A Review of Healthy Dietary Choices for Cardiovascular Disease: From Individual Nutrients and Foods to Dietary Patterns
Abstract
:1. Introduction
2. Literature Search Strategy
3. Macronutrients
3.1. Fat
3.2. Carbohydrate
3.3. Protein
3.4. Vitamins and Minerals
3.5. Dietary Fiber
| Macronutrients | Study | Research Object | MA (No. of Studies) | Exposure Measure | Follow-Up Period | Main Findings | Reference |
|---|---|---|---|---|---|---|---|
| Polyunsaturated fat and saturated fat | Meta-analysis of randomized controlled trials | Studies that randomized adults to increased total or n-6 PUFA consumption for at least 1 year and reported incidence of CHD. | YES (8) | Consuming PUFA in place of SFA can reduce the occurrence of clinical CHD events. | [16] | ||
| Saturated fat | Meta-analysis of prospective cohort studies | Studies of CHD, stroke, and cardiovascular disease of 347,747 participants. | YES (21) | 5–23 years | No significant evidence supports associations between the intake of dietary saturated fat and the risk of CHD or CVD. | [10] | |
| Saturated fat | The Japan Collaborative Cohort Study | 58,453 Japanese men and women aged 40–79 | No | FFQ | 14.1 years | Saturated fatty acid intake is inversely associated with mortality from total stroke. | [8] |
| Saturated fat | The Multi-Ethnic Study | Participants who were 45–84 y old at baseline (n = 5209). | No | FFQ | 10 years | Meat saturated fats are positively associated with CVD risk, whereas dairy saturated fats are inversely associated with CVD risk. | [6] |
| Linoleic acid | Meta-analysis of prospective cohort studies | Studies of linoleic acid and CHD with 310,602 participants. | YES (13) | 5.3–30 years | Increased intake of dietary linoleic acid is associated with a low risk of CHD in a dose–response manner. | [15] | |
| Fatty acids | Meta-analysis of observational studies | Studies of fatty acids, unsaturated fatty acids, and coronary disease of 659,298 participants. | YES (76) | There are no significant associations in prospective studies of coronary disease that involved assessment of dietary intake of long-chain w-3 and w-6 polyunsaturated fatty acids. | [9] | ||
| Dietary fat | The PREvención con DIeta MEDiterránea (PREDIMED) study | 7038 participants with men (aged 55–80 y) and women (aged 60–80 y) at high CVD risk | NO | FFQ | 6 years | Saturated fatty acid and trans fat intake are associated with a high risk of CVD, whereas MUFA and PUFA intake are inversely associated with CVD death. | [12] |
| Low-fat dietary pattern | The Women’s Health Initiative randomized controlled trial | Participants comprised 48,835 postmenopausal women aged 50–79 y. | NO | FFQ and performing laboratory analysis of blood specimens | 8.3 years | Low-fat diets do not have beneficial effects on CVD risk and total mortality. | [11] |
| Fat | Prospective Urban Rural Epidemiology (PURE) study | A study of 135,335 individuals aged 35–70 years in 18 countries from five continents. | NO | FFQ | 7.4 years | Total fats are associated with low risk of total mortality and stroke, but not with the risk of CVD, myocardial infarction, or cardiovascular disease mortality. | [7] |
| A Mediterranean diet supplemented with extra-Virgin olive oil or nuts | The PREDIMED study | A multicenter trial in Spain with 7447 participants (55 to 80 years of age, 57% women) who were at high cardiovascular risk. | NO | FFQ | 4.8 years | The Mediterranean diet effectively prevents the risk of major cardiovascular events. | [14] |
| Dietary total fat | A dose–response meta-analysis of cohort studies | Studies of cohort studies reporting associations of dietary fat intake and risk of CVDs. | YES (43) | Total fat, SFA, MUFA, and PUFA intake are not associated with the risk of cardiovascular disease. | [13] | ||
| Low-carbohydrate diet | Randomized trial | A multicenter, controlled trial involving 63 obese men and women. | NO | participants met with registered dietitian to review dietary issues | 1 years | In the first six months, the low-carbohydrate diet produced a greater weight loss compared with the conventional diet, but the differences are not significant at one year. | [19] |
| Low carbohydrate–high protein diet | A population-based prospective study | A cohort of 42,237 Swedish women (30–49 years old at baseline) | NO | FFQ | 12 years | Low carbohydrate–high protein diet is associated with increased total and particularly cardiovascular mortality among women. | [17] |
| Low in carbohydrate diet | Randomized trial | Participants with 311 free-living, overweight nondiabetic, premenopausal women | NO | Received weekly instruction for 2 months, then an additional 10-month follow-up | 1 years | Low-Carbohydrate diet benefit to weight loss and metabolic effects outcomes. | [20] |
| Low-carbohydrate diets | A prospective cohort study | Nurses’ Health Study and Health Professionals’ Follow-up Study with 85,168 women (aged 34–59 years at baseline) and 44,548 men (aged 40–75 years at baseline) without heart disease, cancer, or diabetes. | NO | FFQ | 20–26 years | A low-carbohydrate diet based on animal sources is associated with higher all-cause mortality, whereas a vegetable-based low-carbohydrate diet is associated with lower cardiovascular disease mortality rates. | [18] |
| Diet with high glycemic index and load | Meta-analysis of prospective cohort studies | Studies showed associations of glycemic index and glycemic load with incidence of CHD including 240,936 participants. | YES (10) | 6–25 years | Diet with high glycemic index and glycemic load diets are significantly associated with CHD events in women but not in men. | [27] | |
| Low-carbohydrate diets | Meta-analysis of randomized controlled trials | Studies showed associations of low-carbohydrate diet, low-fat diet, weight loss, and cardiovascular disease with 1369 participants. | YES (11) | 6–24 months | Low-carbohydrate diets have greater weight loss but increased LDL cholesterol. | [21] | |
| Carbohydrate | Prospective Urban Rural Epidemiology (PURE) study | A large, epidemiological cohort study of 135,335 individuals aged 35–70 years | NO | FFQ | 7.4 years | High intake of carbohydrate is associated with an increased risk of total mortality but is not associated with the risk of CVD or CVD mortality. | [7] |
| Carbohydrate | A prospective cohort study and meta-analysis | 15,428 adults aged 45–64 years, in four US communities | YES (7) | 25 years | Both high and low carbohydrate intake are associated with increased mortality. | [25] | |
| Carbohydrate | Prospective population-based study of UK Biobank participants | The UK Biobank cohort of general population with 195,658 participants | NO | 24-h recall | 3 years | There were nonlinear associations between macronutrient intakes and health (mortality and CVD risk). | [26] |
| Diet with a high glycemic index | Prospective study on five continents | The study included 137,851 participants between the ages of 35 and 70 years living on five continents. | NO | FFQ | 9.5 years | Diet with high glycemic index is associated with an increased risk of CVD and CVD mortality. | [28] |
| Carbohydrate and saturated fat | Prospective cohort study | 9899 women (aged 50–55 years) were recruited into the Australian Longitudinal Study on Women’s Health. | NO | 15 years | A moderate carbohydrate intake is associated with reduced risk of heart disease and stroke. | [22] | |
| Low-carbohydrate diet | An open-label randomized controlled trial in Denmark | Study included 73 patients older than 18 years with type 2 diabetes. | NO | visits and telephone call | 6 months | A non-calorie-restricted low-carbohydrate diet high in fat is significantly beneficial for glycemic control and body composition, without adversely affecting CVD risk factors in patients with T2D. | [23] |
| Carbohydrate | A dose–response meta-analysis | Studies about the relationship between dietary carbohydrate and the incidence of cardiovascular events and mortality. | YES (19) | Higher carbohydrate intake is associated with a slight increase in CVD risk in women but no association is found in men. | [24] | ||
