The Association between Tooth Loss and Insulin Resistance Mediated by Diet Quality and Systemic Immunoinflammatory Index
Abstract
:1. Introduction
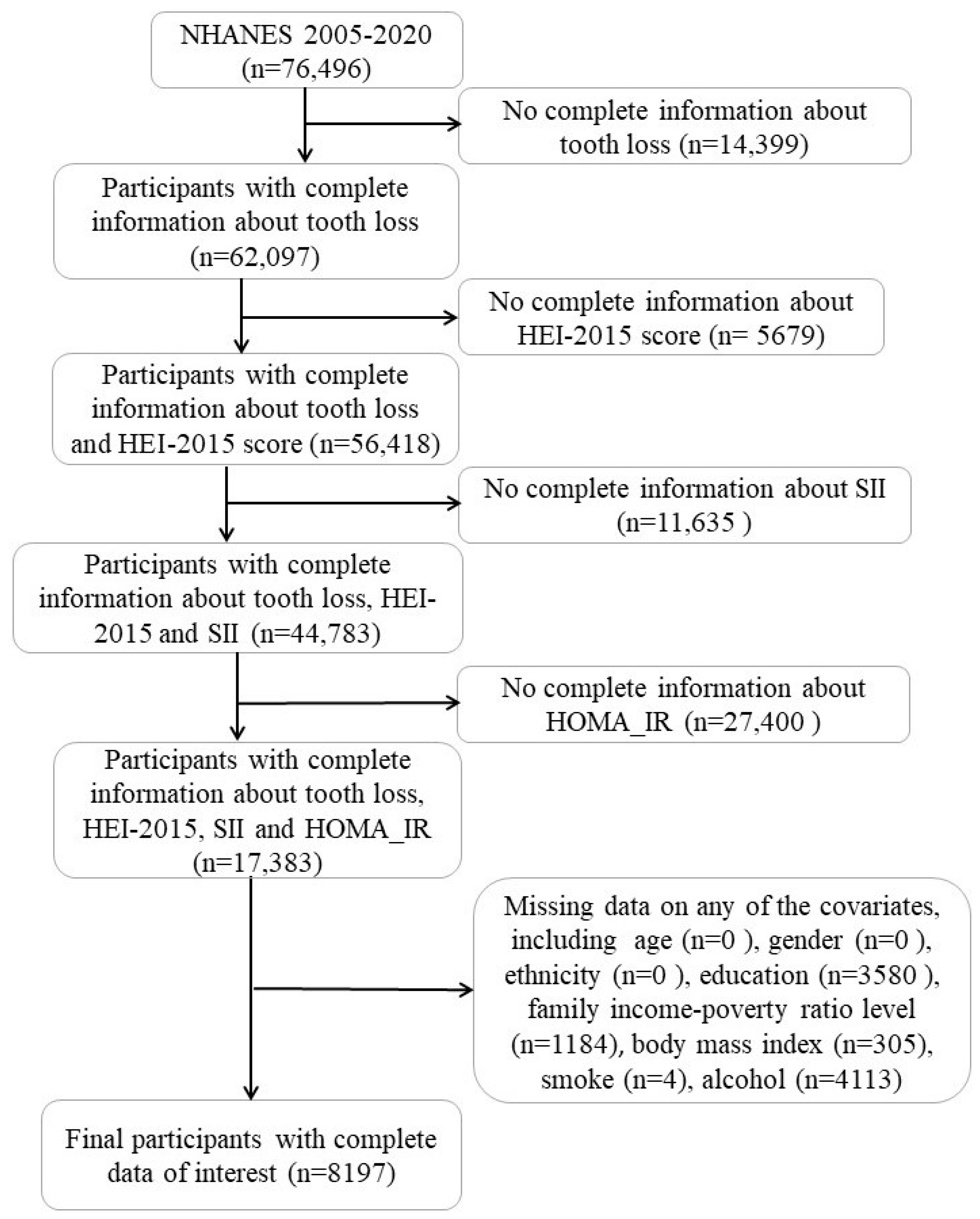
2. Method
2.1. Study Population
2.2. Measures
3. Result
3.1. Baseline Characteristics of the Studied Population
3.2. Association between Tooth Loss and HOMA-IR
3.3. Involvement of Diet Quality in the Association between Tooth Loss and HOMA-IR
3.4. Involvement of SII in the Association between Tooth Loss and HOMA-IR
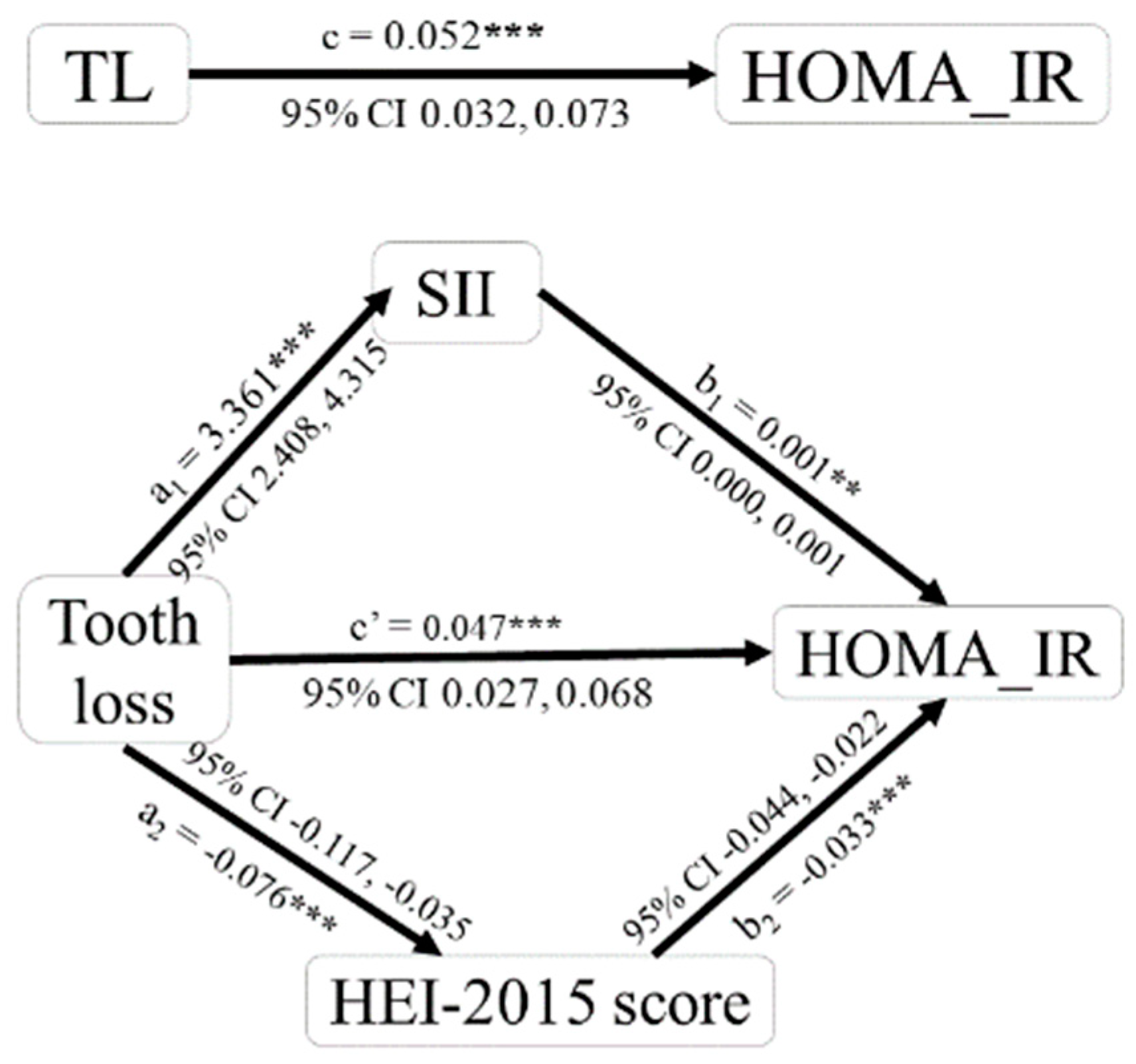
3.5. Analysis of Mediating Effects of Diet Quality and SII in the Association between Tooth Loss and HOMA-IR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimmet, P.; Alberti, K.G.M.M.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef] [PubMed]
- Abnet, C.C.; Qiao, Y.-L.; Dawsey, S.M.; Dong, Z.-W.; Taylor, P.R.; Mark, S.D. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int. J. Epidemiol. 2005, 34, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Raju, K.; Taylor, G.W.; Tahir, P.; Hyde, S. Association of tooth loss with morbidity and mortality by diabetes status in older adults: A systematic review. BMC Endocr. Disord. 2021, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, T.; Kida, M. Tooth loss leads to reduced nutrient intake in middle-aged and older Japanese individuals. Environ. Health Prev. Med. 2019, 24, 15. [Google Scholar] [CrossRef]
- Zelig, R.; Goldstein, S.; Touger-Decker, R.; Firestone, E.; Golden, A.; Johnson, Z.; Kaseta, A.; Sackey, J.; Tomesko, J.; Parrott, J.S. Tooth Loss and Nutritional Status in Older Adults: A Systematic Review and Meta-analysis. JDR Clin. Transl. Res. 2020, 7, 4–15. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Oikonomou, C.; Nychas, G.; Dimitriadis, G.D. Effects of Diet, Lifestyle, Chrononutrition and Alternative Dietary Interventions on Postprandial Glycemia and Insulin Resistance. Nutrients 2022, 14, 823. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Q.; Zhu, L.; Zhang, Y.; Lu, X.; Wu, Y.; Liu, L. Prognostic Value of Preoperative Systemic Immune-Inflammation Index in Patients with Cervical Cancer. Sci. Rep. 2019, 9, 3284. [Google Scholar] [CrossRef]
