Implication of Vegetable Oil-Derived Hydroxynonenal in the Lysosomal Cell Death for Lifestyle-Related Diseases
Abstract
1. Introduction
2. Cultured Cells Exposed to Oxidative Stress
3. C. elegans scav-3 Mutation Models
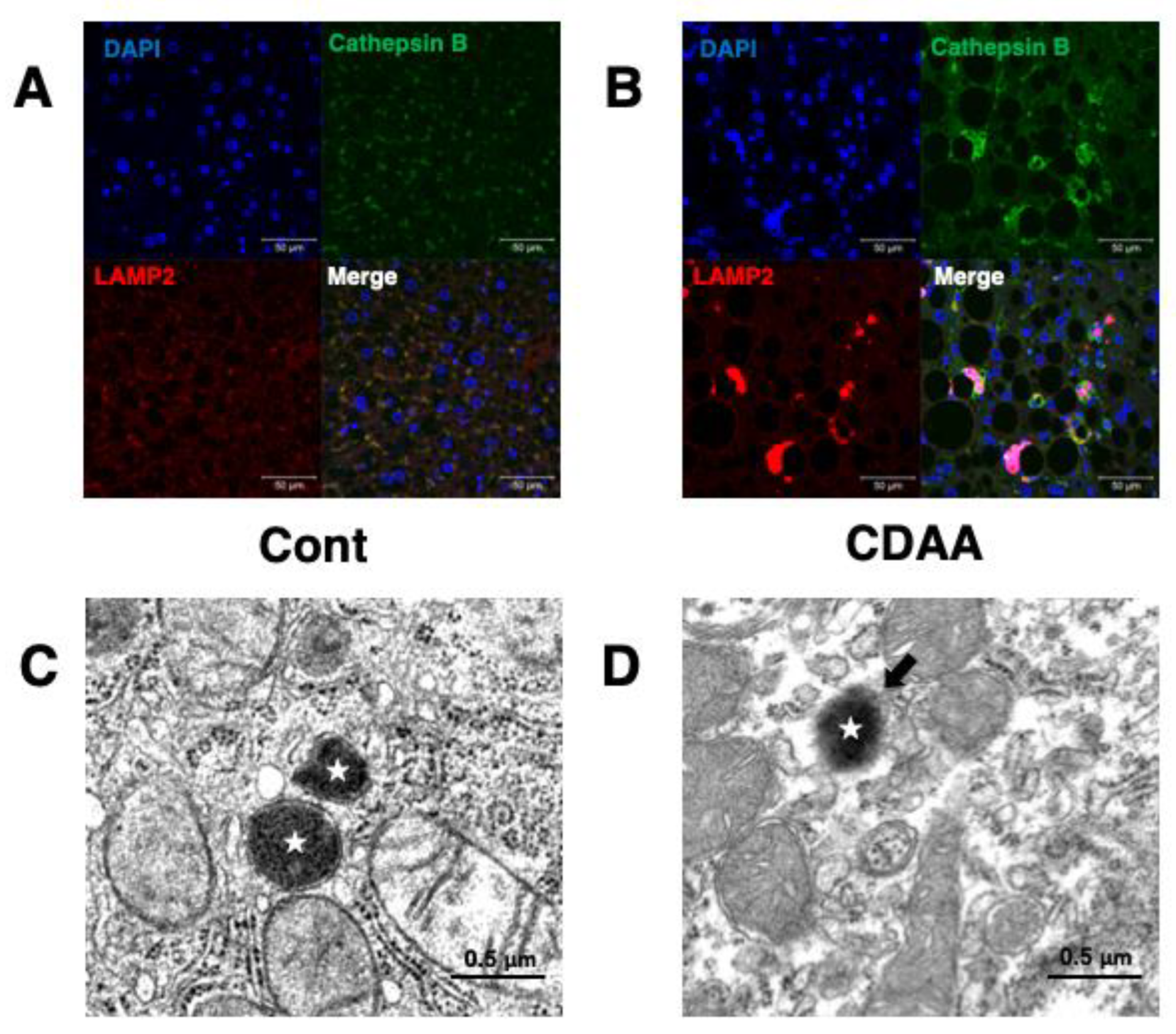
4. CDAA-Diet-Fed NASH Model Mice
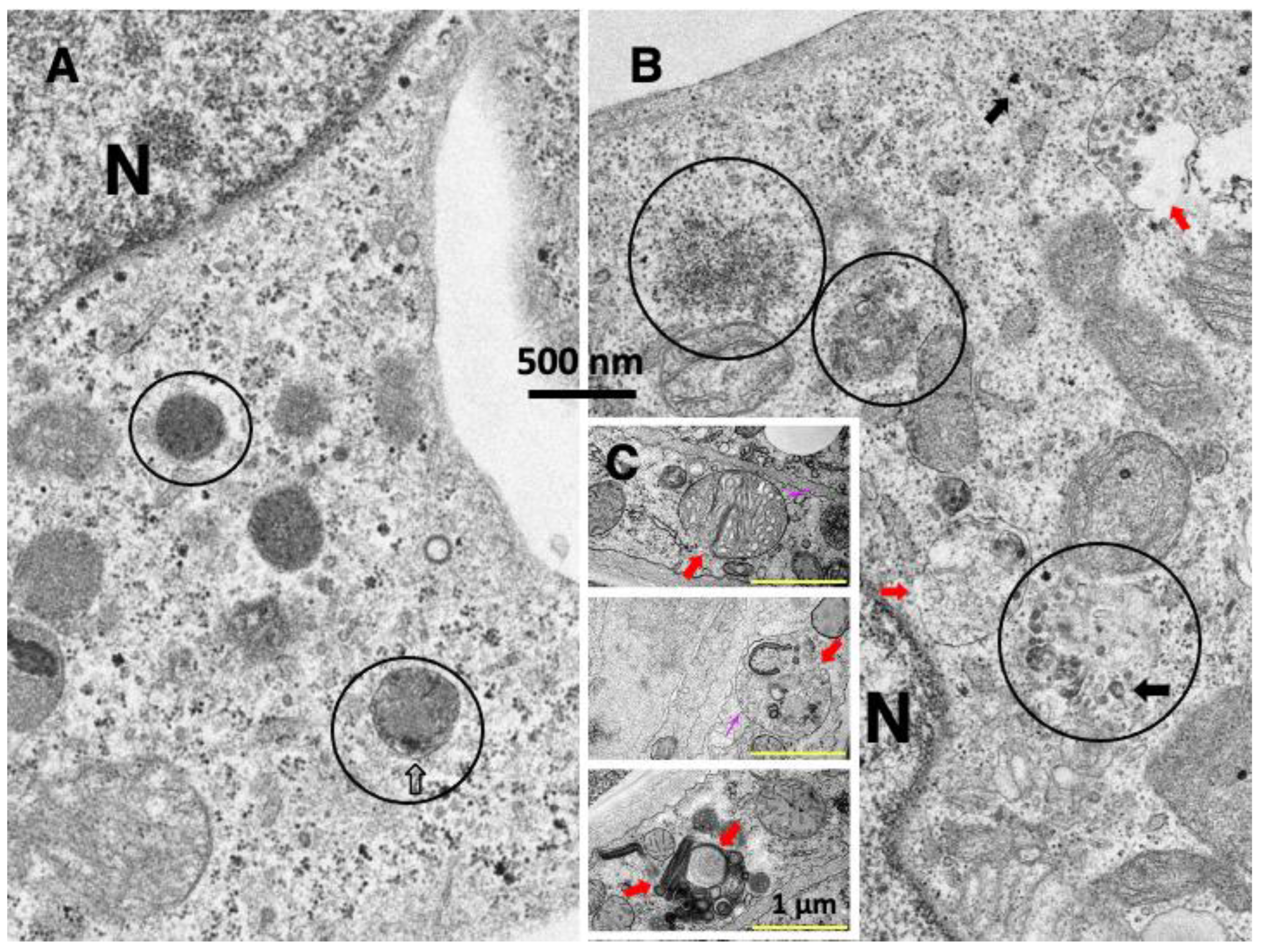
5. Monkey Organ Damage after Hydroxynonenal Injections
5.1. Liver

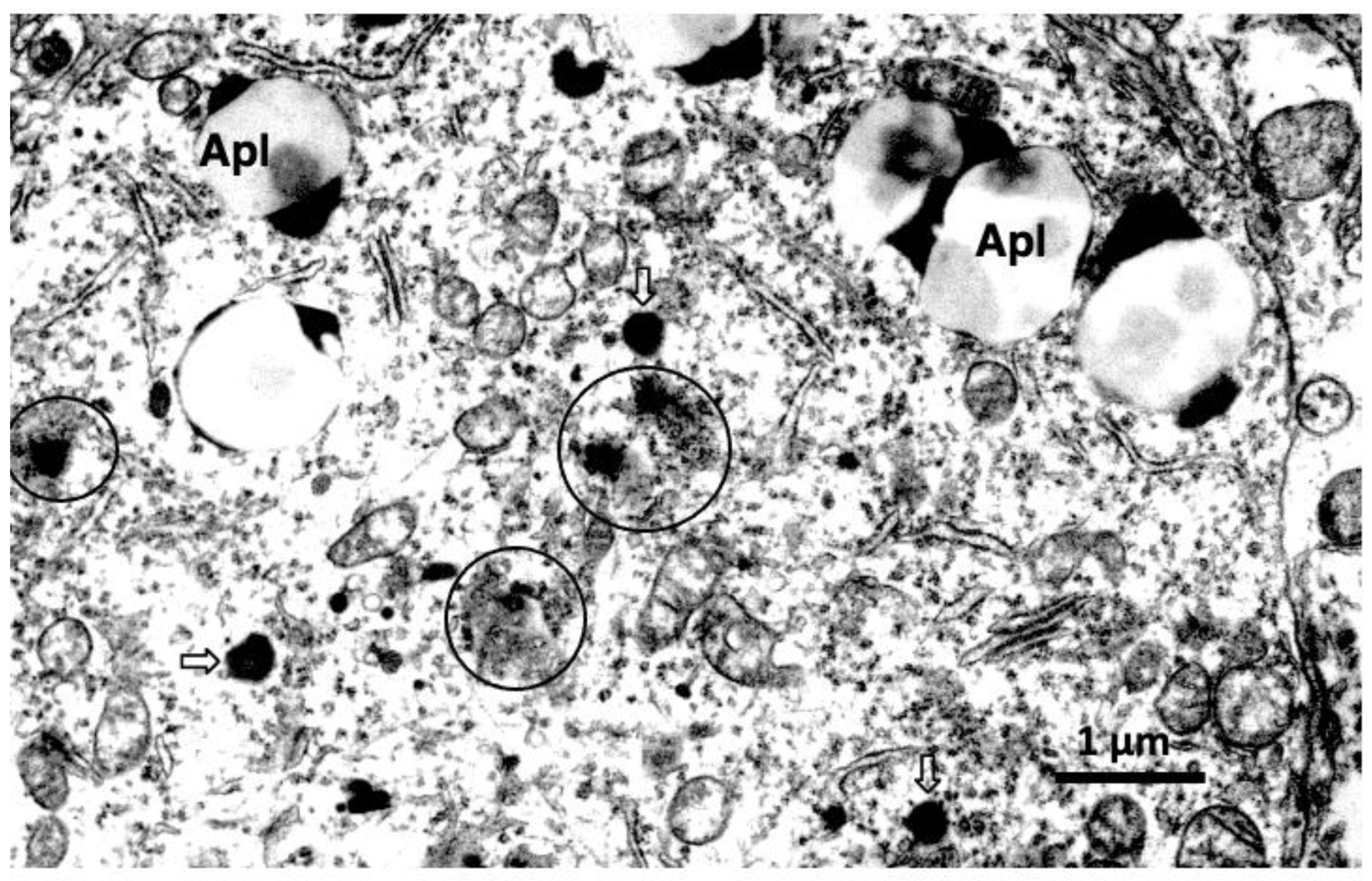
5.2. Pancreas
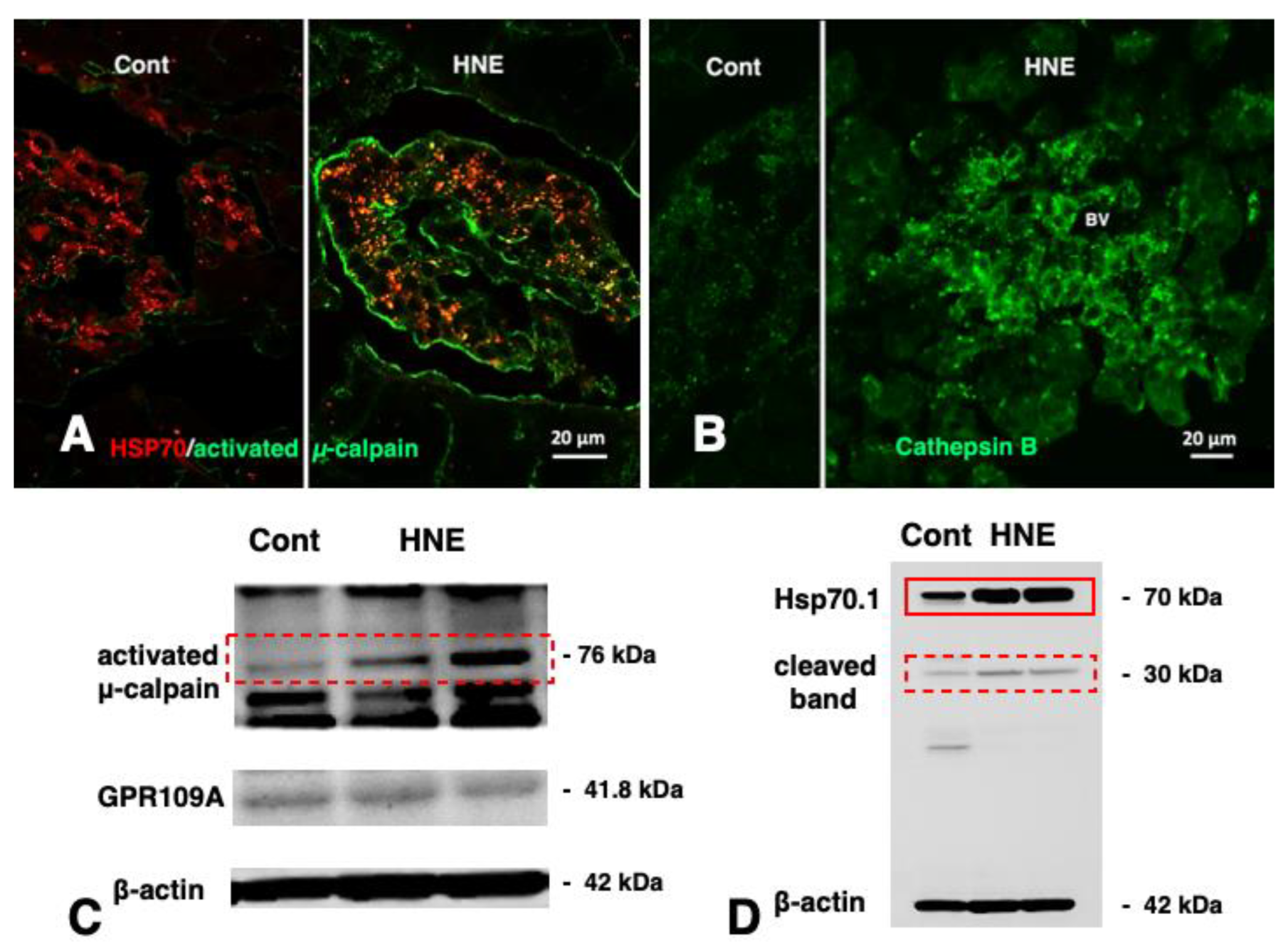
5.3. Brain
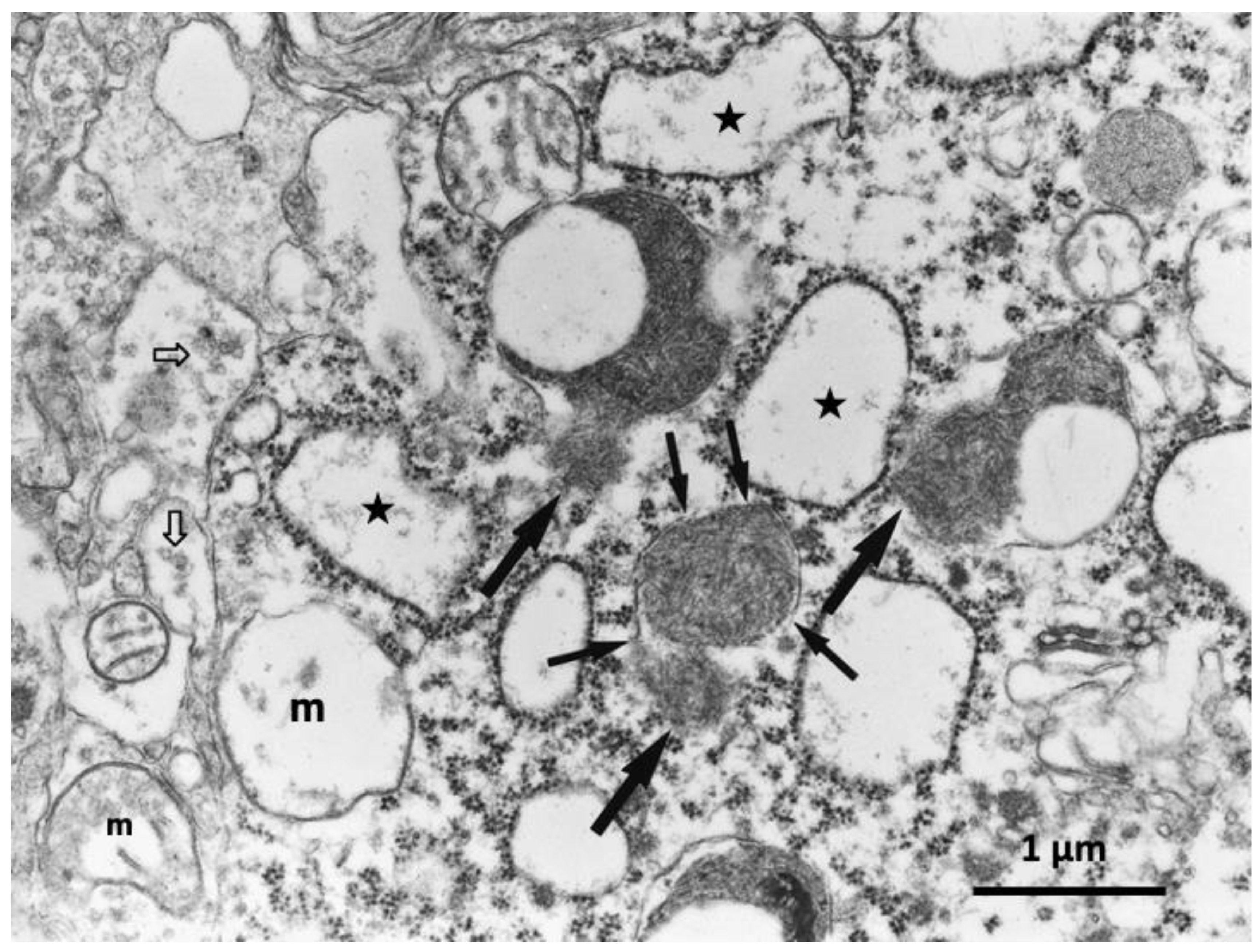
6. Monkey Brain after Transient Ischemia
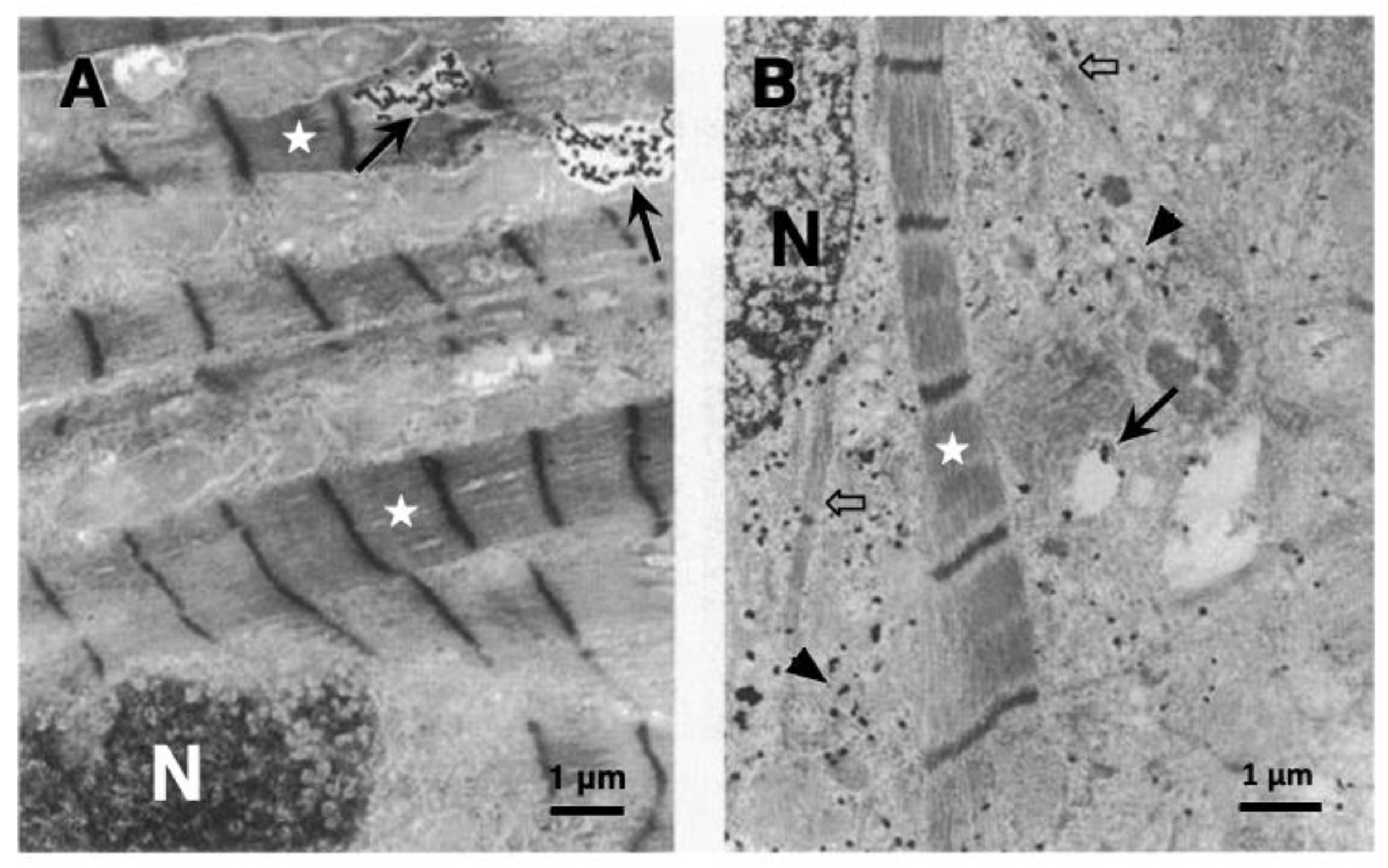
7. Human Alzheimer Brain and NASH Liver
8. Role of Lysosomal Membrane Proteins for Its Integrity
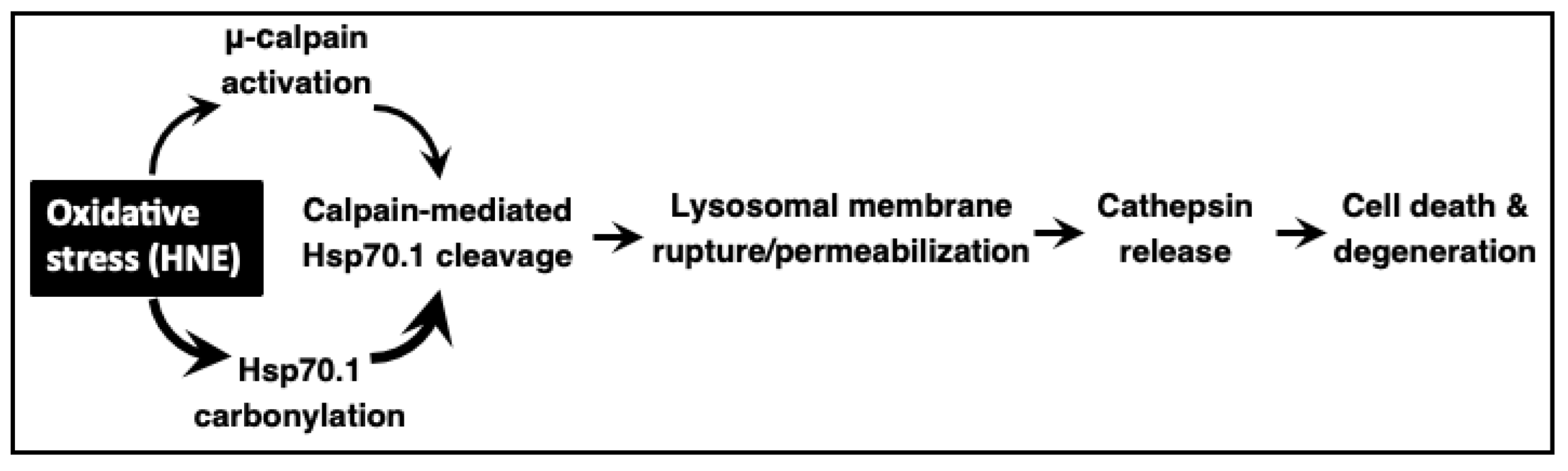
9. Role of Hsp70.1 in the Calpain–Cathepsin Cascade
10. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALLN | N-acetyl-Leu-Leu-Nle-CHO |
| C. elegans | Caenorhabditis elegans |
| CDAA-diet | choline-deficient, amino-acid defined-diet |
| CA1 | cornu Ammonis 1 |
| Hsp70.1 | heat-shock protein 70.1 |
| DAPI | 4’,6-diamidino-2-phenylindole |
| HNE | hydroxynonenal |
| LAMP-2 | lysosome-associated membrane protein 2 |
| LIMP-2 | lysosomal integral membrane protein type 2 |
| LMP | lysosomal membrane permeabilization |
| NASH | nonalcoholic steatohepatitis |
| PUFA | polyunsaturated fatty acid |
| zVAD-fmk | Z-Val-Ala-Asp-fmk |
References
- Appelmans, F.; Wattiaux, R.; de Duve, C. Tissue fractionation studies. 5. The association of acid phosphatase with a special class of cytoplasmic granules in rat liver. Biochem. J. 1955, 59, 438–445. [Google Scholar] [CrossRef] [PubMed]
