The Effects of Amorphous Calcium Carbonate (ACC) Supplementation on Resistance Exercise Performance in Women
Abstract
1. Introduction
2. Materials and Methods
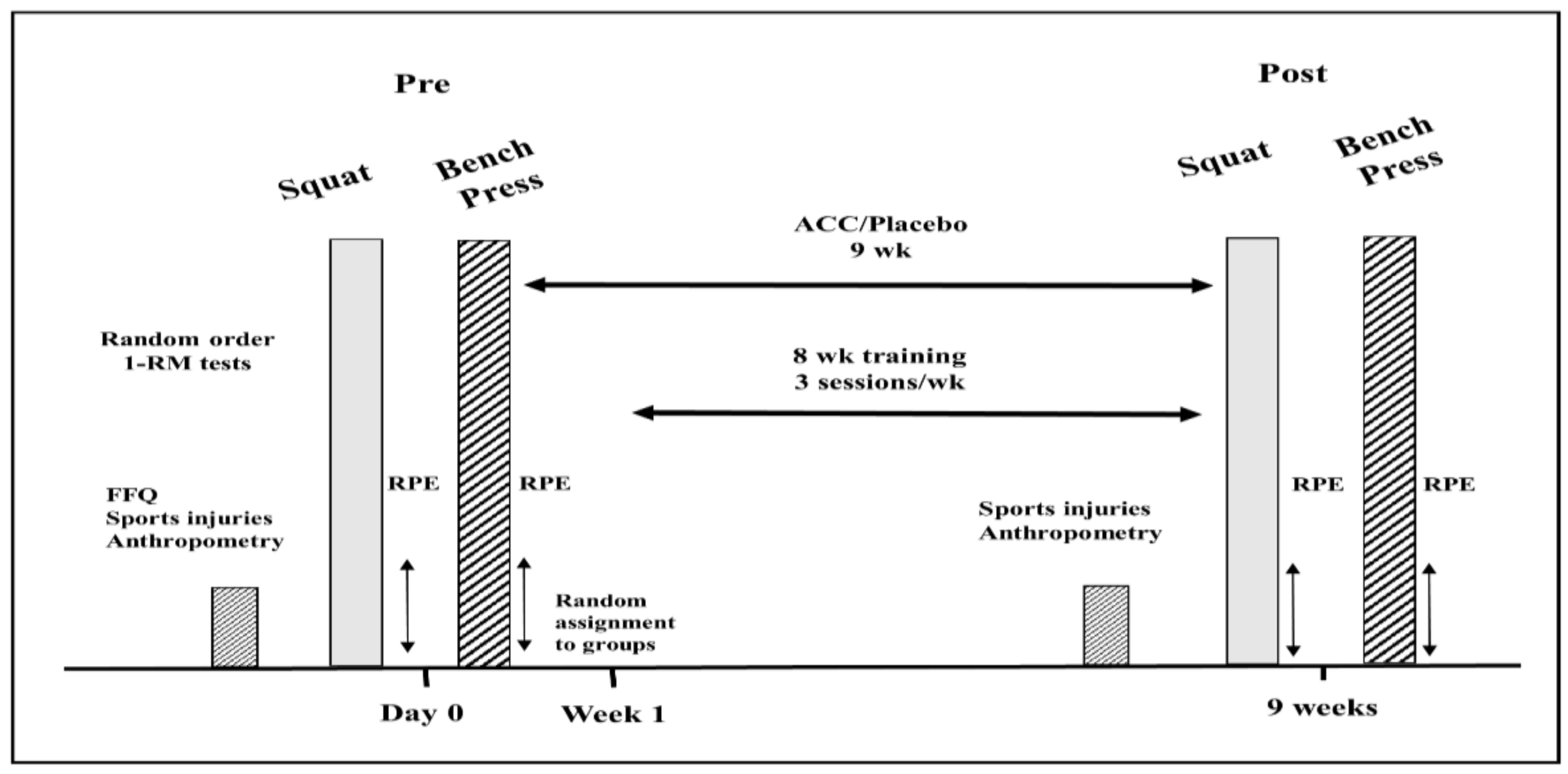
2.1. Experimental Approach
2.2. Participants
2.3. Study Protocol
2.4. Supplement Protocol
2.5. Training
2.6. Maximal Strength Testing
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Baron, J.A.; Burckhardt, P.; Li, R.; Spiegelman, D.; Willett, W.C. Calcium intake and hip fracture risk in men and women: A meta-analysis of prospective cohort studies and randomized controlled trials. Am. J. Clin. Nutr. 2007, 86, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Leung, W.; Tai, V.; Bastin, S.; Gamble, G.D.; Grey, A.; Reid, I.R. Calcium intake and risk of fracture: Systematic review. BMJ 2015, 351, h4580. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.M.; Eslick, G.D.; Nowson, C.; Smith, C.; Bensoussan, A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: A meta-analysis. Lancet 2007, 370, 657–666. [Google Scholar] [CrossRef]
- Arroyo, E.; Jajtner, A.R. Vitamins and Minerals. In Dietary Supplementation in Sport and Exercise; Hoffman, J.R., Ed.; Routledge: New York, NY, USA, 2019; pp. 22–46. [Google Scholar]
- Harvey, N.C.; Biver, E.; Kaufman, J.M.; Bauer, J.; Branco, J.; Brandi, M.L.; Cooper, C. The role of calcium supplementation in healthy musculoskeletal ageing: An expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos. Int. 2017, 28, 447–462. [Google Scholar] [PubMed]
- Chandran, M.; Tay, D.; Mithal, A. Supplemental calcium intake in the aging individual: Implications on skeletal and cardiovascular health. Aging Clin. Exp. Res. 2019, 31, 765–781. [Google Scholar] [CrossRef]
- Weaver, C.M.; Alexander, D.D.; Boushey, C.J.; Dawson-Hughes, B.; Lappe, J.M.; LeBoff, M.S.; Looker, A.C.; Wallace, T.C.; Wang, D.D. Calcium plus vitamin D supplementation and risk of fractures: An updated meta-analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016, 27, 367–376. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Ben-Zeev, T.; Zamir, A.; Levi, C.; Ostfeld, I. Examination of amorphous calcium carbonate on the inflammatory and muscle damage response in experienced resistance trained individuals. Nutrients 2022, 14, 1894. [Google Scholar] [CrossRef]