| Dietary protein | A prospective cohort study | In the Nurses’ Health Study cohort of 80,082 women aged 34–59 y and without a previous diagnosis of ischemic heart disease, stroke, cancer, hypercholesterolemia, or diabetes. | NO | FFQ | 14 years | Replacing carbohydrates with protein may be associated with a lower risk of ischemic heart disease. | [31] |
| Dietary protein | A prospective cohort study | 84,136 women aged 30–55 years in the Nurses’ Health Study with no known cancer, diabetes, angina, myocardial infarction, stroke, or other cardiovascular disease | NO | FFQ | 26 years | Higher intake of red meat and high-fat dairy are significantly associated with elevated risk of CHD, and CHD risk may be reduced by replacing sources of protein. | [33] |
| Energy-restricted high-protein, low-fat diet | Meta-analysis of randomized controlled trials | Studies that compared energy-restricted, isocaloric, high-protein, low-fat (HP) diets with standard-protein, low-fat (SP) diets included 1063 individuals. | YES (24) | Compared with an energy-restricted standard-protein, low-fat diet, an isocalorically prescribed high-protein, low-fat diet provided more benefits in reducing body weight, fat mass, and triglycerides. | [32] | ||
| Animal and plant protein | Two prospective US cohort studies | 85,013 women and 46,329 men from the Nurses’ Health Study (1980–2012) and Health Professionals Follow-up Study (1986–2012) | NO | FFQ | 32 years | Higher animal protein intake is positively, whereas plant protein is inversely, associated with all-cause mortality. | [35] |
| Plant and animal protein | The Adventist Health Study-2 cohort | 81,337 men and women in the Adventist Health Study-2 | NO | FFQ | 9.4 years | Higher animal protein intake is associated with high CVD mortality, but no associations between plant protein intake and CVD mortality. | [36] |
| Animal and plant protein | A large prospective cohort study | Study included 70,696 participants in the Japan Public Health Center–based Prospective Cohort who were aged 45 to 74 years. | NO | FFQ | 18 years | Higher plant protein intake is associated with lower total and CVD-related mortality, but animal protein intake is not associated with mortality outcomes. | [38] |
| Dietary protein | Meta-analysis of prospective cohort studies | Studies about associations of dietary protein from different sources with all-cause and cause-specific mortality with 350,452 participants. | YES (11) | 12–28 years | Total protein intake is positively associated with all-cause mortality, driven mainly by a harmful association of animal protein with CVD mortality. Plant protein intake is inversely associated with all-cause and CVD mortality. | [34] | |
| Plant and animal protein | A large prospective cohort study | Study included 416,104 men and women aged 50 to 71 in the US National Institutes of Health–AARP Diet and Health Study. | NO | FFQ | 16 years | There are small but significant associations between high intake of plant protein and low overall and CVD mortality. | [39] |
| Total, animal, and plant proteins | Dose–response meta-analysis of prospective cohort studies | Studies of the dose–response relation between intake of total, animal, and plant protein and the risk of mortality from all causes, cardiovascular disease, and cancer. | YES (32) | 3.5–32 years | Intake of plant protein is associated with low CVD mortality risk. | [37] | |
| Dietary antioxidant vitamins | A prospective study | 34,486 postmenopausal women aged 55 to 69 years with no cardiovascular disease | NO | FFQ and 24-h recall | 7 years | The intake of vitamin E, but not vitamins A and C, from food is inversely associated with the risk of death from CHD. | [40] |
| Vitamin D | Framingham Offspring Study | 1739 participants (mean age 59 years; 55% women; all white) | NO | FFQ | Deficiency of vitamin D is associated with incident cardiovascular disease. | [47] | |
| Vitamin D | Cross-sectional study | The data from the National Health and Nutrition Examination Survey (NHANES) with 8351 participants | NO | Vitamin D deficiency is associated with increased risk of CVD. | [45] | ||
| Vitamin D | Cross-sectional analysis | The data from the Third National Health and Nutrition Examination Survey (1988–1994) with 16,603 men and women aged 18 years or older | NO | 25-hydroxyvitamin D deficiency is found to be associated with high prevalence of angina, myocardial infarction, and heart failure. | [46] | ||
| Vitamin D | A randomized clinical trial | The study recruited participants mostly from family practices in Auckland, New Zealand, with 5110 participants aged 50 to 84 years. | NO | questionnaire | 3.3 years | Monthly high-dose vitamin D supplementation does not prevent CVD. | [49] |
| Supplemental vitamins and minerals | Meta-analyses of randomized controlled trials | Studies of dietary supplements and cardiovascular disease outcomes and all-cause mortality | YES (179) | Folic acid and B vitamins had preventive benefits for stroke. | [41] | ||
| Vitamin D | Randomized trials | Among men 50 years of age or older and women 55 years of age or older in the United States | NO | FFQ | 5.3 years | Supplementation with vitamin D does not result in a lower incidence of invasive cancer or cardiovascular events compared with placebo. | [50] |
| Supplemental vitamins and minerals | Meta-analyses of randomized controlled trials | Studies of dietary supplements and cardiovascular disease outcomes and all-cause mortality | YES (35) | Niacin shows an increased risk of all-cause mortality. However, multivitamins, vitamins C and D, β-carotene, calcium, and selenium do not exhibit positive effects in reducing the risk of CVD. | [28] | ||
| Vitamin and mineral supplements | Pooled analyses of RCTs and observational cohort studies | RCTs of vitamin or mineral use among adults without cardiovascular disease or cancer and with no known vitamin or mineral deficiencies; observational cohort studies examining serious harms. | YES (84) | Supplementation with vitamins and minerals is associated with little or no benefit in preventing cancer, CVD, and death. | [43] | ||
| vitamin D | Non-linear Mendelian randomization analyses | The analysis was conducted in the UK Biobank with 44 519 CVD cases and 251,269 controls. | NO | Vitamin D deficiency can increase the risk of CVD. | [48] | ||
| Vitamin D | A randomized controlled trial | The randomized, placebo-controlled trial among 2495 male participants ≥60 years and postmenopausal female participants ≥65 years from a general Finnish population who were free of prior CVD or cancer. | NO | Annual study questionnaires and national registry data | 5 years | Supplementation with vitamin D3 does not lower the incidences of major CVD events or invasive cancer. | [51] |
| Dietary fiber | A pooled analysis of cohort studies | Studies about the association between dietary fiber intake and the risk of coronary heart disease | YES (10) | There is an inverse relationship between intake of dietary fiber and the risk of CHD. | [57] | ||
| Dietary fibre | Meta-analysis | Studies about the association of dietary fiber and cardiovascular or coronary heart disease | YES (19) | ≥3 years | A higher intake of dietary fiber is associated with a lower risk of both CVD and CHD. | [58] | |
| Rice bran extract | Randomized controlled trial | Single-blind design study with 60 postmenopausal Vietnamese women (45–65 y old) with high LDL cholesterol levels (over 140 mg/dL) | NO | questionnaires | 6 months | Pre-germinated brown rice bran extract containing acylated steryl glucosides associated with the reduction in the risk of atherosclerosis. | [56] |
| Barley β-glucan | Randomized controlled trial | Crossover study with mild hypercholesterolemia participants (n = 45) | NO | 5 weeks | Consumption of barley β-glucan is found to be effective in circulating cholesterol levels. | [54] | |
| Quinoa | Randomized controlled trial | Crossover designed study with 37 healthy overweight men (35–70 years) completed a 4-week crossover intervention. | NO | FFQ | 6 months | Daily consumption of 20 g quinoa can reduce CVD risk markers including blood cholesterol and blood glucose in overweight participants. | [53] |