- de Duve, C.; Pressman, B.C.; Gianetto, R.; Wattiaux, R.; Appelmans, F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem. J. 1955, 60, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Novikoff, A.B.; Beaufay, H.; de Duve, C. Electon microscopy of lysosome-rich fractions from rat liver. J. Biophys. Biochem. Cytol. 1956, 2 (Suppl. 4), 179–184. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2229688/pdf/179.pdf. (accessed on 20 January 2022). [CrossRef] [PubMed]
- Allison, A.C. Lysosomes. In The Biological Basis of Medicine; Bittar, E.E., Bittar, N., Eds.; Academic Press: London, UK; New York, NY, USA, 1968; Volume I, p. 209. [Google Scholar]
- de Duve, C.; Wattiaux, R. Functions of lysosomes. Ann. Rev. Physiol. 1966, 28, 435–492. [Google Scholar] [CrossRef]
- Brunk, U.T.; Ericsson, J.L. Cytochemical evidence for the leakage of acid phosphatase through ultrastructurally intact lysosomal membranes. Histochem. J. 1972, 4, 479–491. [Google Scholar] [CrossRef]
- Antunes, F.; Cadenas, E.; Brunk, U.T. Apoptosis induced by exposure to a low steady-state concentration of H2O2 is a consequence of lysosomal rupture. Biochem J. 2001, 356 Pt 2, 549–555. [Google Scholar] [CrossRef]
- Brunk, U.T.; Dalen, H.; Roberg, K.; Hellquist, H.B. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Rad. Biol. Med. 1997, 23, 616–626. [Google Scholar] [CrossRef]
- Brunk, U.T.; Svensson, I. Oxidative stress, growth factor starvation and Fas activation may all cause apoptosis through lysosomal leak. Redox Rep. 1999, 4, 3–11. [Google Scholar] [CrossRef]
- Kågedal, K.; Zhao, M.; Svensson, I.; Brunk, U.T. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem. J. 2001, 359, 335–343. [Google Scholar] [CrossRef]
- Li, W.; Yuan, X.; Nordgren, G.; Dalen, H.; Dubowchik, G.M.; Firestone, R.A.; Brunk, U.T. Induction of cell death by the lysosomotropic detergent MSDH. FEBS Lett. 2000, 470, 35–39. [Google Scholar] [CrossRef]