- Meiron, O.E.; Bar-David, E.; Aflalo, E.D.; Shechter, A.; Stepensky, D.; Berman, A.; Sagi, A. Solubility and bioavailability of stabilized amorphous calcium carbonate. J. Bone Miner. Res. 2011, 26, 364–372. [Google Scholar] [CrossRef]
- Straub, D.A. Calcium supplementation in clinical practice: A review of forms, doses, and indications. Nutr. Clin. Pract. 2007, 22, 286–296. [Google Scholar] [CrossRef]
- Heaney, R.P.; Dowell, M.S.; Bierman, J.; Hale, C.A.; Bendich, A. Absorbability and cost effectiveness in calcium supplementation. J. Am. Coll. Nutr. 2001, 20, 239–246. [Google Scholar] [CrossRef]
- Nebel, H.; Neumann, M.; Mayer, C.; Epple, M. On the structure of amorphous calcium carbonate--a detailed study by solidstate NMR spectroscopy. Inorg. Chem. 2008, 47, 7874–7879. [Google Scholar] [CrossRef]
- Gal, J.Y.; Bollinger, J.C.; Tolosa, H.; Gache, N. Calcium carbonate solubility: A reappraisal of scale formation and inhibition. Talanta 1996, 43, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Shechter, A.; Berman, A.; Singer, A.; Freiman, A.; Grinstein, M.; Erez, J.; Sagi, A. Reciprocal changes in calcification of the gastrolith and cuticle during the molt cycle of the red claw crayfish Cherax quadricarinatus. Biol. Bull. 2008, 214, 122–134. [Google Scholar] [CrossRef]
- Shaltiel, G.; Bar-David, E.; Meiron, O.E.; Waltman, E.; Shechter, A.; Aflalo, E.D.; Sagi, A. Bone loss prevention in ovariectomized rats using stable amorphous calcium carbonate. Health 2013, 5, 18–29. [Google Scholar] [CrossRef]
- Vaisman, N.; Shaltiel, G.; Daniely, M.; Meiron, O.E.; Shechter, A.; Abrams, S.A.; Sagi, A. Increased calcium absorption from synthetic stable amorphous calcium carbonate. J. Bone Mineral Res. 2014, 29, 2203–2209. [Google Scholar] [CrossRef] [PubMed]