| Dietary fiber | Meta-analyses | Studies about indicators of carbohydrate quality and noncommunicable disease incidence, mortality, and risk factors | YES (243) | High intake of dietary fiber is associated with lower risk of mortality and incidence of cardiometabolic events. | [59] | ||
| Dietary fiber | Meta-analyses | Studies about dietary fiber in hypertension and cardiovascular disease | YES (15) | Higher fiber intake is shown to be associated with an improvement in cardiometabolic risk factors. | [60] |
4. Foods and Food Products
4.1. Sugar-Sweetened Beverages (SSBs)
4.2. Red Meat and Processed Meat
4.3. Poultry and Fish
4.4. Nuts
4.5. Fruits and Vegetables
4.6. Salt and Sodium
4.7. Dairy Products
5. Dietary Patterns
5.1. Mediterranean Diet
5.2. Vegetarian and Vegan Diets
5.3. Ultra-Processed Foods
5.4. Ketogenic Diet
5.5. Intermittent Fasting
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global status report on noncommunicable diseases 2014. Women 2011, 47, 2562–2563. [Google Scholar]
- Yusuf, S.; Rangarajan, S.; Teo, K.; Islam, S.; Li, W.; Liu, L.; Bo, J.; Lou, Q.; Lu, F.; Liu, T.; et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N. Engl. J. Med. 2014, 371, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.D. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- De Oliveira Otto, M.C.; Mozaffarian, D.; Kromhout, D.; Bertoni, A.G.; Sibley, C.T.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary intake of saturated fat by food source and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2012, 96, 397–404. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Yamagishi, K.; Iso, H.; Yatsuya, H.; Tanabe, N.; Date, C.; Kikuchi, S.; Yamamoto, A.; Inaba, Y.; Tamakoshi, A. Dietary intake of saturated fatty acids and mortality from cardiovascular disease in Japanese: The Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC) Study. Am. J. Clin. Nutr. 2010, 92, 759–765. [Google Scholar] [CrossRef]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef]
- Prentice, R.L.; Aragaki, A.K.; Van Horn, L.; Thomson, C.A.; Beresford, S.A.; Robinson, J.; Snetselaar, L.; Anderson, G.L.; Manson, J.E.; Allison, M.A.; et al. Low-fat dietary pattern and cardiovascular disease: Results from the Women’s Health Initiative randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 35–43. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Babio, N.; Martínez-González, M.A.; Corella, D.; Ros, E.; Martín-Peláez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef]
- Zhu, Y.; Bo, Y.; Liu, Y. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: A dose-response meta-analysis of cohort studies. Lipids Health Dis. 2019, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary linoleic acid and risk of coronary heart disease: A systematic review and meta-analysis of prospective cohort studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef]
- Lagiou, P.; Sandin, S.; Weiderpass, E.; Lagiou, A.; Mucci, L.; Trichopoulos, D.; Adami, H.O. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J. Intern. Med. 2007, 261, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; van Dam, R.M.; Hankinson, S.E.; Stampfer, M.; Willett, W.C.; Hu, F.B. Low-carbohydrate diets and all-cause and cause-specific mortality: Two cohort studies. Ann. Intern. Med. 2010, 153, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; McGuckin, B.G.; Brill, C.; Mohammed, B.S.; Szapary, P.O.; Rader, D.J.; Edman, J.S.; Klein, S. A randomized trial of a low-carbohydrate diet for obesity. N. Engl. J. Med. 2003, 348, 2082–2090. [Google Scholar] [CrossRef]
- Gardner, C.D.; Kiazand, A.; Alhassan, S.; Kim, S.; Stafford, R.S.; Balise, R.R.; Kraemer, H.C.; King, A.C. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: The A TO Z Weight Loss Study: A randomized trial. JAMA 2007, 297, 969–977. [Google Scholar] [CrossRef]
- Mansoor, N.; Vinknes, K.J.; Veierød, M.B.; Retterstøl, K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2016, 115, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Gribbin, S.; Enticott, J.; Hodge, A.M.; Moran, L.; Thong, E.; Joham, A.; Zaman, S. Association of carbohydrate and saturated fat intake with cardiovascular disease and mortality in Australian women. Heart 2022, 108, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Gram-Kampmann, E.M.; Hansen, C.D.; Hugger, M.B.; Jensen, J.M.; Brønd, J.C.; Hermann, A.P.; Krag, A.; Olsen, M.H.; Beck-Nielsen, H.; Højlund, K. Effects of a 6-month, low-carbohydrate diet on glycaemic control, body composition, and cardiovascular risk factors in patients with type 2 diabetes: An open-label randomized controlled trial. Diabetes Obes. Metab. 2022, 24, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifard, N.; Mansourian, M.; Firouzi, S.; Taheri, M.; Haghighatdoost, F. Longitudinal association of dietary carbohydrate and the risk cardiovascular disease: A dose-response meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 6277–6292. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Ho, F.K.; Gray, S.R.; Welsh, P.; Petermann-Rocha, F.; Foster, H.; Waddell, H.; Anderson, J.; Lyall, D.; Sattar, N.; Gill, J.M.R.; et al. Associations of fat and carbohydrate intake with cardiovascular disease and mortality: Prospective cohort study of UK Biobank participants. BMJ 2020, 368, m688. [Google Scholar] [CrossRef]
- Mirrahimi, A.; de Souza, R.J.; Chiavaroli, L.; Sievenpiper, J.L.; Beyene, J.; Hanley, A.J.; Augustin, L.S.; Kendall, C.W.; Jenkins, D.J. Associations of glycemic index and load with coronary heart disease events: A systematic review and meta-analysis of prospective cohorts. J. Am. Heart Assoc. 2012, 1, e000752. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Dehghan, M.; Mente, A.; Bangdiwala, S.I.; Rangarajan, S.; Srichaikul, K.; Mohan, V.; Avezum, A.; Díaz, R.; Rosengren, A.; et al. Glycemic Index, Glycemic Load, and Cardiovascular Disease and Mortality. N. Engl. J. Med. 2021, 384, 1312–1322. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Lemmens, S.G.; Westerterp, K.R. Dietary protein—Its role in satiety, energetics, weight loss and health. Br. J. Nutr. 2012, 108 (Suppl. S2), S105–S112. [Google Scholar] [CrossRef]
- Leidy, H.J.; Clifton, P.M.; Astrup, A.; Wycherley, T.P.; Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.D.; Woods, S.C.; Mattes, R.D. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 2015, 101, 1320s–1329s. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.; Colditz, G.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Dietary protein and risk of ischemic heart disease in women. Am. J. Clin. Nutr. 1999, 70, 221–227. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 1281–1298. [Google Scholar] [CrossRef]
- Bernstein, A.M.; Sun, Q.; Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Willett, W.C. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010, 122, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Glisic, M.; Song, M.; Aliahmad, H.A.; Zhang, X.; Moumdjian, A.C.; Gonzalez-Jaramillo, V.; van der Schaft, N.; Bramer, W.M.; Ikram, M.A.; et al. Dietary protein intake and all-cause and cause-specific mortality: Results from the Rotterdam Study and a meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2020, 35, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of Animal and Plant Protein Intake With All-Cause and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Tharrey, M.; Mariotti, F.; Mashchak, A.; Barbillon, P.; Delattre, M.; Fraser, G.E. Patterns of plant and animal protein intake are strongly associated with cardiovascular mortality: The Adventist Health Study-2 cohort. Int. J. Epidemiol. 2018, 47, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Naghshi, S.; Sadeghi, O.; Willett, W.C.; Esmaillzadeh, A. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2020, 370, m2412. [Google Scholar] [CrossRef] [PubMed]
- Budhathoki, S.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Goto, A.; Kotemori, A.; Ishihara, J.; Takachi, R.; Charvat, H.; Mizoue, T.; et al. Association of Animal and Plant Protein Intake With All-Cause and Cause-Specific Mortality in a Japanese Cohort. JAMA Intern. Med. 2019, 179, 1509–1518. [Google Scholar] [CrossRef]
- Huang, J.; Liao, L.M.; Weinstein, S.J.; Sinha, R.; Graubard, B.I.; Albanes, D. Association Between Plant and Animal Protein Intake and Overall and Cause-Specific Mortality. JAMA Intern. Med. 2020, 180, 1173–1184. [Google Scholar] [CrossRef]