- Chen, J.-H.; Zhai, E.-T.; Yuan, Y.-J.; Wu, K.-M.; Xu, J.-B.; Peng, J.-J.; Chen, C.-Q.; He, Y.-L.; Cai, S.-R. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J. Gastroenterol. 2017, 23, 6261. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.-R.; Xu, Y.; Sun, Y.-F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.-M.; Qiu, S.-J.; Zhou, J.; et al. Systemic Immune-Inflammation Index Predicts Prognosis of Patients after Curative Resection for Hepatocellular Carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Amar, S. Periodontal disease and systemic conditions: A bidirectional relationship. Odontology 2006, 94, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef] [PubMed]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- De La Cruz, N.; Shabaneh, O.; Appiah, D. The Association of Ideal Cardiovascular Health and Ocular Diseases Among US Adults. Am. J. Med. 2021, 134, 252–259.e1. [Google Scholar] [CrossRef] [PubMed]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Homa, D.M.; Gentzke, A.S.; Mahoney, M.; Sharapova, S.R.; Sosnoff, C.S.; Caron, K.T.; Wang, L.Q.; Melstrom, P.C.; Trivers, K.F. Exposure to Secondhand Smoke Among Nonsmokers-United States, 1988–2014. Morb. Mortal. Wkly. Rep. 2018, 67, 1342–1346. [Google Scholar] [CrossRef]
- Scholes, S.; Bann, D. Education-related disparities in reported physical activity during leisure-time, active transportation, and work among US adults: Repeated cross-sectional analysis from the National Health and Nutrition Examination Surveys, 2007 to 2016. BMC Public Health 2018, 18, 926. [Google Scholar] [CrossRef]
- SSY, A.L.; Natto, Z.S.; Midle, J.B.; Gyurko, R.; O’Neill, R.; Steffensen, B. Association between time since quitting smoking and periodontitis in former smokers in the National Health and Nutrition Examination Surveys (NHANES) 2009 to 2012. J. Periodontol. 2019, 90, 16–25. [Google Scholar] [CrossRef]
- Stöhr, J.; Barbaresko, J.; Neuenschwander, M.; Schlesinger, S. Bidirectional association between periodontal disease and diabetes mellitus: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2021, 11, 13686. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Diabetes as a potential risk for periodontitis: Association studies. Periodontology 2000 2020, 83, 40–45. