Effects of the Complex of Panicum miliaceum Extract and Triticum aestivum Extract on Hair Condition
Abstract
1. Introduction
2. Materials and Methods
2.1. MWC Preparation
2.2. Cell Culture
2.3. Chemicals
2.4. Cell Viability
2.5. Mouse Breeding and Anagen-Synchronized Mouse Model
2.6. RNA Extraction and Quantitative RT-PCR
2.7. H&E Staining
2.8. Statistics
3. Results
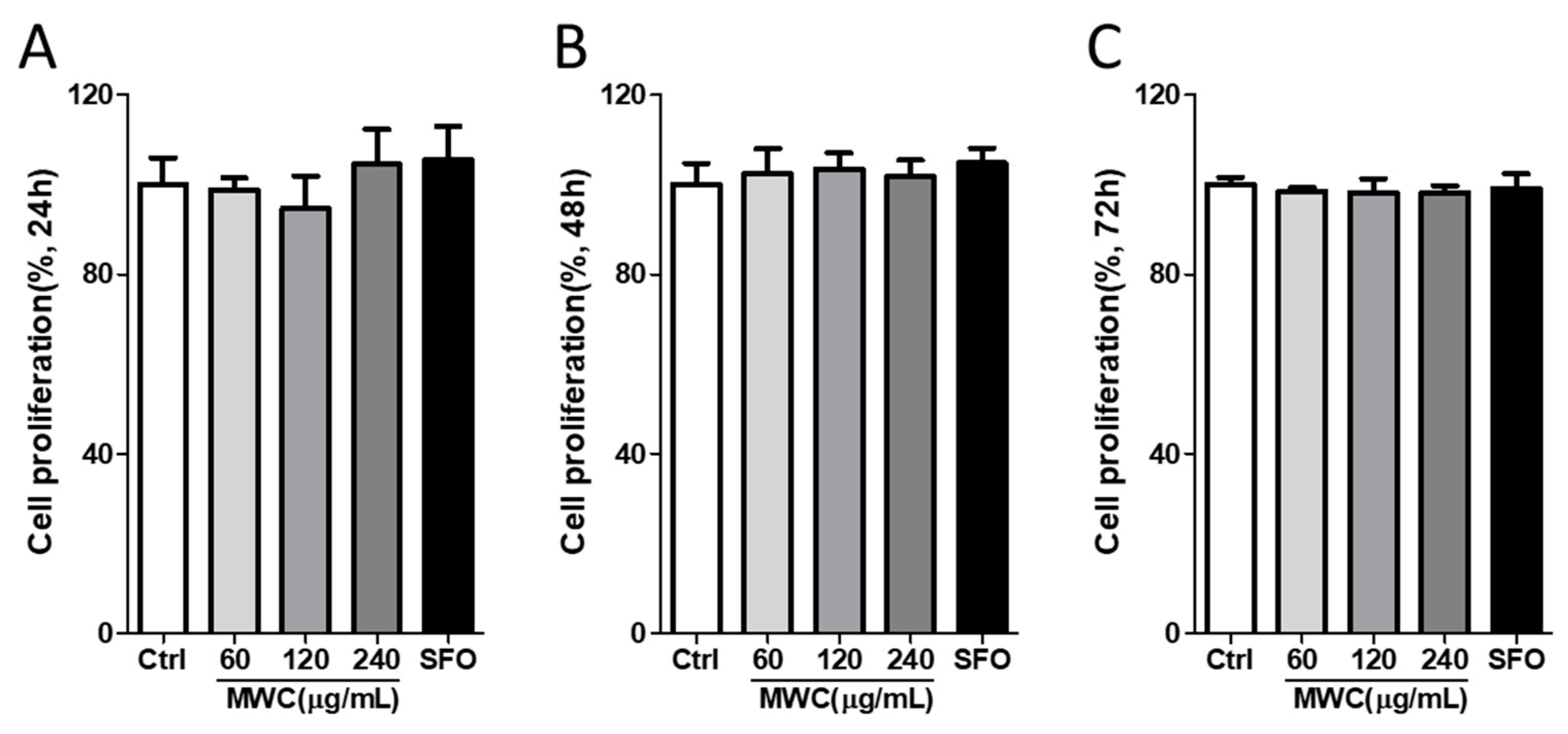
3.1. Effects of MWC on Cell Proliferation in Human iDPCs
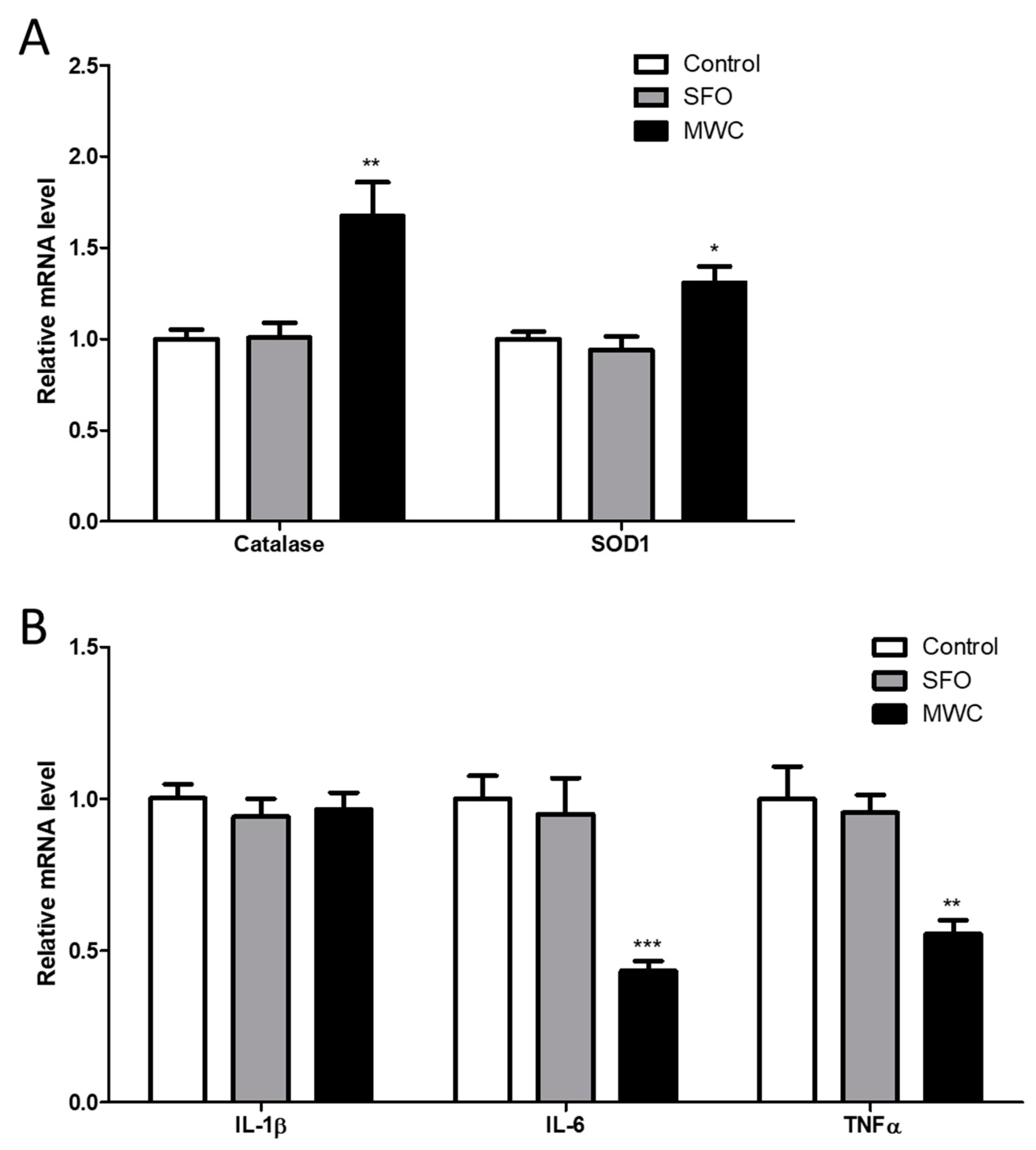
3.2. Effect of MWC on Gene Expressions of