- Kushi, L.H.; Folsom, A.R.; Prineas, R.J.; Mink, P.J.; Wu, Y.; Bostick, R.M. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N. Engl. J. Med. 1996, 334, 1156–1162. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Spence, J.D.; Giovannucci, E.L.; Kim, Y.I.; Josse, R.; Vieth, R.; Blanco Mejia, S.; Viguiliouk, E.; Nishi, S.; Sahye-Pudaruth, S.; et al. Supplemental Vitamins and Minerals for CVD Prevention and Treatment. J. Am. Coll. Cardiol. 2018, 71, 2570–2584. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Spence, J.D.; Giovannucci, E.L.; Kim, Y.I.; Josse, R.G.; Vieth, R.; Sahye-Pudaruth, S.; Paquette, M.; Patel, D.; Blanco Mejia, S.; et al. Supplemental Vitamins and Minerals for Cardiovascular Disease Prevention and Treatment: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 423–436. [Google Scholar] [CrossRef]
- O’Connor, E.A.; Evans, C.V.; Ivlev, I.; Rushkin, M.C.; Thomas, R.G.; Martin, A.; Lin, J.S. Vitamin and Mineral Supplements for the Primary Prevention of Cardiovascular Disease and Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 327, 2334–2347. [Google Scholar] [CrossRef] [PubMed]
- Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Jaén, C.R.; et al. Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 327, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Sabour, S.; Sagar, U.N.; Adams, S.; Whellan, D.J. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am. J. Cardiol. 2008, 102, 1540–1544. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.; Targher, G.; Smits, G.; Chonchol, M. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis 2009, 205, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Selvanayagam, J.B.; Hyppönen, E. Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur. Heart J. 2022, 43, 1731–1739. [Google Scholar] [CrossRef]
- Scragg, R.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Sluyter, J.; Murphy, J.; Khaw, K.T.; Camargo, C.A., Jr. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 608–616. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Virtanen, J.K.; Nurmi, T.; Aro, A.; Bertone-Johnson, E.R.; Hyppönen, E.; Kröger, H.; Lamberg-Allardt, C.; Manson, J.E.; Mursu, J.; Mäntyselkä, P.; et al. Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish Vitamin D Trial: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 115, 1300–1310. [Google Scholar] [CrossRef]
- Stephen, A.M.; Champ, M.M.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef]
- Li, L.; Lietz, G.; Bal, W.; Watson, A.; Morfey, B.; Seal, C. Effects of Quinoa (Chenopodium quinoa Willd.) Consumption on Markers of CVD Risk. Nutrients 2018, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Harding, S.V.; Thandapilly, S.J.; Tosh, S.M.; Jones, P.J.H.; Ames, N.P. Barley β-glucan reduces blood cholesterol levels via interrupting bile acid metabolism. Br. J. Nutr. 2017, 118, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Nhung, B.T.; Tuyen, L.D.; Linh, V.A.; Anh, N.D.; Nga, T.T.; Thuc, V.T.; Yui, K.; Ito, Y.; Nakashima, Y.; Yamamoto, S. Rice Bran Extract Reduces the Risk of Atherosclerosis in Post-Menopausal Vietnamese Women. J. Nutr. Sci. Vitaminol. 2016, 62, 295–302. [Google Scholar] [CrossRef]
- Pereira, M.A.; O’Reilly, E.; Augustsson, K.; Fraser, G.E.; Goldbourt, U.; Heitmann, B.L.; Hallmans, G.; Knekt, P.; Liu, S.; Pietinen, P.; et al. Dietary fiber and risk of coronary heart disease: A pooled analysis of cohort studies. Arch. Intern. Med. 2004, 164, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.; Kumar, S.; Diep Pham, H.T.; Coffey, S.; Mann, J. Dietary fibre in hypertension and cardiovascular disease management: Systematic review and meta-analyses. BMC Med. 2022, 20, 139. [Google Scholar] [CrossRef]
- Huang, C.; Huang, J.; Tian, Y.; Yang, X.; Gu, D. Sugar sweetened beverages consumption and risk of coronary heart disease: A meta-analysis of prospective studies. Atherosclerosis 2014, 234, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.B.; Kaar, J.L.; Welsh, J.A.; Van Horn, L.V.; Feig, D.I.; Anderson, C.A.M.; Patel, M.J.; Cruz Munos, J.; Krebs, N.F.; Xanthakos, S.A.; et al. Added Sugars and Cardiovascular Disease Risk in Children: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e1017–e1034. [Google Scholar] [CrossRef]
- Duffey, K.J.; Gordon-Larsen, P.; Steffen, L.M.; Jacobs, D.R., Jr.; Popkin, B.M. Drinking caloric beverages increases the risk of adverse cardiometabolic outcomes in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am. J. Clin. Nutr. 2010, 92, 954–959. [Google Scholar] [CrossRef] [PubMed]
- De Koning, L.; Malik, V.S.; Kellogg, M.D.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 2012, 125, 1735–1741.s1731. [Google Scholar] [CrossRef]
- Te Morenga, L.A.; Howatson, A.J.; Jones, R.M.; Mann, J. Dietary sugars and cardiometabolic risk: Systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am. J. Clin. Nutr. 2014, 100, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Z.; Gregg, E.W.; Flanders, W.D.; Merritt, R.; Hu, F.B. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern. Med. 2014, 174, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ley, S.H.; Sun, Q.; Hu, F.B.; Malik, V.S. Cross-sectional association between sugar-sweetened beverage intake and cardiometabolic biomarkers in US women. Br. J. Nutr. 2018, 119, 570–580. [Google Scholar] [CrossRef]
- Malik, V.S.; Li, Y.; Pan, A.; De Koning, L.; Schernhammer, E.; Willett, W.C.; Hu, F.B. Long-Term Consumption of Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Mortality in US Adults. Circulation 2019, 139, 2113–2125. [Google Scholar] [CrossRef]
- Popkin, B.M.; Hawkes, C. Sweetening of the global diet, particularly beverages: Patterns, trends, and policy responses. Lancet Diabetes Endocrinol. 2016, 4, 174–186. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Koh, W.P.; Yuan, J.M.; Pereira, M.A. Beverage habits and mortality in Chinese adults. J. Nutr. 2015, 145, 595–604. [Google Scholar] [CrossRef]
- Chen, C.H.; Tsai, M.K.; Lee, J.H.; Wen, C.; Wen, C.P. Association of Sugar-Sweetened Beverages and Cardiovascular Diseases Mortality in a Large Young Cohort of Nearly 300,000 Adults (Age 20–39). Nutrients 2022, 14, 2720. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, N.D.; Khan, T.A.; Wang, L.; Zhang, R.; Chiavaroli, L.; Au-Yeung, F.; Lee, J.J.; Noronha, J.C.; Comelli, E.M.; Blanco Mejia, S.; et al. Association of Low- and No-Calorie Sweetened Beverages as a Replacement for Sugar-Sweetened Beverages With Body Weight and Cardiometabolic Risk: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e222092. [Google Scholar] [CrossRef] [PubMed]
- Debras, C.; Chazelas, E.; Sellem, L.; Porcher, R.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaësse, C.; De Sa, A.; Lutchia, R.; et al. Artificial sweeteners and risk of cardiovascular diseases: Results from the prospective NutriNet-Santé cohort. BMJ 2022, 378, e071204. [Google Scholar] [CrossRef] [PubMed]
- Narain, A.; Kwok, C.S.; Mamas, M.A. Soft drinks and sweetened beverages and the risk of cardiovascular disease and mortality: A systematic review and meta-analysis. Int. J. Clin. Pract. 2016, 70, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Chazelas, E.; Debras, C.; Srour, B.; Fezeu, L.K.; Julia, C.; Hercberg, S.; Deschasaux, M.; Touvier, M. Sugary Drinks, Artificially-Sweetened Beverages, and Cardiovascular Disease in the NutriNet-Santé Cohort. J. Am. Coll. Cardiol. 2020, 76, 2175–2177. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhu, Y.; Malik, V.; Li, X.; Peng, X.; Zhang, F.F.; Shan, Z.; Liu, L. Intake of Sugar-Sweetened and Low-Calorie Sweetened Beverages and Risk of Cardiovascular Disease: A Meta-Analysis and Systematic Review. Adv. Nutr. 2021, 12, 89–101. [Google Scholar] [CrossRef]
- Wang, X.; Lin, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G.; Pan, A.; Hu, F.B. Red and processed meat consumption and mortality: Dose-response meta-analysis of prospective cohort studies. Public Health Nutr. 2016, 19, 893–905. [Google Scholar] [CrossRef]