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2011, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Liljestrand, J.M.; Havulinna, A.S.; Paju, S.; Männistö, S.; Salomaa, V.; Pussinen, P.J. Missing Teeth Predict Incident Cardiovascular Events, Diabetes, and Death. J. Dent. Res. 2015, 94, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hollis, J.H. Tooth loss and its association with dietary intake and diet quality in American adults. J. Dent. 2014, 42, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Qian, S.J.; Huang, L.Y.; Tao, Y.; Chen, H.; Deng, K.; Yang, F.; Zong, G.; Zheng, Y.; Wang, X.F.; et al. Association of the number of natural teeth with dietary diversity and nutritional status in older adults: A cross-sectional study in China. J. Clin. Periodontol. 2023, 50, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.C.; Willett, W.; Ascherio, A.; Rosner, B.A.; Rimm, E.; Joshipura, K.J. Tooth loss and dietary intake. J. Am. Dent. Assoc. 2003, 134, 1185–1192. [Google Scholar] [CrossRef]
- He, D.; Qiao, Y.; Xiong, S.; Liu, S.; Ke, C.; Shen, Y. Association between Dietary Quality and Prediabetes based on the Diet Balance Index. Sci. Rep. 2020, 10, 3190. [Google Scholar] [CrossRef]
- Ley, S.H.; Pan, A.; Li, Y.; Manson, J.E.; Willett, W.C.; Sun, Q.; Hu, F.B. Changes in Overall Diet Quality and Subsequent Type 2 Diabetes Risk: Three U.S. Prospective Cohorts. Diabetes Care 2016, 39, 2011–2018. [Google Scholar] [CrossRef]
- Suh, J.S.; Kim, S.; Boström, K.I.; Wang, C.Y.; Kim, R.H.; Park, N.H. Periodontitis-induced systemic inflammation exacerbates atherosclerosis partly via endothelial-mesenchymal transition in mice. Int. J. Oral Sci. 2019, 11, 21. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Taylor, J.J.; Jaedicke, K.M.; De Jager, M.; Bikker, J.W.; Selten, W.; Bissett, S.M.; Whall, K.M.; van de Merwe, R.; Areibi, A.; et al. Treatment of periodontitis reduces systemic inflammation in type 2 diabetes. J. Clin. Periodontol. 2020, 47, 737–746. [Google Scholar] [CrossRef]
- Cardoso, E.M.; Reis, C.; Manzanares-Céspedes, M.C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 2017, 130, 98–104. [Google Scholar] [CrossRef]
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral Sci. 2020, 28, e20190248. [Google Scholar] [CrossRef] [PubMed]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.H.; Yang, Y.J.; Hu, X.; Zhang, R.H.; Li, X. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: A systematic review and meta-analysis. J. Transl. Med. 2023, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Li, C.; Geng, F.; Pan, Y. J-shaped association between systemic immune-inflammation index and periodontitis: Results from NHANES 2009–2014. J. Periodontol. 2023. [Google Scholar] [CrossRef]