- Boya, P.; Andreau, K.; Poncet, D.; Zamzami, N.; Perfettini, J.L.; Metivier, D.; Ojcius, D.M.; Jäättelä, M.; Kroemer, G. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J. Exp. Med. 2003, 197, 1323–1334. [Google Scholar] [CrossRef]
- Terman, A.; Kurz, T.; Gustafsson, B.; Brunk, U.T. Lysosomal labilization. IUBMB Life 2006, 58, 531–539. [Google Scholar] [CrossRef]
- Yamashima, T.; Saido, T.C.; Takita, M.; Miyazawa, A.; Yamano, J.; Miyakawa, A.; Nishijyo, H.; Yamashita, J.; Kawashima, S.; Ono, T.; et al. Transient brain ischaemia provokes Ca2+, PIP2 and calpain responses prior to delayed neuronal death in monkeys. Eur. J. Neurosci. 1996, 8, 1932–1944. [Google Scholar] [CrossRef]
- Bursch, W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 2001, 8, 569–581. [Google Scholar] [CrossRef]
- Kirkegaard, T.; Jäättelä, M. Lysosomal involvement in cell death and cancer. Biochim. Biophys. Acta 2009, 1793, 746–754. [Google Scholar] [CrossRef]
- Nylandsted, J.; Gyrd-Hansen, M.; Danielewicz, A.; Fehrenbacher, N.; Lademann, U.; Høyer-Hansen, M.; Weber, E.; Multhoff, G.; Rohde, M.; Jäättelä, M. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J. Exp. Med. 2004, 200, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Seike, T.; Boontem, P.; Kido, H.; Yanagi, M.; Yamamiya, D.; Nakagawa, H.; Yamashita, T.; Li, S.; Okada, H.; Harada, K.; et al. Hydroxynonenal causes hepatocyte death by disrupting lysosomal integrity in non-alcoholic steatohepatitis. Cell. Mol. Gastro. Hepatol. 2022, 14, 925–944. [Google Scholar] [CrossRef]
- Syntichaki, P.; Xu, K.; Driscoll, M.; Tavernarakis, N. Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature 2002, 419, 939–944. [Google Scholar] [CrossRef]
- Tardy, C.; Codogno, P.; Autefage, H.; Levade, T.; Andrieu-Abadie, N. Lysosomes and lysosomal proteins in cancer cell death (new players of an old struggle). Biochim. Biophys. Acta 2006, 1765, 101–125. [Google Scholar] [CrossRef]