- Zhong, V.W.; Van Horn, L.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Allen, N.B. Associations of Processed Meat, Unprocessed Red Meat, Poultry, or Fish Intake With Incident Cardiovascular Disease and All-Cause Mortality. JAMA Intern. Med. 2020, 180, 503–512. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Grau, N.; Mohammadifard, N.; Hassannejhad, R.; Haghighatdoost, F.; Sadeghi, M.; Talaei, M.; Sajjadi, F.; Mavrommatis, Y.; Sarrafzadegan, N. Red and processed meat consumption and risk of incident cardiovascular disease and mortality: Isfahan cohort study. Int. J. Food Sci. Nutr. 2022, 73, 503–512. [Google Scholar] [CrossRef]
- Al-Shaar, L.; Satija, A.; Wang, D.D.; Rimm, E.B.; Smith-Warner, S.A.; Stampfer, M.J.; Hu, F.B.; Willett, W.C. Red meat intake and risk of coronary heart disease among US men: Prospective cohort study. BMJ 2020, 371, m4141. [Google Scholar] [CrossRef] [PubMed]
- Vernooij, R.W.M.; Zeraatkar, D.; Han, M.A.; El Dib, R.; Zworth, M.; Milio, K.; Sit, D.; Lee, Y.; Gomaa, H.; Valli, C.; et al. Patterns of Red and Processed Meat Consumption and Risk for Cardiometabolic and Cancer Outcomes: A Systematic Review and Meta-analysis of Cohort Studies. Ann. Intern. Med. 2019, 171, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.C.; Zeraatkar, D.; Han, M.A.; Vernooij, R.W.M.; Valli, C.; El Dib, R.; Marshall, C.; Stover, P.J.; Fairweather-Taitt, S.; Wójcik, G.; et al. Unprocessed Red Meat and Processed Meat Consumption: Dietary Guideline Recommendations From the Nutritional Recommendations (NutriRECS) Consortium. Ann. Intern. Med. 2019, 171, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Zeraatkar, D.; Han, M.A.; Guyatt, G.H.; Vernooij, R.W.M.; El Dib, R.; Cheung, K.; Milio, K.; Zworth, M.; Bartoszko, J.J.; Valli, C.; et al. Red and Processed Meat Consumption and Risk for All-Cause Mortality and Cardiometabolic Outcomes: A Systematic Review and Meta-analysis of Cohort Studies. Ann. Intern. Med. 2019, 171, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, R.; Dehghan, M.; Mente, A.; Rangarajan, S.; Wielgosz, A.; Avezum, A.; Seron, P.; AlHabib, K.F.; Lopez-Jaramillo, P.; Swaminathan, S.; et al. Associations of unprocessed and processed meat intake with mortality and cardiovascular disease in 21 countries [Prospective Urban Rural Epidemiology (PURE) Study]: A prospective cohort study. Am. J. Clin. Nutr. 2021, 114, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, A.; Sinha, R.; Ward, M.H.; Graubard, B.I.; Inoue-Choi, M.; Dawsey, S.M.; Abnet, C.C. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: Population based cohort study. BMJ 2017, 357, j1957. [Google Scholar] [CrossRef]
- Kim, K.; Hyeon, J.; Lee, S.A.; Kwon, S.O.; Lee, H.; Keum, N.; Lee, J.K.; Park, S.M. Role of Total, Red, Processed, and White Meat Consumption in Stroke Incidence and Mortality: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Abete, I.; Romaguera, D.; Vieira, A.R.; Lopez de Munain, A.; Norat, T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: A meta-analysis of cohort studies. Br. J. Nutr. 2014, 112, 762–775. [Google Scholar] [CrossRef]
- Lupoli, R.; Vitale, M.; Calabrese, I.; Giosuè, A.; Riccardi, G.; Vaccaro, O. White Meat Consumption, All-Cause Mortality, and Cardiovascular Events: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2021, 13, 676. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Parra-Soto, S.; Gray, S.; Anderson, J.; Welsh, P.; Gill, J.; Sattar, N.; Ho, F.K.; Celis-Morales, C.; Pell, J.P. Vegetarians, fish, poultry, and meat-eaters: Who has higher risk of cardiovascular disease incidence and mortality? A prospective study from UK Biobank. Eur. Heart J. 2021, 42, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Streppel, M.T.; Ocké, M.C.; Boshuizen, H.C.; Kok, F.J.; Kromhout, D. Long-term fish consumption and n-3 fatty acid intake in relation to (sudden) coronary heart disease death: The Zutphen study. Eur. Heart J. 2008, 29, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Stevens, S.; Gorman, D.; Pan, A.; Warnakula, S.; Chowdhury, S.; Ward, H.; Johnson, L.; Crowe, F.; Hu, F.B.; et al. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: Systematic review and meta-analysis. BMJ 2012, 345, e6698. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zhou, C.H.; Pei, H.J.; Zhou, X.L.; Li, L.H.; Wu, Y.J.; Hui, R.T. Fish consumption and incidence of heart failure: A meta-analysis of prospective cohort studies. Chin. Med. J. 2013, 126, 942–948. [Google Scholar] [PubMed]
- Tong, T.Y.N.; Appleby, P.N.; Bradbury, K.E.; Perez-Cornago, A.; Travis, R.C.; Clarke, R.; Key, T.J. Risks of ischaemic heart disease and stroke in meat eaters, fish eaters, and vegetarians over 18 years of follow-up: Results from the prospective EPIC-Oxford study. BMJ 2019, 366, l4897. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Appleby, P.N.; Bradbury, K.E.; Sweeting, M.; Wood, A.; Johansson, I.; Kühn, T.; Steur, M.; Weiderpass, E.; Wennberg, M.; et al. Consumption of Meat, Fish, Dairy Products, and Eggs and Risk of Ischemic Heart Disease. Circulation 2019, 139, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Mente, A.; Dehghan, M.; Rangarajan, S.; O’Donnell, M.; Hu, W.; Dagenais, G.; Wielgosz, A.; Lear, S.; Wei, L.; et al. Associations of Fish Consumption With Risk of Cardiovascular Disease and Mortality Among Individuals With or Without Vascular Disease From 58 Countries. JAMA Intern. Med. 2021, 181, 631–649. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Petsini, F.; Fragopoulou, E.; Antonopoulou, S. Fish consumption and cardiovascular disease related biomarkers: A review of clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2061–2071. [Google Scholar] [CrossRef]
- Ros, E. Health benefits of nut consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef]
- Fraser, G.E.; Sabaté, J.; Beeson, W.L.; Strahan, T.M. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch. Intern. Med. 1992, 152, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Colditz, G.A.; Rosner, B.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Frequent nut consumption and risk of coronary heart disease in women: Prospective cohort study. BMJ 1998, 317, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.M.; Gaziano, J.M.; Willett, W.C.; Manson, J.E. Nut consumption and decreased risk of sudden cardiac death in the Physicians’ Health Study. Arch. Intern. Med. 2002, 162, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Han, J.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Association of nut consumption with total and cause-specific mortality. N. Engl. J. Med. 2013, 369, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhang, Y.; Ding, Y.; Shan, Z.; Chen, S.; Yu, M.; Hu, F.B.; Liu, L. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Hshieh, T.T.; Petrone, A.B.; Gaziano, J.M.; Djoussé, L. Nut consumption and risk of mortality in the Physicians’ Health Study. Am. J. Clin. Nutr. 2015, 101, 407–412. [Google Scholar] [CrossRef]
- Afshin, A.; Micha, R.; Khatibzadeh, S.; Mozaffarian, D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 278–288. [Google Scholar] [CrossRef]
- Grosso, G.; Yang, J.; Marventano, S.; Micek, A.; Galvano, F.; Kales, S.N. Nut consumption on all-cause, cardiovascular, and cancer mortality risk: A systematic review and meta-analysis of epidemiologic studies. Am. J. Clin. Nutr. 2015, 101, 783–793. [Google Scholar] [CrossRef]
- Luu, H.N.; Blot, W.J.; Xiang, Y.B.; Cai, H.; Hargreaves, M.K.; Li, H.; Yang, G.; Signorello, L.; Gao, Y.T.; Zheng, W.; et al. Prospective evaluation of the association of nut/peanut consumption with total and cause-specific mortality. JAMA Intern. Med. 2015, 175, 755–766. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: A systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016, 14, 207. [Google Scholar] [CrossRef]