Antioxidant Enzymes and Inflammatory Cytokines in Human iDPCs
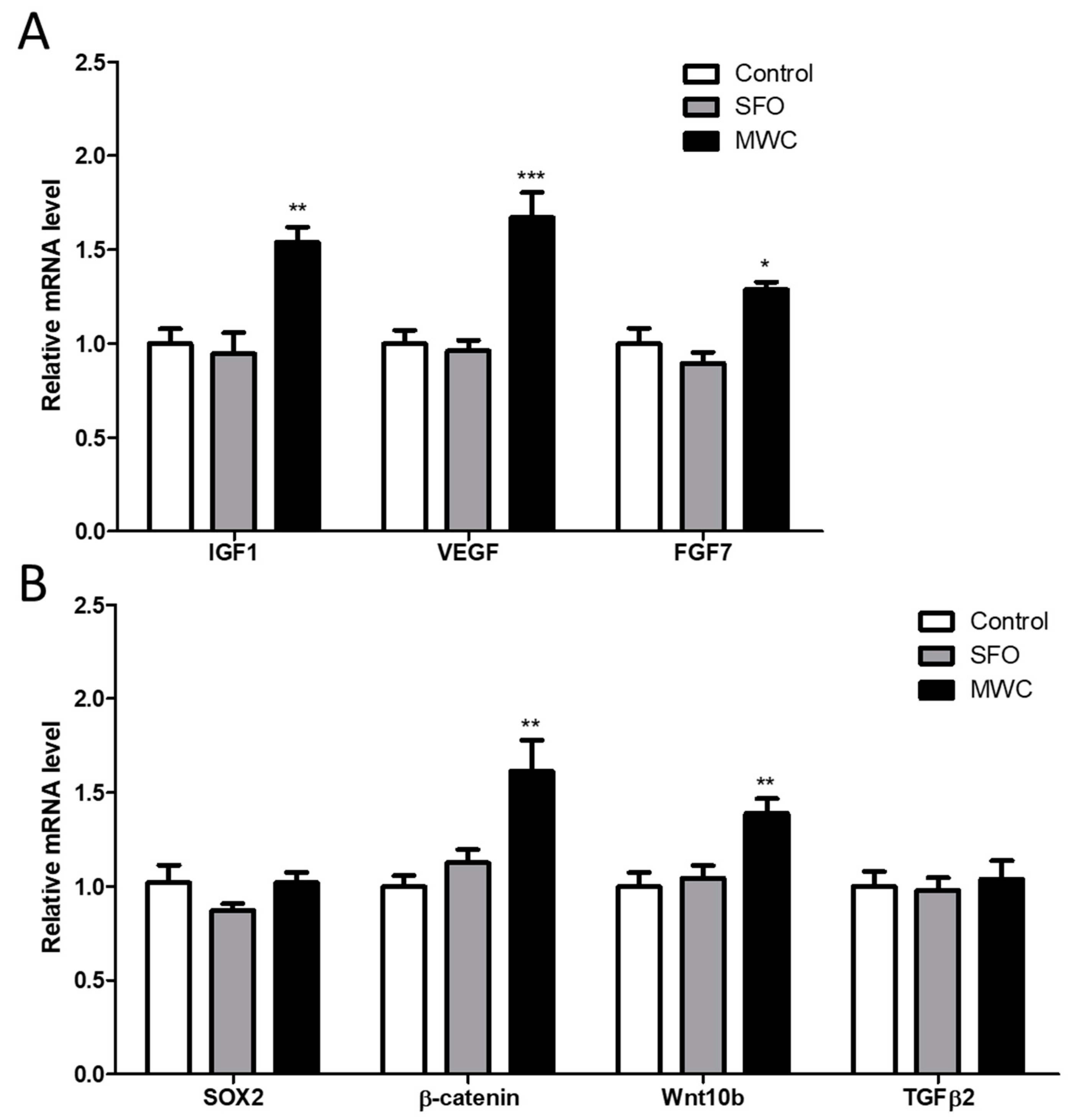
3.3. Effects of MWC on Growth and Hair-Growth-Related Gene Expression in Human iDPCs