- Yamashima, T. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Progress Neurobiol. 2000, 62, 273–295. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, T. Reconsider Alzheimer’s disease by the ‘calpain–cathepsin hypothesis’—A perspective review. Prog. Neurobiol. 2013, 105, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, T.; Ota, T.; Mizukoshi, E.; Nakamura, H.; Yamamoto, Y.; Kikuchi, M.; Yamashita, T.; Kaneko, S. Intake of ω-6 polyunsaturated fatty acid-rich vegetable oils and risk of lifestyle diseases. Adv. Nutr. 2020, 11, 1489–1509. [Google Scholar] [CrossRef] [PubMed]
- Firestone, R.A.; Pisano, J.M.; Bonney, R.J. Lysosomotropic agents. 1. Synthesis and cytotoxic action of lysosomotropic detergents. J. Med. Chem. 1979, 22, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, T.; Kohda, Y.; Tsuchiya, K.; Ueno, T.; Yamashita, J.; Yoshioka, T.; Kominami, E. Inhibition of ischaemic hippocampal neuronal death in primates with catepsin B inhibitor CA-074: A novel strategy for neuroprotection based on `calpain-cathepsin hypothesis’. Eur. J. Neurosci. 1998, 10, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, G.; Tzaneva, M.; Bratoeva, K.; Ivanova, I.; Kotzev, A.; Hristova, M.; Krastev, D.; Kindekov, I.; Mileva, M. 4-Hydroxynonenal (HNE) and hepatic injury related to chronic oxidative stress. Biotechnol. Biotechnol. Equip. 2019, 33, 1544–1552. [Google Scholar] [CrossRef]
- Humphries, K.M.; Yoo, Y.; Szweda, L.I. Inhibition of NADH-linked mitochondrial respiration by 4-hydroxy-2-nonenal. Biochemistry 1998, 37, 552–557. [Google Scholar] [CrossRef]
- Lashin, O.M.; Szweda, P.A.; Szweda, L.I.; and Romani, A.M.P. Decreased complex II respiration and HNE-modified SDH subunit in diabetic heart. Free Radic. Biol. Med. 2006, 40, 886–896. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, S.; Tang, Z.; Tang, J.; Takhar, P.S. Microwave frying and post-frying of French fries. Food Res. Int. 2022, 159, 111663. [Google Scholar] [CrossRef] [PubMed]