- Balakrishna, R.; Bjørnerud, T.; Bemanian, M.; Aune, D.; Fadnes, L.T. Consumption of Nuts and Seeds and Health Outcomes Including Cardiovascular Disease, Diabetes and Metabolic Disease, Cancer, and Mortality: An Umbrella Review. Adv. Nutr. 2022, 13, 2136–2148. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Liu, X.; Malik, V.S.; Sun, Q.; Willett, W.C.; Manson, J.E.; Rexrode, K.M.; Li, Y.; Hu, F.B.; Bhupathiraju, S.N. Nut Consumption and Risk of Cardiovascular Disease. J. Am. Coll. Cardiol. 2017, 70, 2519–2532. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Guasch-Ferré, M.; Hu, Y.; Li, Y.; Hu, F.B.; Rimm, E.B.; Manson, J.E.; Rexrode, K.M.; Sun, Q. Nut Consumption in Relation to Cardiovascular Disease Incidence and Mortality Among Patients With Diabetes Mellitus. Circ. Res. 2019, 124, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Q.; Tang, J.J.; Wu, H.; Xie, C.Y.; He, Z.Z. Consumption of nuts and legumes and risk of stroke: A meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, G.; Wei, Y.; Zhu, W.; Liu, X. Nut consumption and risk of stroke. Eur. J. Epidemiol. 2015, 30, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Djoussé, L.; Gaziano, J.M.; Kase, C.S.; Kurth, T. Nut consumption and risk of stroke in US male physicians. Clin. Nutr. 2010, 29, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, R.; Fjeld, M.K.; Dierkes, J.; Theoflylaktopoulou, D.; Arregui, M.; Boeing, H.; Weikert, C. The association between nut consumption and the risk of total and ischemic stroke in a German cohort study. Eur. J. Clin. Nutr. 2015, 69, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Vupputuri, S.; Myers, L.; Whelton, P.K. Fruit and vegetable intake and risk of cardiovascular disease in US adults: The first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am. J. Clin. Nutr. 2002, 76, 93–99. [Google Scholar] [CrossRef]
- Steffen, L.M.; Jacobs, D.R., Jr.; Stevens, J.; Shahar, E.; Carithers, T.; Folsom, A.R. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2003, 78, 383–390. [Google Scholar] [CrossRef]
- Dauchet, L.; Amouyel, P.; Hercberg, S.; Dallongeville, J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J. Nutr. 2006, 136, 2588–2593. [Google Scholar] [CrossRef]
- Gan, Y.; Tong, X.; Li, L.; Cao, S.; Yin, X.; Gao, C.; Herath, C.; Li, W.; Jin, Z.; Chen, Y.; et al. Consumption of fruit and vegetable and risk of coronary heart disease: A meta-analysis of prospective cohort studies. Int. J. Cardiol. 2015, 183, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): A prospective cohort study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef]
- Wang, J.; Liu, F.; Li, J.; Huang, K.; Yang, X.; Chen, J.; Liu, X.; Cao, J.; Chen, S.; Shen, C.; et al. Fruit and vegetable consumption, cardiovascular disease, and all-cause mortality in China. Sci. China Life Sci. 2022, 65, 119–128. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Nowson, C.A.; Lucas, M.; MacGregor, G.A. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: Meta-analysis of cohort studies. J. Hum. Hypertens. 2007, 21, 717–728. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Bhupathiraju, S.N.; Rosner, B.A.; Sun, Q.; Giovannucci, E.L.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; et al. Fruit and Vegetable Intake and Mortality: Results From 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation 2021, 143, 1642–1654. [Google Scholar] [CrossRef]
- Dauchet, L.; Amouyel, P.; Dallongeville, J. Fruit and vegetable consumption and risk of stroke: A meta-analysis of cohort studies. Neurology 2005, 65, 1193–1197. [Google Scholar] [CrossRef]
- Joshipura, K.J.; Ascherio, A.; Manson, J.E.; Stampfer, M.J.; Rimm, E.B.; Speizer, F.E.; Hennekens, C.H.; Spiegelman, D.; Willett, W.C. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA 1999, 282, 1233–1239. [Google Scholar] [CrossRef]
- Johnsen, S.P.; Overvad, K.; Stripp, C.; Tjønneland, A.; Husted, S.E.; Sørensen, H.T. Intake of fruit and vegetables and the risk of ischemic stroke in a cohort of Danish men and women. Am. J. Clin. Nutr. 2003, 78, 57–64. [Google Scholar] [CrossRef]
- Sauvaget, C.; Nagano, J.; Allen, N.; Kodama, K. Vegetable and fruit intake and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Stroke 2003, 34, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Nowson, C.A.; MacGregor, G.A. Fruit and vegetable consumption and stroke: Meta-analysis of cohort studies. Lancet 2006, 367, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.Y.N.; Appleby, P.N.; Key, T.J.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjønneland, A.; Katzke, V.; Kühn, T.; Boeing, H.; et al. The associations of major foods and fibre with risks of ischaemic and haemorrhagic stroke: A prospective study of 418 329 participants in the EPIC cohort across nine European countries. Eur. Heart J. 2020, 41, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J. Global sodium consumption and death from cardiovascular causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018, 138, e426–e483. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Intersalt Cooperative Research Group. Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 1988, 297, 319–328. [Google Scholar] [CrossRef]
- MacGregor, G.A.; Markandu, N.D.; Sagnella, G.A.; Singer, D.R.; Cappuccio, F.P. Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet 1989, 2, 1244–1247. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., 3rd; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- He, F.J.; Li, J.; Macgregor, G.A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013, 346, f1325. [Google Scholar] [CrossRef] [PubMed]
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliott, P.; Cappuccio, F.P.; Meerpohl, J.J. Effect of lower sodium intake on health: Systematic review and meta-analyses. BMJ 2013, 346, f1326. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Jousilahti, P.; Rastenyte, D.; Moltchanov, V.; Tanskanen, A.; Pietinen, P.; Nissinen, A. Urinary sodium excretion and cardiovascular mortality in Finland: A prospective study. Lancet 2001, 357, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; D’Elia, L.; Kandala, N.B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef]
- Poggio, R.; Gutierrez, L.; Matta, M.G.; Elorriaga, N.; Irazola, V.; Rubinstein, A. Daily sodium consumption and CVD mortality in the general population: Systematic review and meta-analysis of prospective studies. Public Health Nutr. 2015, 18, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Cutler, J.A.; Obarzanek, E.; Buring, J.E.; Rexrode, K.M.; Kumanyika, S.K.; Appel, L.J.; Whelton, P.K. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: Observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007, 334, 885–888. [Google Scholar] [CrossRef]
- Mente, A.; O’Donnell, M.; Rangarajan, S.; Dagenais, G.; Lear, S.; McQueen, M.; Diaz, R.; Avezum, A.; Lopez-Jaramillo, P.; Lanas, F.; et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: A pooled analysis of data from four studies. Lancet 2016, 388, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Oparil, S.; Whelton, P.K.; McKee, M.; Dominiczak, A.; Luft, F.C.; AlHabib, K.; Lanas, F.; Damasceno, A.; Prabhakaran, D.; et al. The technical report on sodium intake and cardiovascular disease in low- and middle-income countries by the joint working group of the World Heart Federation, the European Society of Hypertension and the European Public Health Association. Eur. Heart J. 2017, 38, 712–719. [Google Scholar] [CrossRef]
- Welsh, C.E.; Welsh, P.; Jhund, P.; Delles, C.; Celis-Morales, C.; Lewsey, J.D.; Gray, S.; Lyall, D.; Iliodromiti, S.; Gill, J.M.R.; et al. Urinary Sodium Excretion, Blood Pressure, and Risk of Future Cardiovascular Disease and Mortality in Subjects Without Prior Cardiovascular Disease. Hypertension 2019, 73, 1202–1209. [Google Scholar] [CrossRef]
- Ma, H.; Wang, X.; Li, X.; Heianza, Y.; Qi, L. Adding Salt to Foods and Risk of Cardiovascular Disease. J. Am. Coll. Cardiol. 2022, 80, 2157–2167. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, Q.; Kwame Amakye, W.; Su, Y.; Zhang, Z. Biomarkers of dairy fat intake and risk of cardiovascular disease: A systematic review and meta analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Trieu, K.; Bhat, S.; Dai, Z.; Leander, K.; Gigante, B.; Qian, F.; Korat, A.V.A.; Sun, Q.; Pan, X.F.; Laguzzi, F.; et al. Biomarkers of dairy fat intake, incident cardiovascular disease, and all-cause mortality: A cohort study, systematic review, and meta-analysis. PLoS Med. 2021, 18, e1003763. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Mente, A.; Rangarajan, S.; Sheridan, P.; Mohan, V.; Iqbal, R.; Gupta, R.; Lear, S.; Wentzel-Viljoen, E.; Avezum, A.; et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): A prospective cohort study. Lancet 2018, 392, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.Q.; Xu, J.Y.; Han, S.F.; Zhang, Z.L.; Zhao, Y.Y.; Szeto, I.M. Dairy consumption and risk of cardiovascular disease: An updated meta-analysis of prospective cohort studies. Asia Pac. J. Clin. Nutr. 2015, 24, 90–100. [Google Scholar] [CrossRef]