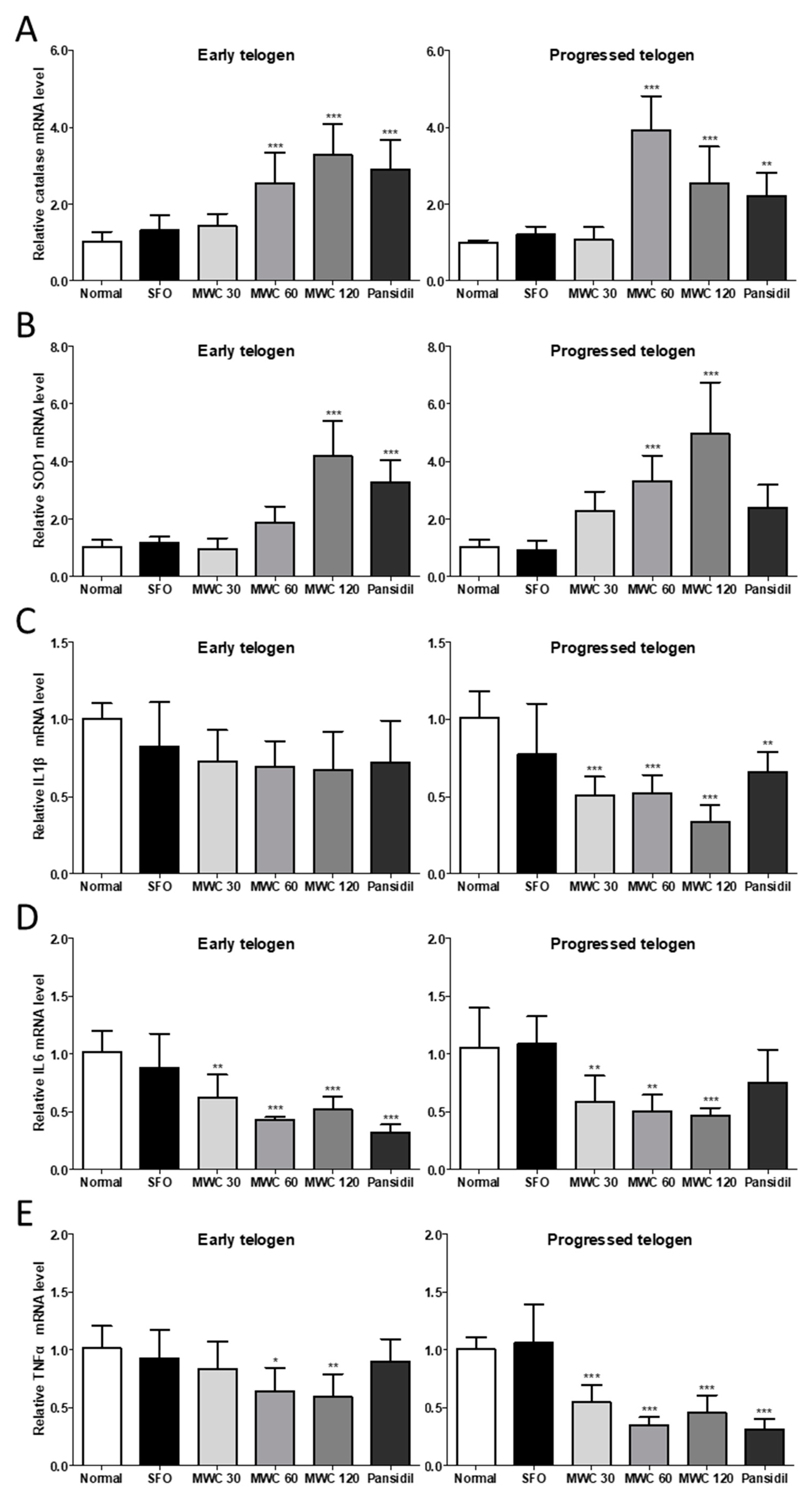
3.4. Effects of MWC on Gene Expression of Antioxidant Enzymes and Inflammatory Cytokines in an Anagen-Synchronized Mouse Model