- Boontem, P.; Yamashima, T. Hydroxynonenal causes Langerhans cell degeneration in the pancreas of Japanese macaque monkeys. PLoS ONE 2021, 16, e0245702. [Google Scholar] [CrossRef]
- Yamashima, T.; Boontem, P.; Shimizu, H.; Ota, T.; Kikuchi, M.; Yamashita, T.; Mizukoshi, E.; Kaneko, S. Vegetable oil-derived ‘hydroxynonenal’ causes diverse cell death possibly leading to Alzheimer’s and related lifestyle diseases. J. Alzheimers Dis. Park. 2020, 10, 483. Available online: http://www.arimatu-clinic.com/img/20110430paper.pdf. (accessed on 1 October 2022).
- Mrschtik, M.; Ryan, K.M. Lysosomal proteins in cell death and autophagy. FEBS J. 2015, 282, 1858–1870. [Google Scholar] [CrossRef] [PubMed]
- Aits, S.; Jäättelä, M. Lysosomal cell death at a glance. J. Cell Sci. 2013, 126, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yuan, X.M.; Olsson, A.G.; Brunk, U.T. Uptake of oxidized LDL by macrophages results in partial lysosomal enzyme inactivation and relocation. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 177–184. [Google Scholar] [CrossRef]
- Roberg, K.; Öllinger, K. Oxidative stress causes relocation of the lysosomal enzyme cathepsin D with ensuing apoptosis in neonatal rat cardiomyocytes. Am. J. Pathol. 1998, 152, 1151–1156. [Google Scholar]
- Heybrock, S.; Kanerva, K.; Meng, Y.; Ing, C.; Liang, A.; Xiong, Z.-J.; Weng, X.; Kim, Y.A.; Collins, R.; Trimble, W.; et al. Lysosomal integral membrane protein-2 (LIMP-2/SCARB2) is involved in lysosomal cholesterol export. Nat. Commun. 2019, 10, 3521. [Google Scholar] [CrossRef]