- Alexander, D.D.; Bylsma, L.C.; Vargas, A.J.; Cohen, S.S.; Doucette, A.; Mohamed, M.; Irvin, S.R.; Miller, P.E.; Watson, H.; Fryzek, J.P. Dairy consumption and CVD: A systematic review and meta-analysis. Br. J. Nutr. 2016, 115, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Astrup, A.; Lovegrove, J.A.; Gijsbers, L.; Givens, D.I.; Soedamah-Muthu, S.S. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: Dose-response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2017, 32, 269–287. [Google Scholar] [CrossRef]
- Jakobsen, M.U.; Trolle, E.; Outzen, M.; Mejborn, H.; Grønberg, M.G.; Lyndgaard, C.B.; Stockmarr, A.; Venø, S.K.; Bysted, A. Intake of dairy products and associations with major atherosclerotic cardiovascular diseases: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2021, 11, 1303. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, X.; Zhang, L.; Deng, Z. Fermented dairy foods intake and risk of cardiovascular diseases: A meta-analysis of cohort studies. Crit. Rev. Food Sci. Nutr. 2020, 60, 1189–1194. [Google Scholar] [CrossRef]
- Koskinen, T.T.; Virtanen, H.E.K.; Voutilainen, S.; Tuomainen, T.P.; Mursu, J.; Virtanen, J.K. Intake of fermented and non-fermented dairy products and risk of incident CHD: The Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2018, 120, 1288–1297. [Google Scholar] [CrossRef]
- Johansson, I.; Esberg, A.; Nilsson, L.M.; Jansson, J.H.; Wennberg, P.; Winkvist, A. Dairy Product Intake and Cardiometabolic Diseases in Northern Sweden: A 33-Year Prospective Cohort Study. Nutrients 2019, 11, 284. [Google Scholar] [CrossRef]
- Tutunchi, H.; Naghshi, S.; Naemi, M.; Naeini, F.; Esmaillzadeh, A. Yogurt consumption and risk of mortality from all causes, CVD and cancer: A comprehensive systematic review and dose-response meta-analysis of cohort studies. Public Health Nutr. 2023, 26, 1196–1209. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402s–1406s. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, A.J.; Suter-Zimmermann, K.; Bucher, H.C.; Shai, I.; Tuttle, K.R.; Estruch, R.; Briel, M. Meta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factors. Am. J. Med. 2011, 124, 841–851.e842. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Galbete, C.; Schwingshackl, L.; Schwedhelm, C.; Boeing, H.; Schulze, M.B. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: An umbrella review of meta-analyses. Eur. J. Epidemiol. 2018, 33, 909–931. [Google Scholar] [CrossRef] [PubMed]
- Tognon, G.; Lissner, L.; Sæbye, D.; Walker, K.Z.; Heitmann, B.L. The Mediterranean diet in relation to mortality and CVD: A Danish cohort study. Br. J. Nutr. 2014, 111, 151–159. [Google Scholar] [CrossRef]
- Rosato, V.; Temple, N.J.; La Vecchia, C.; Castellan, G.; Tavani, A.; Guercio, V. Mediterranean diet and cardiovascular disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019, 58, 173–191. [Google Scholar] [CrossRef]
- Grosso, G.; Marventano, S.; Yang, J.; Micek, A.; Pajak, A.; Scalfi, L.; Galvano, F.; Kales, S.N. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: Are individual components equal? Crit. Rev. Food Sci. Nutr. 2017, 57, 3218–3232. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Blanco Mejía, S.; Viguiliouk, E.; Khan, T.; Kendall, C.W.C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1207–1227. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, C. Vegetarian nutrition: Past, present, future. Am. J. Clin. Nutr. 2014, 100 (Suppl. S1), 496S–502S. [Google Scholar] [CrossRef] [PubMed]
- Van Horn, L.; Carson, J.A.; Appel, L.J.; Burke, L.E.; Economos, C.; Karmally, W.; Lancaster, K.; Lichtenstein, A.H.; Johnson, R.K.; Thomas, R.J.; et al. Recommended Dietary Pattern to Achieve Adherence to the American Heart Association/American College of Cardiology (AHA/ACC) Guidelines: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e505–e529. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Yang, B.; Zheng, J.; Li, G.; Wahlqvist, M.L.; Li, D. Cardiovascular disease mortality and cancer incidence in vegetarians: A meta-analysis and systematic review. Ann. Nutr. Metab. 2012, 60, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Umar, S.; Myint, P.K.; Mamas, M.A.; Loke, Y.K. Vegetarian diet, Seventh Day Adventists and risk of cardiovascular mortality: A systematic review and meta-analysis. Int. J. Cardiol. 2014, 176, 680–686. [Google Scholar] [CrossRef]
- Dybvik, J.S.; Svendsen, M.; Aune, D. Vegetarian and vegan diets and the risk of cardiovascular disease, ischemic heart disease and stroke: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Nutr. 2023, 62, 51–69. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef]
- Baden, M.Y.; Liu, G.; Satija, A.; Li, Y.; Sun, Q.; Fung, T.T.; Rimm, E.B.; Willett, W.C.; Hu, F.B.; Bhupathiraju, S.N. Changes in Plant-Based Diet Quality and Total and Cause-Specific Mortality. Circulation 2019, 140, 979–991. [Google Scholar] [CrossRef]
- Khosravi-Boroujeni, H.; Mohammadifard, N.; Sarrafzadegan, N.; Sajjadi, F.; Maghroun, M.; Khosravi, A.; Alikhasi, H.; Rafieian, M.; Azadbakht, L. Potato consumption and cardiovascular disease risk factors among Iranian population. Int. J. Food Sci. Nutr. 2012, 63, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Baden, M.Y.; Shan, Z.; Wang, F.; Li, Y.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B.; Rexrode, K.M. Quality of Plant-Based Diet and Risk of Total, Ischemic, and Hemorrhagic Stroke. Neurology 2021, 96, e1940–e1953. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.H.T.; Chang, H.R.; Wang, L.Y.; Chang, C.C.; Lin, M.N.; Lin, C.L. Vegetarian diet and incidence of total, ischemic, and hemorrhagic stroke in 2 cohorts in Taiwan. Neurology 2020, 94, e1112–e1121. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; van Daalen, K.R.; Thayyil, A.; Cocco, M.; Caputo, D.; Oliver-Williams, C. A Systematic Review of the Association Between Vegan Diets and Risk of Cardiovascular Disease. J. Nutr. 2021, 151, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Desmond, M.A.; Sobiecki, J.G.; Jaworski, M.; Płudowski, P.; Antoniewicz, J.; Shirley, M.K.; Eaton, S.; Książyk, J.; Cortina-Borja, M.; De Stavola, B.; et al. Growth, body composition, and cardiovascular and nutritional risk of 5- to 10-y-old children consuming vegetarian, vegan, or omnivore diets. Am. J. Clin. Nutr. 2021, 113, 1565–1577. [Google Scholar] [CrossRef]
- Weikert, C.; Trefflich, I.; Menzel, J.; Obeid, R.; Longree, A.; Dierkes, J.; Meyer, K.; Herter-Aeberli, I.; Mai, K.; Stangl, G.I.; et al. Vitamin and Mineral Status in a Vegan Diet. Dtsch. Arztebl. Int. 2020, 117, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public. Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Monteiro, C.A. Nutrition and health. The issue is not food, nor nutrients, so much as processing. Public Health Nutr. 2009, 12, 729–731. [Google Scholar] [CrossRef]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Andrianasolo, R.M.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultra-processed food intake and risk of cardiovascular disease: Prospective cohort study (NutriNet-Santé). BMJ 2019, 365, l1451. [Google Scholar] [CrossRef]