- Mishra, S.; Johnson, L.; Gazala, M.P.; Dahiya, S.; Rahman, W.; Sreeraj, V.S. Systemic immune-inflammation index in patients with generalized stage III grade C periodontitis. Oral Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 4, 851–863. [Google Scholar] [CrossRef]
- Klöting, N.; Blüher, M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Dis. 2014, 15, 277–287. [Google Scholar] [CrossRef]
- Parimisetty, A.; Dorsemans, A.C.; Awada, R.; Ravanan, P.; Diotel, N.; d’Hellencourt, C.L. Secret talk between adipose tissue and central nervous system via secreted factors-an emerging frontier in the neurodegenerative research. J. Neuroinflamm. 2016, 13, 67. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Qiang, J.; Wang, X.; Chen, L.; Zou, D. Experimental immunology Diet-induced obesity mediates a proinflammatory response in pancreatic β cell via toll-like receptor 4. Cent. Eur. J. Immunol. 2014, 3, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Vianna, C.R.; Fukuda, M.; Berglund, E.D.; Liu, C.; Tao, C.; Sun, K.; Liu, T.M.; Harper, M.J.; Lee, C.E.; et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat. Commun. 2014, 5, 3878. [Google Scholar] [CrossRef] [PubMed]
- Maedler, K.; Zhang, N.; Liang, H.; Farese, R.V.; Li, J.; Musi, N.; Hussey, S.E. Pharmacological TLR4 Inhibition Protects against Acute and Chronic Fat-Induced Insulin Resistance in Rats. PLoS ONE 2015, 10, e0132575. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 8197) | HOMA-IR < 2.5 (n = 4336) | HOMA-IR ≥ 2.5 (n = 3861) | p-Value |
|---|---|---|---|---|
| Age | 47.33 ± 0.18 | 45.96 ± 0.26 | 48.87 ± 0.26 | <0.001 |
| Sex | <0.001 | |||
| Female | 3761 (45.9) | 2106 (48.6) | 1655 (42.9) | |
| Male | 4436 (54.1) | 2230 (51.4) | 2206 (57.1) | |
| Race and ethnicity | <0.001 | |||
| Non-Hispanic White | 3981 (48.6) | 2282 (52.6) | 1699 (44.0) | |
| Non-Hispanic Black | 1659 (20.2) | 833 (19.2) | 826 (21.4) | |
| Mexican American | 1097 (13.4) | 434 (10.0) | 663 (17.2) | |
| Other | 1460 (17.8) | 787 (18.2) | 673 (17.4) | |
| Education level | <0.001 | |||
| Less than high school | 1440 (17.6) | 697 (16.1) | 743 (19.2) | |
| High school or equivalent | 1847 (22.5) | 908 (20.9) | 939 (24.3) | |
| College or above | 4910 (59.9) | 2731 (63.0) | 2179 (56.5) | |
| Ratio of family income to poverty | <0.001 | |||
| 0–1.0 (including 1.0) | 1380 (16.8) | 703 (16.2) | 677 (17.5) | |