- Reczek, D.; Schwake, M.; Schroöder, J.; Hughes, H.; Blanz, J.; Jin, X.; Brondyk, W.; Patten, S.V.; Edmunds, T.; Saftig. P. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell 2007, 131, 770–783. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B.; Zou, W.; Wang, X.; Wu, Y.; Zhao, D.; Sun, Y.; Liu, Y.; Chen, L.; Miao, L.; et al. The lysosomal membrane protein SCAV-3 maintains lysosome integrity and adult longevity. J. Cell Biol. 2016, 215, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Koteish, A.; Diehl, A.M. Animal models of steatosis. Semin. Liver Dis. 2001, 21, 89–104. [Google Scholar] [CrossRef]
- McGrath, L.T.; McGleenon, B.M.; Brennan, S.; McColl, D.; McILroy, S.; Passmore, A.P. Increased oxidative stress in Alzheimer’s disease as assessed with 4-hydroxynonenal but not malondialdehyde. QJ Med. 2001, 94, 485–490. [Google Scholar] [CrossRef]
- Yamashima, T. Focus on lysosomal rupture in Alzheimer neuron. J. Alzheimers. Dis. Park. 2020, 10, 7. [Google Scholar]
- Yamashima, T. Can ‘calpain-cathepsin hypothesis’ explain Alzheimer neuronal death? Age. Res. Rev. 2016, 32, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J. Biol. Chem. 1991, 266, 21327–21330. Available online: https://www.jbc.org/article/S0021-9258(18)54636-6/pdf. (accessed on 20 January 2022). [CrossRef] [PubMed]
- Lewis, V.; Green, S.A.; Marsh, M.; Vihko, P.; Helenius, A.; Mellman, I. Glycoproteins of the lysosomal membrane. J. Cell Biol. 1985, 100, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Valeiras, M.; Sidransky, E.; Tayebi, N. Lysosomal integral membrane protein-2: A new player in lysosome-related pathology. Mol. Genet. Metab. 2014, 111, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.A. A “protease activation cascade” in the pathogenesis of Alzheimer’s disease. Ann. N.Y. Acad. Sci. 2000, 924, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Fujita, Y.; Hayashi, S.; Kakita, A.; Takahashi, H.; Murayama, S.; Saido, T.C.; Hisanaga, S.; Iwatsubo, T.; Hasegawa, M. Calpain-mediated degradation of p35 to p25 in postmortem human and rat brains. FEBS Lett. 2001, 489, 46–50. [Google Scholar] [CrossRef]
