendsen, N.; Christensen, R.; Bartels, E.; Astrup, A. Consumption of industrial and ruminant trans fatty acids and risk of coronary heart disease: A systematic review and meta-analysis of cohort studies. Eur. J. Clin. Nutr. 2011, 65, 773–783. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Aro, A.; Willett, W.C. Health effects of trans-fatty acids: Experimental and observational evidence. Eur. J. Clin. Nutr. 2009, 63, S5–S21. [Google Scholar] [CrossRef]
- Abd El-Aal, Y.A.; Abdel-Fattah, D.M.; Ahmed, K.E.-D. Some biochemical studies on trans fatty acid-containing diet. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1753–1757. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Cao, H.; King, I.B.; Lemaitre, R.N.; Song, X.; Siscovick, D.S.; Hotamisligil, G.S. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in US adults: A cohort study. Ann. Intern. Med. 2010, 153, 790–799. [Google Scholar] [CrossRef]
- Zhu, W.; Niu, X.; Wang, M.; Li, Z.; Jiang, H.-K.; Li, C.; Caton, S.J.; Bai, Y. Endoplasmic reticulum stress may be involved in insulin resistance and lipid metabolism disorders of the white adipose tissues induced by high-fat diet containing industrial trans-fatty acids. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1625. [Google Scholar] [CrossRef] [PubMed]
- Pfeuffer, M.; Jahreis, G. Trans-Fettsäuren: Herkunft, Stoffwechsel, gesundheitliche Risiken. Ernahr. Umsch. 2018, 65, 196–203. [Google Scholar]
- Kuhnt, K.; Degen, C.; Jahreis, G. Evaluation of the impact of ruminant trans fatty acids on human health: Important aspects to consider. Crit. Rev. Food Sci. Nutr. 2016, 56, 1964–1980. [Google Scholar] [CrossRef]
- Da Silva, M.S.; Julien, P.; Bilodeau, J.-F.; Barbier, O.; Rudkowska, I. Trans fatty acids suppress TNF-α-induced inflammatory gene expression in endothelial (HUVEC) and hepatocellular carcinoma (HepG2) cells. Lipids 2017, 52, 315–325. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.; Rudkowska, I. Dietary Fatty Acids and the Metabolic Syndrome: A Personalized Nutrition Approach. Adv. Food Nutr. Res. 2019, 87, 43–146. [Google Scholar]
- Micha, R.; Mozaffarian, D. Trans fatty acids: Effects on metabolic syndrome, heart disease and diabetes. Nat. Rev. Endocrinol. 2009, 5, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Sawano, S.; Imaizumi, M.; Fushiki, T. Preference for corn oil in olfactory-blocked mice in the conditioned place preference test and the two-bottle choice test. Life Sci. 2001, 69, 847–854. [Google Scholar] [CrossRef]
- Laugerette, F.; Passilly-Degrace, P.; Patris, B.; Niot, I.; Febbraio, M.; Montmayeur, J.-P.; Besnard, P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Investig. 2005, 115, 3177–3184. [Google Scholar] [CrossRef]
- Chotard, É.; Mohammadi, F.; Julien, P.; Berthiaume, L.; Rudkowska, I.; Bertrand, N. Drinkable lecithin nanovesicles to study the biological effects of individual hydrophobic macronutrients and food preferences. Food Chem. 2020, 322, 126736. [Google Scholar] [CrossRef] [PubMed]
- Schumann, L.; Wilken-Schmitz, A.; Trautmann, S.; Vogel, A.; Schreiber, Y.; Hahnefeld, L.; Gurke, R.; Geisslinger, G.; Tegeder, I. Increased Fat Taste Preference in Progranulin-Deficient Mice. Nutrients 2021, 13, 4125. [Google Scholar] [CrossRef] [PubMed]