| 1.0–3.0(including 3.0) | 3172 (38.7) | 1578 (36.4) | 1594 (41.3) | |
| >3.0 | 3645 (44.5) | 2055 (47.4) | 1590 (41.2) | |
| Body mass index | <0.001 | |||
| Normal (<25.0) | 2626 (32.0) | 2145 (49.5) | 481 (12,5) | |
| Overweight (25.0–30.0) | 2922 (35.6) | 1576 (36.3) | 1346 (34.8) | |
| Obese (>30.0) | 2649 (32.3) | 615 (14.2) | 2034 (52.7) | |
| Smoking status | <0.001 | |||
| Non-smoker | 4148 (50.6) | 2204 (50.8) | 1944 (50.3) | |
| Former smoker | 2159 (26.3) | 1048 (24.2) | 1111 (28.8) | |
| Current smoker | 1890 (23.1) | 1084 (25.0) | 806 (20.9) | |
| Alcohol drinking | <0.05 | |||
| ≥5 drinks/day | 1159 (14.1) | 574 (13.2) | 585 (15.2) | |
| <5 drinks/day | 7038 (85.9) | 3762 (86.8) | 3276 (84.8) | |
| Number of Tooth Loss | Linear Regression Analysis | Logistic Regression Analysis | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| β (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Continuous | 0.052 (0.031, 0.073) | <0.001 | 1.018 (1.012, 1.025) | <0.001 | 1.013 (1.005, 1.022) | <0.01 |
| Q1 (0) | Reference | - | Reference | - | Reference | - |
| Q2 (1) | 0.023 (0.008, 1.035) | <0.05 | 1.308 (1.127, 1.519) | <0.001 | 1.078 (0.906, 1.282) | 0.396 |
| Q3 (2–5) | 0.031 (0.107, 0.859) | <0.05 | 1.366 (1.225, 1.524) | <0.001 | 1.199 (1.048, 1.371) | <0.01 |
| Q4 (6–28) | 0.073 (0.802, 1.584) | <0.001 | 1.646 (1.469, 1.845) | <0.001 | 1.301 (1.102, 1.537) | <0.01 |
| Number of Tooth Loss | β (95%CI) | |||
|---|---|---|---|---|
| Model 1 | p-Value | Model 2 | p-Value | |
| Continuous | −0.040 (−0.116, −0.035) | <0.001 | −0.112 (−0.258, −0.164) | <0.001 |
| Q1 (0) | Reference | - | Reference | - |
| Q2 (1) | 0.017 (−0.249, 1.786) | 0.139 | −0.005 (−1.179, 0.780) | 0.689 |
| Q3 (2–5) | 0.014 (−0.299, 1.191) | 0.240 | −0.047 (−2.203, −0.699) | <0.001 |
| Q4 (6–28) | −0.027 (−1.642, −0.092) | <0.05 | −0.121 (−4.839, −2.974) | <0.001 |
| HEI-2015 Score | Model 1 | Model 2 | ||
|---|---|---|---|---|
| β (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Continuous | 0.040 (0.000, 0.001) | <0.001 | 0.986 (0.982, 0.990) | <0.001 |
| A1 (average) | Reference | - | Reference | - |
| A2 (best) | −0.025 (−1.182, 0.062) | <0.05 | 0.776 (0.641, 0.939) | <0.01 |
| A3 (inadequate) | 0.052 (0.408, 1.039) | <0.001 | 1.267 (1.138, 1.411) | <0.001 |
| Number of Tooth Loss | β (95%CI) | |||
|---|---|---|---|---|