- Som, A.; Raliya, R.; Tian, L.; Akers, W.; Ippolito, J.E.; Singamaneni, S.; Achilefu, S. Monodispersed calcium carbonate nanoparticles modulate local pH and inhibit tumor growth in vivo. Nanoscale 2016, 8, 12639–12647. [Google Scholar] [CrossRef]
- De Oliveira, L.F.; Saunders, B.; Artioli, G.G. Is bypassing the stomach a means to optimize sodium bicarbonate supplementation? A case study with a postbariatric surgery individual. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 660–663. [Google Scholar] [CrossRef]
- Hadzic, M.; Eckstein, M.L.; Schugardt, M. The impact of sodium bicarbonate on performance in response to exercise duration in athletes: A systematic review. J. Sports Sci. Med. 2019, 18, 271. [Google Scholar]
- Lopes-Silva, J.P.; Reale, R.; Franchini, E. Acute and chronic effect of sodium bicarbonate ingestion on Wingate test performance: A systematic review and meta-analysis. J. Sports Sci. 2019, 37, 762–771. [Google Scholar] [CrossRef]
- Burke, L.M.; Pyne, D.B. Bicarbonate loading to enhance training and competitive performance. Int J. Sports Physiol. Performance. 2007, 2, 93–97. [Google Scholar] [CrossRef]
- Seo, D.I.; Kim, E.; Fahs, C.A.; Rossow, L.; Young, K.; Ferguson, S.L.; So, W.Y. Reliability of the one-repetition maximum test based on muscle group and gender. J. Sports Sci. Med. 2012, 11, 221. [Google Scholar] [PubMed]
- Hoffman, J.R. Physiological Aspects of Sport Training and Performance; Human Kinetics: Champaign, IL, USA, 2014. [Google Scholar]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.R.; Wilborn, C.D.; Sale, C.; Antonio, J. International society of sports nutrition position stand: Beta-Alanine. J. Int. Soc. Sports Nutr. 2015, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Santana, J.O.; De Freitas, M.C.; Dos Santos, D.M.; Rossi, F.E.; Lira, F.S.; Rosa-Neto, J.C.; Caperuto, E.C. Beta-alanine supplementation improved 10-km running time trial in physically active adults. Front. Physiol. 2018, 9, 1105. [Google Scholar] [CrossRef]
- Van Thienen, R.; Van Proeyen, K.; Puype, J.; Lefere, T.; Hespel, P. Beta-alanine improves sprint performance in endurance cycling. Med. Sci. Sports Exerc. 2009, 41, 898–903. [Google Scholar] [CrossRef]