- Reifert, J.; Hartung-Cranston, D.; Feinstein, S.C. Amyloid β-mediated cell death of cultured hippocampal neurons reveals extensive Tau fragmentation without increased full-length Tau phosphorylation. J. Biol. Chem. 2011, 286, 20797–20811. [Google Scholar] [CrossRef]
- Sahara, S.; Yamashima, T. Calpain-mediated Hsp70.1 cleavage in hippocampal CA1 neuronal death. Biochem. Biophys. Res. Commun. 2010, 393, 806–811. [Google Scholar] [CrossRef]
- Gerónimo-Olvera, C.; Montiel, T.; Rincon-Heredia, R.; Castro-Obregón, S.; Massieu, L. Autophagy fails to prevent glucose deprivation/glucose reintroduction-induced neuronal death due to calpain-mediated lysosomal dysfunction in cortical neurons. Cell Death Dis. 2017, 8, e2911. [Google Scholar] [CrossRef]
- Villalpando Rodríguez, G.E.; Torriglia, A. Calpain 1 induce lysosomal permeabilization by cleavage of lysosomal associated membrane protein 2. Biochim. Biophys. Acta 2013, 1833, 2244–2253. [Google Scholar] [CrossRef]
- Arnandis, T.; Ferrer-Vicens, I.; García-Trevijano, E.R.; Miralle, V.J.; García, C.; Torres, L.; Viña, J.R.; Zaragozá, R. Calpains mediate epithelial-cell death duringmammary gland involution:mitochondria and lysosomal destabilization. Cell Death Differ. 2012, 19, 1536–1548. [Google Scholar] [CrossRef]
- Oikawa, S.; Yamada, T.; Minohata, T.; Kobayashi, H.; Furukawa, A.; Tada- Oikawa, S.; Hiraku, Y.; Murata, M.; Kikuchi, M.; Yamashima, T. Proteomic identification of carbonylated proteins in the monkey hippocampus after ischemia-reperfusion. Free Radic. Biol. Med. 2009, 46, 1472–1477. [Google Scholar] [CrossRef]