- Juul, F.; Vaidean, G.; Lin, Y.; Deierlein, A.L.; Parekh, N. Ultra-Processed Foods and Incident Cardiovascular Disease in the Framingham Offspring Study. J. Am. Coll. Cardiol. 2021, 77, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Suksatan, W.; Moradi, S.; Naeini, F.; Bagheri, R.; Mohammadi, H.; Talebi, S.; Mehrabani, S.; Hojjati Kermani, M.A.; Suzuki, K. Ultra-Processed Food Consumption and Adult Mortality Risk: A Systematic Review and Dose-Response Meta-Analysis of 207,291 Participants. Nutrients 2021, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Costanzo, S.; Di Castelnuovo, A.; Persichillo, M.; Magnacca, S.; De Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Ultra-processed food intake and all-cause and cause-specific mortality in individuals with cardiovascular disease: The Moli-sani Study. Eur. Heart J. 2022, 43, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hu, E.A.; Rebholz, C.M. Ultra-processed food intake and mortality in the USA: Results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). Public Health Nutr. 2019, 22, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Honicky, M.; Cardoso, S.M.; Kunradi Vieira, F.G.; Hinnig, P.F.; Back, I.C.; Moreno, Y.M.F. Ultra-processed food intake is associated with children and adolescents with congenital heart disease clustered by high cardiovascular risk factors. Br. J. Nutr. 2022, 129, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Ciaffi, J.; Mitselman, D.; Mancarella, L.; Brusi, V.; Lisi, L.; Ruscitti, P.; Cipriani, P.; Meliconi, R.; Giacomelli, R.; Borghi, C.; et al. The Effect of Ketogenic Diet on Inflammatory Arthritis and Cardiovascular Health in Rheumatic Conditions: A Mini Review. Front. Med. 2021, 8, 792846. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.L.; Esteves, S.S.; da Costa Pereira, A.; Yancy, W.S., Jr.; Nunes, J.P. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes. Rev. 2012, 13, 1048–1066. [Google Scholar] [CrossRef]
- Bueno, N.B.; de Melo, I.S.; de Oliveira, S.L.; da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef]
- Cicero, A.F.; Benelli, M.; Brancaleoni, M.; Dainelli, G.; Merlini, D.; Negri, R. Middle and Long-Term Impact of a Very Low-Carbohydrate Ketogenic Diet on Cardiometabolic Factors: A Multi-Center, Cross-Sectional, Clinical Study. High Blood Press. Cardiovasc. Prev. 2015, 22, 389–394. [Google Scholar] [CrossRef]
- Naude, C.E.; Schoonees, A.; Senekal, M.; Young, T.; Garner, P.; Volmink, J. Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: A systematic review and meta-analysis. PLoS ONE 2014, 9, e100652. [Google Scholar] [CrossRef]
- Valsdottir, T.D.; Henriksen, C.; Odden, N.; Nellemann, B.; Jeppesen, P.B.; Hisdal, J.; Westerberg, A.C.; Jensen, J. Effect of a Low-Carbohydrate High-Fat Diet and a Single Bout of Exercise on Glucose Tolerance, Lipid Profile and Endothelial Function in Normal Weight Young Healthy Females. Front. Physiol. 2019, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Burén, J.; Ericsson, M.; Damasceno, N.R.T.; Sjödin, A. A Ketogenic Low-Carbohydrate High-Fat Diet Increases LDL Cholesterol in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients 2021, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K. Cardiometabolic Benefits of Intermittent Fasting. Annu. Rev. Nutr. 2021, 41, 333–361. [Google Scholar] [CrossRef] [PubMed]
- De Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Kroeger, C.M.; Klempel, M.C.; Bhutani, S.; Trepanowski, J.F.; Tangney, C.C.; Varady, K.A. Improvement in coronary heart disease risk factors during an intermittent fasting/calorie restriction regimen: Relationship to adipokine modulations. Nutr. Metab. 2012, 9, 98. [Google Scholar] [CrossRef]
- Most, J.; Gilmore, L.A.; Smith, S.R.; Han, H.; Ravussin, E.; Redman, L.M. Significant improvement in cardiometabolic health in healthy nonobese individuals during caloric restriction-induced weight loss and weight loss maintenance. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E396–E405. [Google Scholar] [CrossRef] [PubMed]
- Patikorn, C.; Roubal, K.; Veettil, S.K.; Chandran, V.; Pham, T.; Lee, Y.Y.; Giovannucci, E.L.; Varady, K.A.; Chaiyakunapruk, N. Intermittent Fasting and Obesity-Related Health Outcomes: An Umbrella Review of Meta-analyses of Randomized Clinical Trials. JAMA Netw. Open 2021, 4, e2139558. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Schroder, J.D.; Falqueto, H.; Mânica, A.; Zanini, D.; de Oliveira, T.; de Sá, C.A.; Cardoso, A.M.; Manfredi, L.H. Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J. Transl. Med. 2021, 19, 3. [Google Scholar] [CrossRef]
- Jamshed, H.; Steger, F.L.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.J.; Peterson, C.M. Effectiveness of Early Time-Restricted Eating for Weight Loss, Fat Loss, and Cardiometabolic Health in Adults With Obesity: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 953–962. [Google Scholar] [CrossRef]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luo, S.; Ye, Y.; Yin, S.; Fan, J.; Xia, M. Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients. J. Clin. Endocrinol. Metab. 2021, 106, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-obese Humans. Cell Metab. 2019, 30, 462–476.e466. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Seo, Y.G.; Paek, Y.J.; Song, H.J.; Park, K.H.; Noh, H.M. Effect of alternate-day fasting on obesity and cardiometabolic risk: A systematic review and meta-analysis. Metabolism 2020, 111, 154336. [Google Scholar] [CrossRef] [PubMed]
- Templeman, I.; Smith, H.A.; Chowdhury, E.; Chen, Y.C.; Carroll, H.; Johnson-Bonson, D.; Hengist, A.; Smith, R.; Creighton, J.; Clayton, D.; et al. A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci. Transl. Med. 2021, 13, eabd8034. [Google Scholar] [CrossRef]
- O’Hearn, M.; Lara-Castor, L.; Cudhea, F.; Miller, V.; Reedy, J.; Shi, P.; Zhang, J.; Wong, J.B.; Economos, C.D.; Micha, R.; et al. Incident type 2 diabetes attributable to suboptimal diet in 184 countries. Nat. Med. 2023, 29, 982–995. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef]
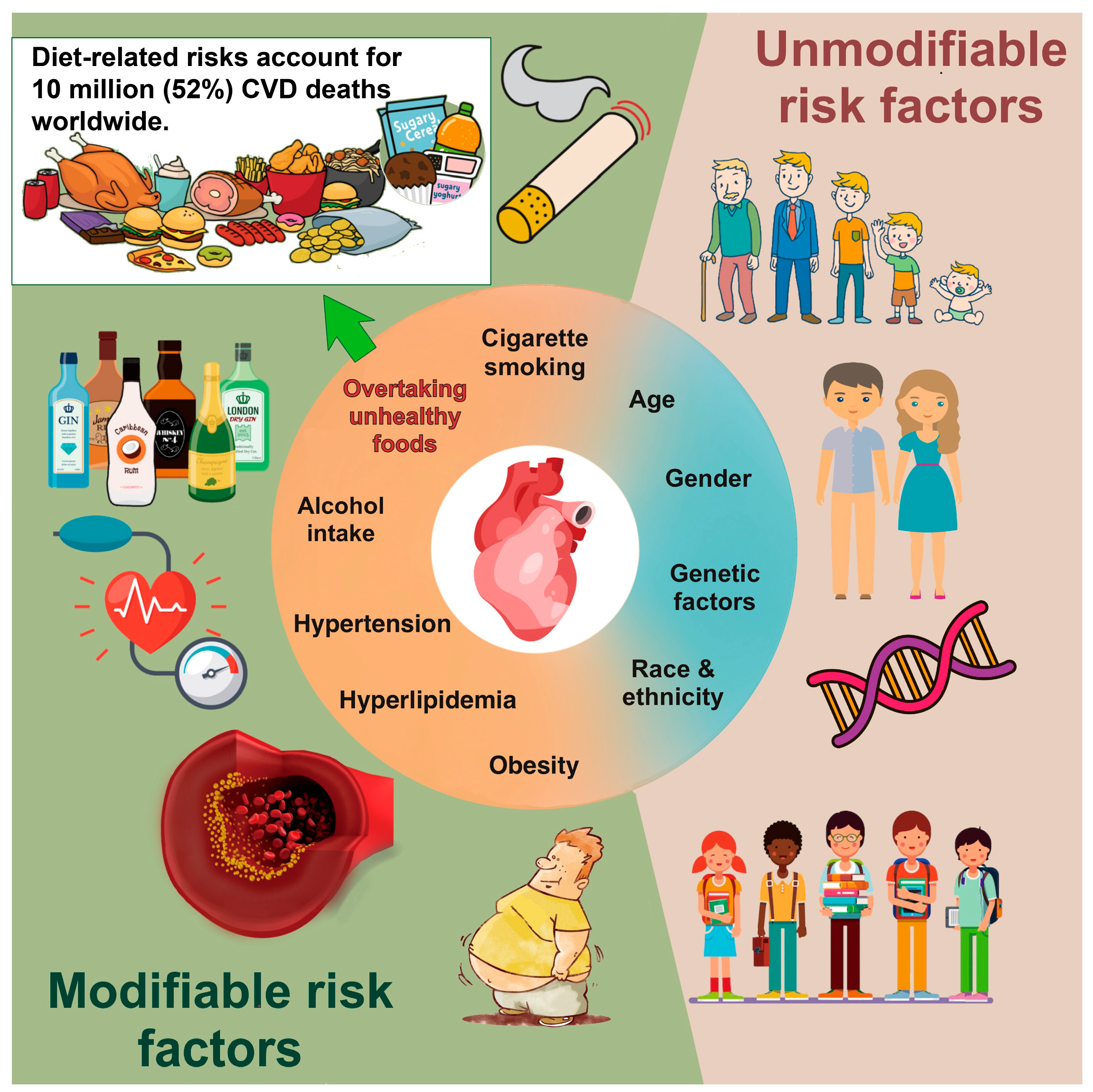

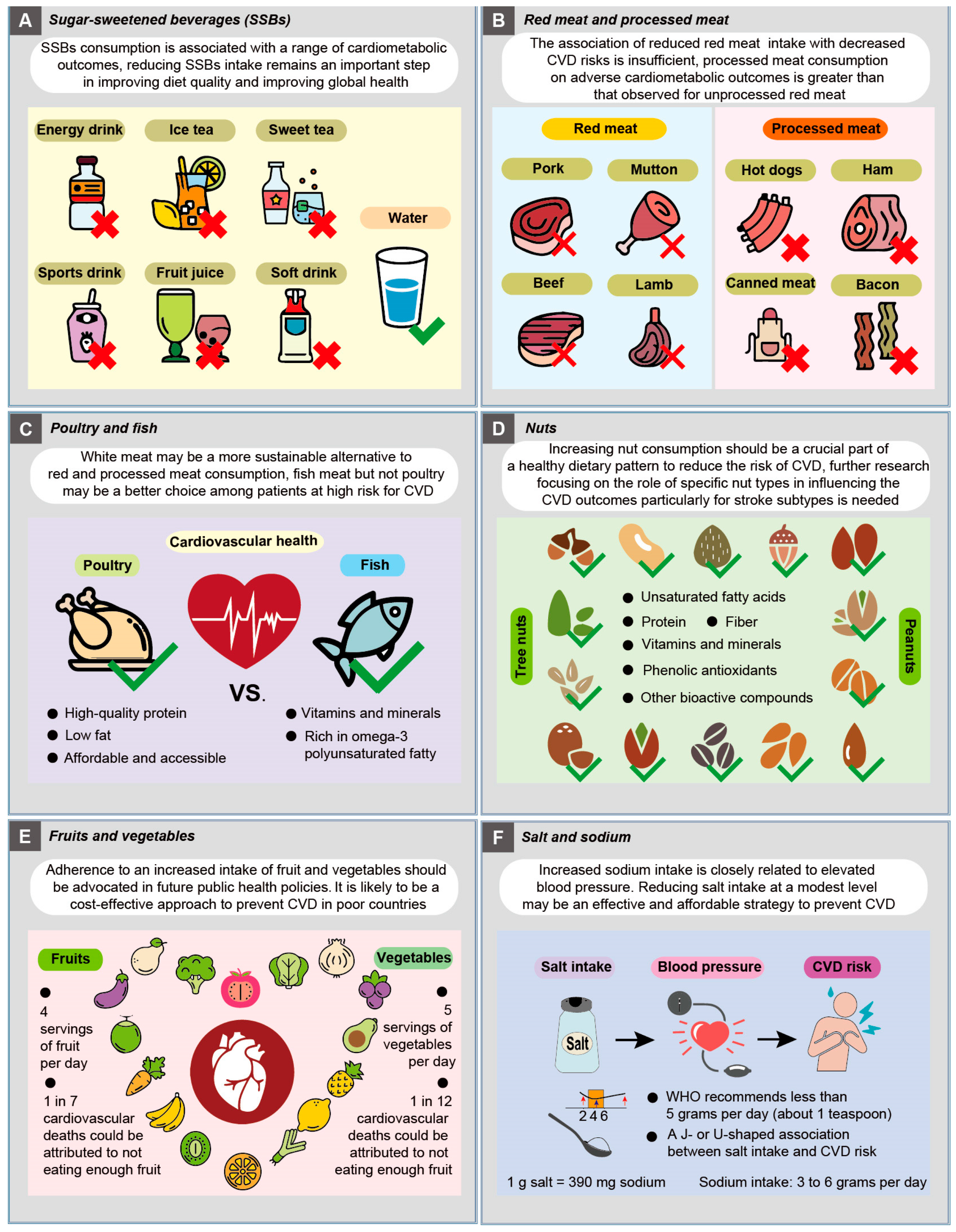
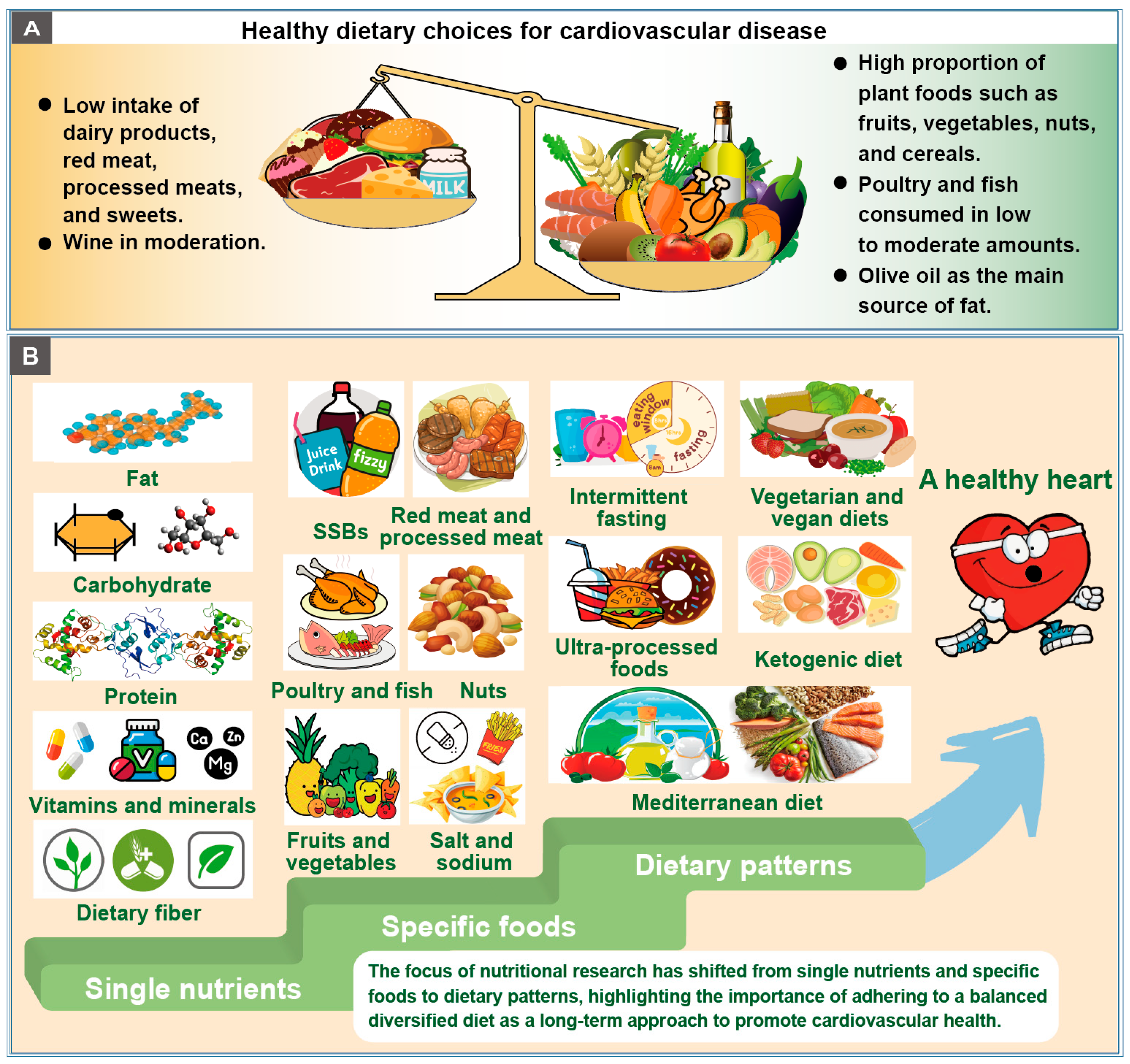
| Dietary Component | ESC [218] | AHA [219] |
|---|---|---|
| Vegetables and fruits | ≥200 g of vegetables per day (≥2–3 servings). ≥200 g of fruit per day (≥2–3 servings). | Eat plenty of fruits and vegetables, and choose a wide variety. |
| Unsalted nuts | 30 g unsalted nuts per day. | |
| Grains | Carbohydrates from whole grains. 30–45 g of fiber of per day, preferably from whole grains. | Choose foods made mostly with whole grains rather than refined grains. |
| Fatty | Saturated fatty acids should account for <10% of total energy intake, through replacement by PUFAs, MUFAs. Trans unsaturated fatty acids should be minimized as far as possible, with none from processed foods. | Use liquid plant oils rather than tropical oils (coconut, palm, and palm kernel), animal fats (e.g., butter and lard), or partially hydrogenated fats. |
| Protein | a. Mostly protein from plants (legumes and nuts). b. Fish and seafood. c. Low-fat or fat-free dairy products instead of full-fat dairy products. d. If meat or poultry are desired, choose lean cuts and avoid processed forms. | |
| Salt | <5 g total salt intake per day. | Choose and prepare foods with little or no salt. |
| Meat | Red meat should be reduced to a maximum of 350−500 g a week; in particular processed meat should be minimized. Fish is recommended 1–2 times per week, in particular fatty fish. | |
| Alcoholic beverages | Consumption of alcohol should be limited to a maximum of 100 g per week. | If you do not drink alcohol, do not start; if you choose to drink alcohol, limit intake. |
| SSBs | Sugar-sweetened beverages, such as soft drinks and fruit juices, must be discouraged. | Minimize intake of beverages and foods with added sugars. |
| Ultra-processed foods | Choose minimally processed foods instead of ultra-processed foods. | |
| Dietary patterns | Adopt a more plant-based and less animal-based food pattern. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhang, S.; Hu, X.; Chen, F.; Li, D. A Review of Healthy Dietary Choices for Cardiovascular Disease: From Individual Nutrients and Foods to Dietary Patterns. Nutrients 2023, 15, 4898. https://doi.org/10.3390/nu15234898
Chen W, Zhang S, Hu X, Chen F, Li D. A Review of Healthy Dietary Choices for Cardiovascular Disease: From Individual Nutrients and Foods to Dietary Patterns. Nutrients. 2023; 15(23):4898. https://doi.org/10.3390/nu15234898
Chicago/Turabian StyleChen, Wenjing, Shuqing Zhang, Xiaosong Hu, Fang Chen, and Daotong Li. 2023. "A Review of Healthy Dietary Choices for Cardiovascular Disease: From Individual Nutrients and Foods to Dietary Patterns" Nutrients 15, no. 23: 4898. https://doi.org/10.3390/nu15234898
APA StyleChen, W., Zhang, S., Hu, X., Chen, F., & Li, D. (2023). A Review of Healthy Dietary Choices for Cardiovascular Disease: From Individual Nutrients and Foods to Dietary Patterns. Nutrients, 15(23), 4898. https://doi.org/10.3390/nu15234898