- Lopez, P.; Radaelli, R.; Taaffe, D.R.; Newton, R.U.; Galvão, D.A.; Trajano, G.S.; Teodoro, J.L.; Kraemer, W.J.; Häkkinen, K.; Pinto, R.S. Resistance training load effects on muscle hypertrophy and strength gain: Systematic review and network meta-analysis. Med. Sci. Sports Exerc. 2021, 53, 1206–1216. [Google Scholar] [CrossRef]
- Stewart, P.A. Modern quantitative acid–base chemistry. Can. J. Physiol. Pharma. 1983, 61, 1444–1461. [Google Scholar] [CrossRef]
- Lappe, J.; Cullen, D.; Haynatzki, G.; Recker, R.; Ahlf, R.; Thompson, K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J. Bone Miner. Res. 2008, 23, 741–749. [Google Scholar] [CrossRef]
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010, 5 (Suppl. S1), S23–S30. [Google Scholar] [CrossRef]
- Mammucari, C.; Raffaello, A.; VecellioReane, D.; Gherardi, G.; De Mario, A.; Rizzuto, R. Mitochondrial calcium uptake in organ physiology: From molecular mechanism to animal models. Pflügers Archiv Europ. J. Physiol. 2018, 470, 1165–1179. [Google Scholar] [CrossRef]
- Cairns, S.P.; Leader, J.P.; Loiselle, D.S.; Higgins, A.; Lin, W.; Renaud, J.M. Extracellular Ca2+-induced force restoration in K+-depressed skeletal muscle of the mouse involves an elevation of [K+] i: Implications for fatigue. J. Appl. Physiol. 2015, 118, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, M.I. Total carbon dioxide in adult standardbred and thoroughbred Horses. J. Equine Veter. Sci. 2021, 106, 103730. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, L.R.; Gough, L.; Deb, S.; Bentley, D.; Sparks, S.A. Recent developments in the use of sodium Bicarbonate as an ergogenic aid. Curr. Sports Med. Rep. 2016, 15, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.J.; Weldon, A.; Price, M.J. The effect of sodium bicarbonate ingestion on back squat and bench press exercise to failure. J. Strength Cond. Res. 2014, 28, 1358–1366. [Google Scholar] [CrossRef]
- Burton, R.F. On calculating concentrations of ”HCO3“ from pH and PCO2. Comp. Biochem. Physiol. A Comp. Physiol. 1987, 87, 417–422. [Google Scholar] [CrossRef]