- Yamashima, T.; Oikawa, S. The role of lysosomal rupture in neuronal death. Prog. Neurobiol. 2009, 89, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Schaur, R.J.; Siems, W.; Bresgen, N.; Eckl, P.M. 4-Hydroxy-nonenal-A bioactive lipid peroxidation product. Biomolecules 2015, 5, 2247–2337. [Google Scholar] [CrossRef]
- Liang, H.; Kurimoto, S.; Shima, K.R.; Shimizu, D.; Ota, T.; Minabe, Y.; Yamashima, T. Why is hippocampal CA1 especially vulnerable to ischemia? SOJ Biochem. 2016, 2, 7. [Google Scholar] [CrossRef]
- Yamashima, T.; Mathivanan, A.; Dazortsava, M.Y.; Sakai, S.; Kurimoto, S.; Zhu, H.; Funaki, N.; Liang, H.; Hullin-Matsuda, F.; Kobayashi, T.; et al. Calpain-mediated Hsp70.1 cleavage in monkey CA1 after ischemia induces similar ‘lysosomal vesiculosis’ to Alzheimer neurons. J. Alzheimers Dis. Parkinsonism. 2014, 4, 1000139. [Google Scholar] [CrossRef]
- Schröder, B.A.; Wrocklage, C.; Hasilik, A.; Saftig, P. The proteome of lysosomes. Proteomics 2010, 10, 4053–4076. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashima, T. Implication of Vegetable Oil-Derived Hydroxynonenal in the Lysosomal Cell Death for Lifestyle-Related Diseases. Nutrients 2023, 15, 609. https://doi.org/10.3390/nu15030609
Yamashima T. Implication of Vegetable Oil-Derived Hydroxynonenal in the Lysosomal Cell Death for Lifestyle-Related Diseases. Nutrients. 2023; 15(3):609. https://doi.org/10.3390/nu15030609
Chicago/Turabian StyleYamashima, Tetsumori. 2023. "Implication of Vegetable Oil-Derived Hydroxynonenal in the Lysosomal Cell Death for Lifestyle-Related Diseases" Nutrients 15, no. 3: 609. https://doi.org/10.3390/nu15030609
APA StyleYamashima, T. (2023). Implication of Vegetable Oil-Derived Hydroxynonenal in the Lysosomal Cell Death for Lifestyle-Related Diseases. Nutrients, 15(3), 609. https://doi.org/10.3390/nu15030609