| Model 1 | p-Value | Model 2 | p-Value | |
| Continuous | 0.076 (2.408, 4.315) | <0.001 | 0.051 (1.121, 3.406) | <0.001 |
| Q1 (0) | Reference | - | Reference | - |
| Q2 (1) | 0.006 (−17.623, 29.890) | 0.613 | −0.006 (−30.274, 17.710) | 0.608 |
| Q3 (2–5) | 0.032 (5.485, 40.268) | <0.05 | 0.010 (−11.046, 25.804) | 0.432 |
| Q4 (6–28) | 0.072 (36.135, 72.336) | <0.001 | 0.032 (1.777, 47.448) | <0.05 |
| SII | Model 1 | Model 2 | ||
|---|---|---|---|---|
| β (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Continuous | 0.040 (0.000, 0.001) | <0.001 | 1.000 (1.000, 1.001) | <0.001 |
| Q1 (≤325.18) | Reference | - | Reference | - |
| Q2 (325.19–452.57) | 0.027 (0.003, 0.851) | <0.05 | 1.130 (0.980, 1.303) | 0.092 |
| Q3 (452.58–640.40) | 0.037 (0.168, 1.015) | <0.01 | 1.234 (1.070, 1.423) | <0.01 |
| Q4 (≥640.40) | 0.060 (0.542, 1.389) | <0.001 | 1.363 (1.179, 1.575) | <0.001 |
| Tooth Loss →HEI-2015 Score → HOMA-IR | Tooth Loss → SII → HOMA-IR | |
|---|---|---|
| C (total effect) | 0.052 *** | 0.052 *** |
| a | −0.076 *** | 3.361 *** |
| b | −0.033 *** | 0.001 ** |
| a × b (mediating effect) | 0.002 | 0.002 |
| a × b (BOOT SE) | 0.001 | 0.001 |
| a × b (z-value) | 3.089 | 2.846 |
| a × b (p-value) | 0.002 | 0.004 |
| a × b (95% Boot CI) | 0.001, 0.004 | 0.001, 0.004 |
| c’ (direct effect) | 0.047 *** | 0.047 *** |
| Effect ratio | 4.731% | 4.576% |
| Test conclusion | Partial mediation | Partial mediation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Li, S.; Dong, S.; Niu, L. The Association between Tooth Loss and Insulin Resistance Mediated by Diet Quality and Systemic Immunoinflammatory Index. Nutrients 2023, 15, 5008. https://doi.org/10.3390/nu15235008
Hao Y, Li S, Dong S, Niu L. The Association between Tooth Loss and Insulin Resistance Mediated by Diet Quality and Systemic Immunoinflammatory Index. Nutrients. 2023; 15(23):5008. https://doi.org/10.3390/nu15235008
Chicago/Turabian StyleHao, Yaqi, Shaoru Li, Shaojie Dong, and Lin Niu. 2023. "The Association between Tooth Loss and Insulin Resistance Mediated by Diet Quality and Systemic Immunoinflammatory Index" Nutrients 15, no. 23: 5008. https://doi.org/10.3390/nu15235008
APA StyleHao, Y., Li, S., Dong, S., & Niu, L. (2023). The Association between Tooth Loss and Insulin Resistance Mediated by Diet Quality and Systemic Immunoinflammatory Index. Nutrients, 15(23), 5008. https://doi.org/10.3390/nu15235008