| Variable | ACC | PL | p-Value |
|---|---|---|---|
| Age (years) | 32.3 ± 7.7 | 33.6 ± 7.2 | 0.765 |
| BM (kg) | 67.1 ± 16.0 | 72.6 ± 18.1 | 0.302 |
| Height (cm) | 162.5 ± 4.9 | 165.5 ± 5.4 | 0.131 |
| BMI (kg/m2) | 25.4 ± 6.4 | 26.4 ± 6.0 | 0.662 |
| LBMI (kg/m2) | 16.5 ± 2.2 | 16.5 ± 1.5 | 0.972 |
| BF (%) | 31.9 ± 8.1 | 35.6 ± 9.1 | 0.258 |
| LBM (kg) | 43.5 ± 4.6 | 45.3 ± 4.9 | 0.306 |
| Exercise | Week 1–2 (Sets × Reps) | Week 3–4 (Sets × Reps) | Week 5–6 (Sets × Reps) | Week 7–8 (Sets × Reps) |
|---|---|---|---|---|
| Barbell back squat | 4 × 8–10 | 4 × 10–12 | 5 × 10–12 | 5 × 12–14 |
| Rubber band pull-ups | 4 × 10–12 | 4 × 12–15 | 4 × 12–15 | 5 × 12–15 |
| Bench press | 4 × 8–10 | 4 × 10–12 | 5 × 10–12 | 5 × 12–14 |
| Push press | - | 4 × 8–10 | 4 × 10–12 | 4 × 12–15 |
| Standing shoulder press | 4 × 8–10 | 4 × 8–10 | 4 × 10–12 | 4 × 10–12 |
| Static squat battle rope shake | 4 × 40–60 | 4 × 40–60 | 4 × 60–70 | 4 × 60–70 |
| Biceps curls | 4 × 8–10 | 4 × 10–12 | 5 × 10–12 | 5 × 12–14 |
| Romanian deadlift | - | 4 × 8–10 | 4 × 10–12 | 5 × 10–12 |
| TRX narrow row | 4 × 8–10 | 4 × 8–10 | 4 × 10–12 | 4 × 10–12 |
| Saw dumbbell single hand row | 4 × 10–12 | 4 × 12–15 | 4 × 12–15 | 5 × 12–15 |
| Wall ball throw | 4 × 10–12 | 4 × 12–15 | 4 × 12–15 | 4 × 12–15 |
| Abdominal routine | 3 × 10 | 3 × 12 | 4 × 10 | 4 × 12 |
| Group | ACC | PL | |
|---|---|---|---|
| Variable, Units/Day | (n = 14) | (n = 17) | p-Value |
| Energy, Kcal | 2723.3 ± 1159.4 | 2914.5 ± 1018.3 | 0.943 |
| Protein, g | 114.0 ± 54.0 | 132.1 ± 54.8 | 0.738 |
| Fat, g | 113.8 ± 50.2 | 104.9 ± 36.5 | 0.585 |
| CHO, g | 285.4 ± 146.9 | 303.9 ± 132.2 | 0.725 |
| Water, g | 3754.5 ± 1390.9 | 4409.5 ± 1280.5 | 0.198 |
| Calcium, mg | 1281.5 ± 602.0 | 1535.2 ± 732.4 | 0.551 |
| Vitamin D, µg | 8.9 ± 6.4 | 11.3 ± 7.2 | 0.354 |
| Exercise | ACC | PL | p-Value |
|---|---|---|---|
| Squat, kg | 55.5 ± 12.4 | 52.4 ± 11.3 | 0.471 |
| Squat, kg/kg BW | 0.88 ± 0.3 | 0.76 ± 0.2 | 0.213 |
| Squat, kg/kg LBM | 1.24 ± 0.3 | 1.12 ± 0.2 | 0.199 |