- Callon, M.C.; Cargo-Froom, C.; DeVries, T.J.; Shoveller, A.K. Canine food preference assessment of animal and vegetable ingredient-based diets using single-pan tests and behavioral observation. Front. Vet. Sci. 2017, 4, 154. [Google Scholar] [CrossRef]
- Le Roux, C.W.; Bueter, M.; Theis, N.; Werling, M.; Ashrafian, H.; Löwenstein, C.; Athanasiou, T.; Bloom, S.R.; Spector, A.C.; Olbers, T. Gastric bypass reduces fat intake and preference. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R1057–R1066. [Google Scholar] [CrossRef] [PubMed]
- Machado, G.; Pezzali, J.; Marx, F.; Kessler, A.; Trevizan, L. Palatability, digestibility, and metabolizable energy of dietary glycerol in adult cats. J. Anim. Sci. 2017, 95, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.M.; Johnson, S.B.; Hommel, J.D. Fat Preference: A novel model of eating behavior in rats. J. Vis. Exp. 2014, 88, e51575. [Google Scholar]
- Kiryk, A.; Janusz, A.; Zglinicki, B.; Turkes, E.; Knapska, E.; Konopka, W.; Lipp, H.-P.; Kaczmarek, L. IntelliCage as a tool for measuring mouse behavior–20 years perspective. Behav. Brain Res. 2020, 388, 112620. [Google Scholar] [CrossRef]
- Dikpati, A.; Mohammadi, F.; Greffard, K.; Quéant, C.; Arnaud, P.; Bastiat, G.; Rudkowska, I.; Bertrand, N. Residual solvents in nanomedicine and lipid-based drug delivery systems: A case study to better understand processes. Pharm. Res. 2020, 37, 149. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Reed, D.R.; Beauchamp, G.K.; Tordoff, M.G. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 2002, 32, 435–443. [Google Scholar] [CrossRef]
- Nakaya, K.; Kohata, T.; Doisaki, N.; Ushio, H.; Ohshima, T. Effect of oil droplet sizes of oil-in-water emulsion on the taste impressions of added tastants. Fish. Sci. 2006, 72, 877–883. [Google Scholar] [CrossRef]
- Takeda, M.; Imaizumi, M.; Fushiki, T. Preference for vegetable oils in the two-bottle choice test in mice. Life Sci. 2000, 67, 197–204. [Google Scholar] [CrossRef]
- Manabe, Y.; Matsumura, S.; Fushiki, T. 10. Preference for High-Fat Food in Animals. In Fat Detection: Taste, Texture, and Post Ingestive Effects; CRC Press: Boca Raton, FL, USA, 2009; p. 243. [Google Scholar]
- Cartoni, C.; Yasumatsu, K.; Ohkuri, T.; Shigemura, N.; Yoshida, R.; Godinot, N.; Le Coutre, J.; Ninomiya, Y.; Damak, S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 2010, 30, 8376–8382. [Google Scholar] [CrossRef]
- Martin, C.; Passilly-Degrace, P.; Gaillard, D.; Merlin, J.-F.; Chevrot, M.; Besnard, P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: Impact on spontaneous fat preference. PLoS ONE 2011, 6, e24014. [Google Scholar] [CrossRef]
- Johnson, A.; Sherwood, A.; Smith, D.; Wosiski-Kuhn, M.; Gallagher, M.; Holland, P. An analysis of licking microstructure in three strains of mice. Appetite 2010, 54, 320–330. [Google Scholar] [CrossRef]
- Mura, E.; Taruno, A.; Yagi, M.; Yokota, K.; Hayashi, Y. Innate and acquired tolerance to bitter stimuli in mice. PloS ONE 2018, 13, e0210032. [Google Scholar] [CrossRef]
- Tsuruta, M.; Kawada, T.; Fukuwatari, T.; Fushiki, T. The orosensory recognition of long-chain fatty acids in rats. Physiol. Behav. 1999, 66, 285–288. [Google Scholar] [CrossRef]
- Gaillard, D.; Passilly-Degrace, P.; Besnard, P. Molecular mechanisms of fat preference and overeating. Ann. N. Y. Acad. Sci. 2008, 1141, 163–175. [Google Scholar] [CrossRef]