| Bench Press, kg | 39.3 ± 10.3 | 38.2 ± 5.6 | 0.749 |
| Bench Press, kg/kg BW | 0.59 ± 0.1 | 0.54 ± 0.1 | 0.259 |
| Bench Press, kg/kg LBM | 0.87 ± 0.2 | 0.83 ± 0.2 | 0.602 |
| Exercise | Group | p-Value | Time | p-Value | Gr X T | p-Value |
|---|---|---|---|---|---|---|
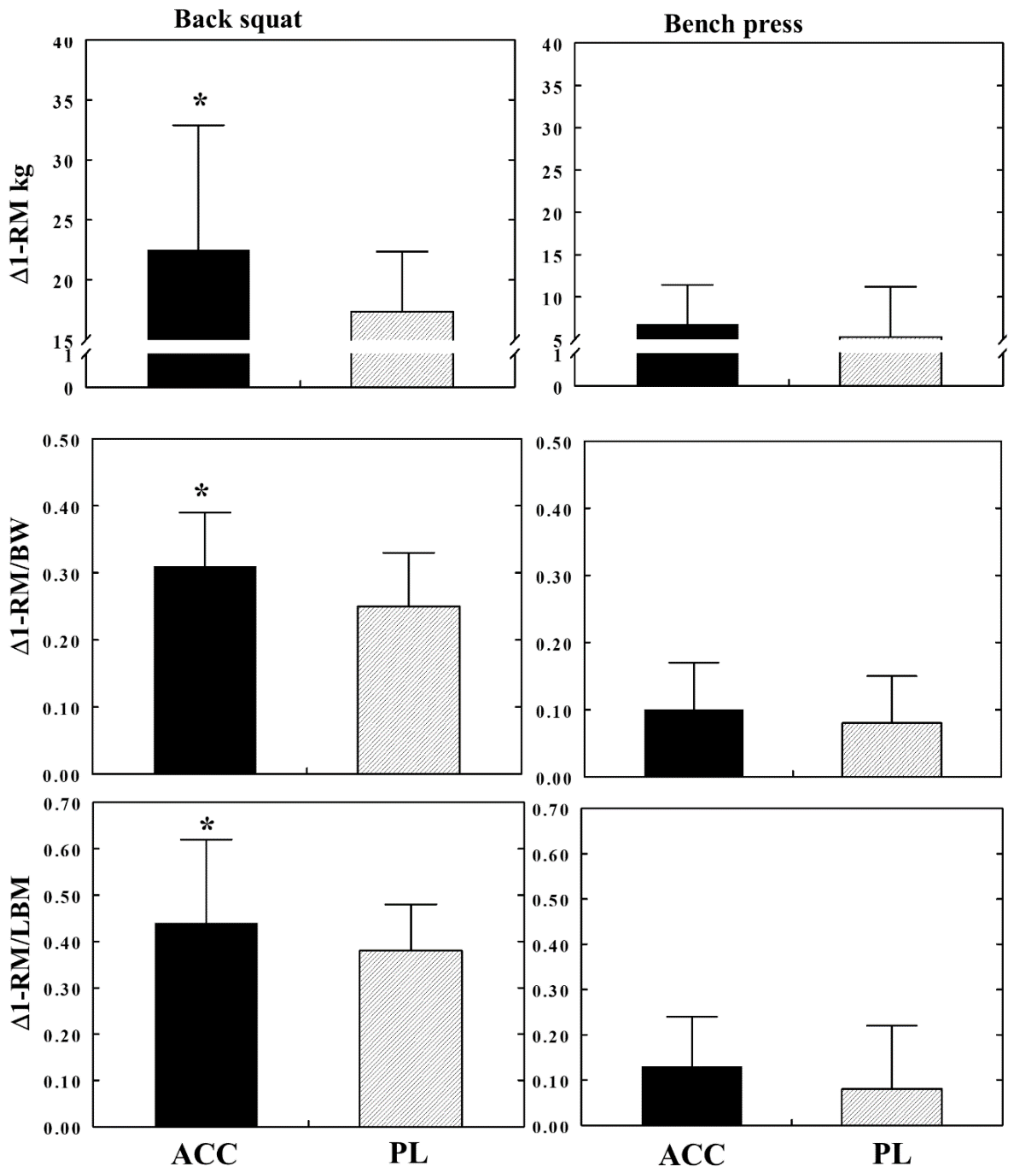
| Squat abs | 3.53 | 0.065 | 44.06 | 0.001 * | 0.66 | 0.421 |
| Squat/BW | 5.61 | 0.021 * | 19.14 | 0.001 * | 0.22 | 0.638 |
| Squat/LBM | 7.85 | 0.007 * | 40.58 | 0.001 * | 0.77 | 0.385 |
| Bench Press abs | 0.64 | 0.429 | 7.48 | 0.008 * | 0.11 | 0.738 |
| Bench Press/BW | 3.63 | 0.062 | 10.05 | 0.002 * | 0.16 | 0.691 |
| Bench Press/LBM | 1.88 | 0.176 | 5.30 | 0.025 * | 0.31 | 0.580 |
| Exercise | Effect | 95% CI | p-Value |
|---|---|---|---|
| Squat | 5.62 | [0.27, 10.96] | 0.049 * |
| Squat/BW | 0.07 | [0.01, 013] | 0.042 * |
| Squat/LBM | 0.14 | [0.02, 0.26] | 0.035 * |
| Bench Press | 1.57 | [−2.29, 5.43] | 0.432 |
| Bench Press/BW | 0.03 | [−0.03, 0.08] | 0.337 |
| Bench Press/LBM | 0.06 | [−0.02, 0.15] | 0.128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weinstein, Y.; Ovadia, Y.; Weinstein, B.; Weinstein, A. The Effects of Amorphous Calcium Carbonate (ACC) Supplementation on Resistance Exercise Performance in Women. Nutrients 2023, 15, 538. https://doi.org/10.3390/nu15030538
Weinstein Y, Ovadia Y, Weinstein B, Weinstein A. The Effects of Amorphous Calcium Carbonate (ACC) Supplementation on Resistance Exercise Performance in Women. Nutrients. 2023; 15(3):538. https://doi.org/10.3390/nu15030538
Chicago/Turabian StyleWeinstein, Yitzhak, Yarden Ovadia, Bar Weinstein, and Ayelet Weinstein. 2023. "The Effects of Amorphous Calcium Carbonate (ACC) Supplementation on Resistance Exercise Performance in Women" Nutrients 15, no. 3: 538. https://doi.org/10.3390/nu15030538
APA StyleWeinstein, Y., Ovadia, Y., Weinstein, B., & Weinstein, A. (2023). The Effects of Amorphous Calcium Carbonate (ACC) Supplementation on Resistance Exercise Performance in Women. Nutrients, 15(3), 538. https://doi.org/10.3390/nu15030538