- Shimizu, Y.; Son, C.; Aotani, D.; Nomura, H.; Hikida, T.; Hosoda, K.; Nakao, K. Role of leptin in conditioned place preference to high-fat diet in leptin-deficient ob/ob mice. Neurosci. Lett. 2017, 640, 60–63. [Google Scholar] [CrossRef]
- Liang, N.-C.; Hajnal, A.; Norgren, R. Sham feeding corn oil increases accumbens dopamine in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1236–R1239. [Google Scholar] [CrossRef]
- Sarkar, S.; Kochhar, K.P.; Khan, N.A. Fat addiction: Psychological and physiological trajectory. Nutrients 2019, 11, 2785. [Google Scholar] [CrossRef]
- Gebauer, S.K.; Chardigny, J.-M.; Jakobsen, M.U.; Lamarche, B.; Lock, A.L.; Proctor, S.D.; Baer, D.J. Effects of ruminant trans fatty acids on cardiovascular disease and cancer: A comprehensive review of epidemiological, clinical, and mechanistic studies. Adv. Nutr. 2011, 2, 332–354. [Google Scholar] [CrossRef]
- De Francesco, P.N.; Cornejo, M.P.; Barrile, F.; García Romero, G.; Valdivia, S.; Andreoli, M.F.; Perello, M. Inter-individual variability for high fat diet consumption in inbred C57BL/6 mice. Front. Nutr. 2019, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Faizi, M.; Bader, P.L.; Tun, C.; Encarnacion, A.; Kleschevnikov, A.; Belichenko, P.; Saw, N.; Priestley, M.; Tsien, R.W.; Mobley, W.C. Comprehensive behavioral phenotyping of Ts65Dn mouse model of Down syndrome: Activation of β1-adrenergic receptor by xamoterol as a potential cognitive enhancer. Neurobiol. Dis. 2011, 43, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Codita, A.; Gumucio, A.; Lannfelt, L.; Gellerfors, P.; Winblad, B.; Mohammed, A.H.; Nilsson, L.N. Impaired behavior of female tg-ArcSwe APP mice in the IntelliCage: A longitudinal study. Behav. Brain Res. 2010, 215, 83–94. [Google Scholar] [CrossRef]
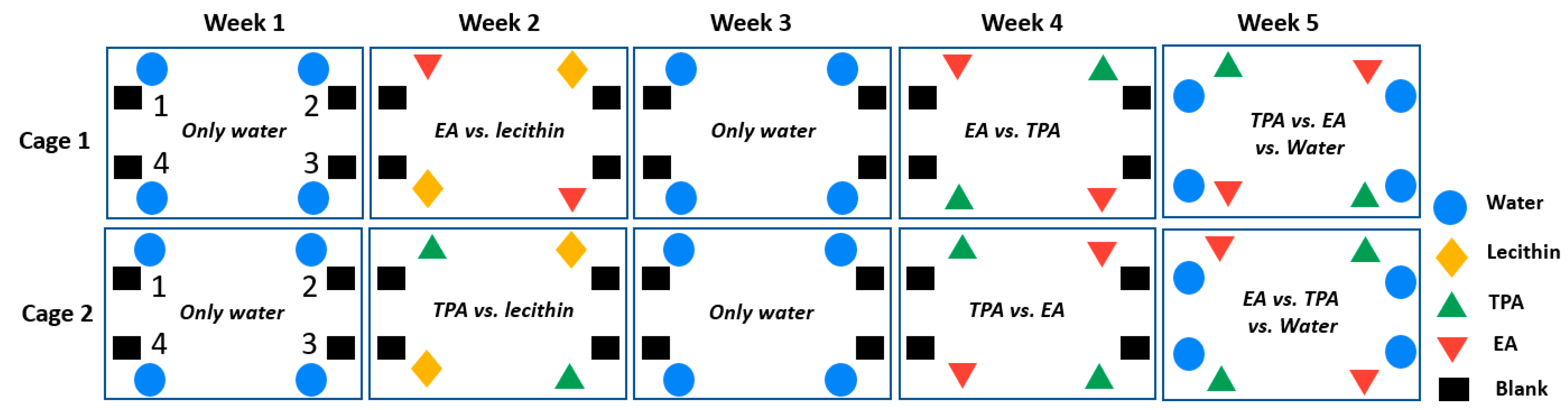
| Cage 1 | Corner 1 | Corner 2 | Corner 3 | Corner 4 |
|---|---|---|---|---|
| Week 1 (water) | 94 | 44 | 73 | 91 |
| Week 2 | EA: 105 | Lecithin: 22 | EA: 105 | Lecithin: 30 |
| Week 3 (water) | 78 | 22 | 104 | 31 |
| Week 4 | EA:120 | TPA: 60 | EA: 112 | TPA: 16 |
| Week 5 | TPA: 106 | EA: 12 | TPA: 46 | EA: 42 |
| Week 5 (water) | 18 | 12 | 20 | 20 |
| Cage 2 | Corner 1 | Corner 2 | Corner 3 | Corner 4 |
| Week 1 | 98 | 30 | 88 | 106 |
| Week 2 | TPA: 100 | Lecithin: 62 | TPA: 106 | Lecithin: 20 |
| Week 3(water) | 47 | 38 | 129 | 54 |
| Week 4 | TPA: 82 | EA: 58 | TPA: 114 | EA: 84 |
| Week 5 | EA: 52 | TPA: 54 | EA: 43 | TPA: 82 |
| Week 5 (water) | 15 | 20 | 20 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, F.; Bertrand, N.; Rudkowska, I. C57bl/6 Mice Show Equivalent Taste Preferences toward Ruminant and Industrial Trans Fatty Acids. Nutrients 2023, 15, 610. https://doi.org/10.3390/nu15030610
Mohammadi F, Bertrand N, Rudkowska I. C57bl/6 Mice Show Equivalent Taste Preferences toward Ruminant and Industrial Trans Fatty Acids. Nutrients. 2023; 15(3):610. https://doi.org/10.3390/nu15030610
Chicago/Turabian StyleMohammadi, Farzad, Nicolas Bertrand, and Iwona Rudkowska. 2023. "C57bl/6 Mice Show Equivalent Taste Preferences toward Ruminant and Industrial Trans Fatty Acids" Nutrients 15, no. 3: 610. https://doi.org/10.3390/nu15030610
APA StyleMohammadi, F., Bertrand, N., & Rudkowska, I. (2023). C57bl/6 Mice Show Equivalent Taste Preferences toward Ruminant and Industrial Trans Fatty Acids. Nutrients, 15(3), 610. https://doi.org/10.3390/nu15030610