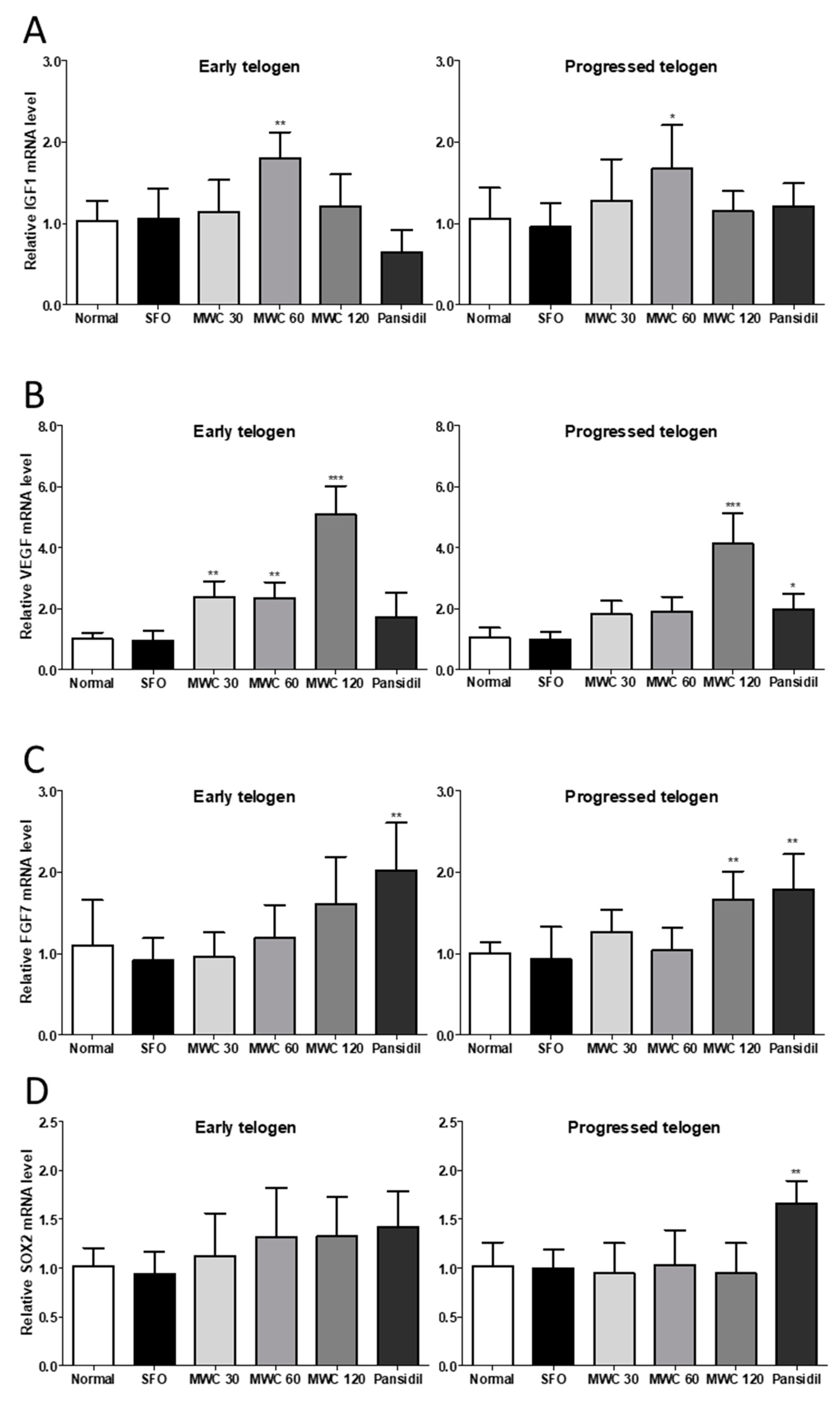
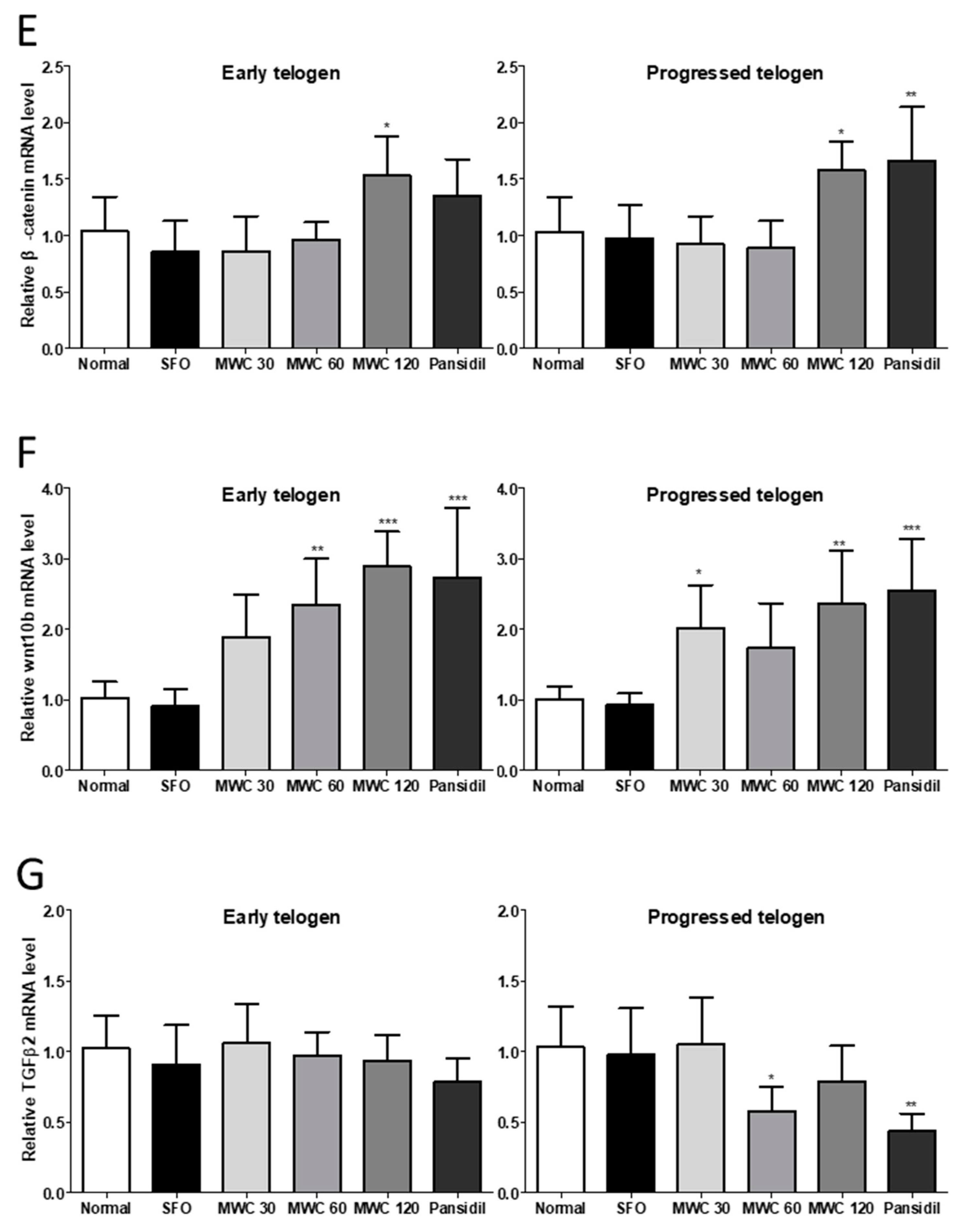
3.5. Effects of MWC on Gene Expression of Growth and Hair-Growth-Related Factors in Anagen-Synchronized Mouse Model
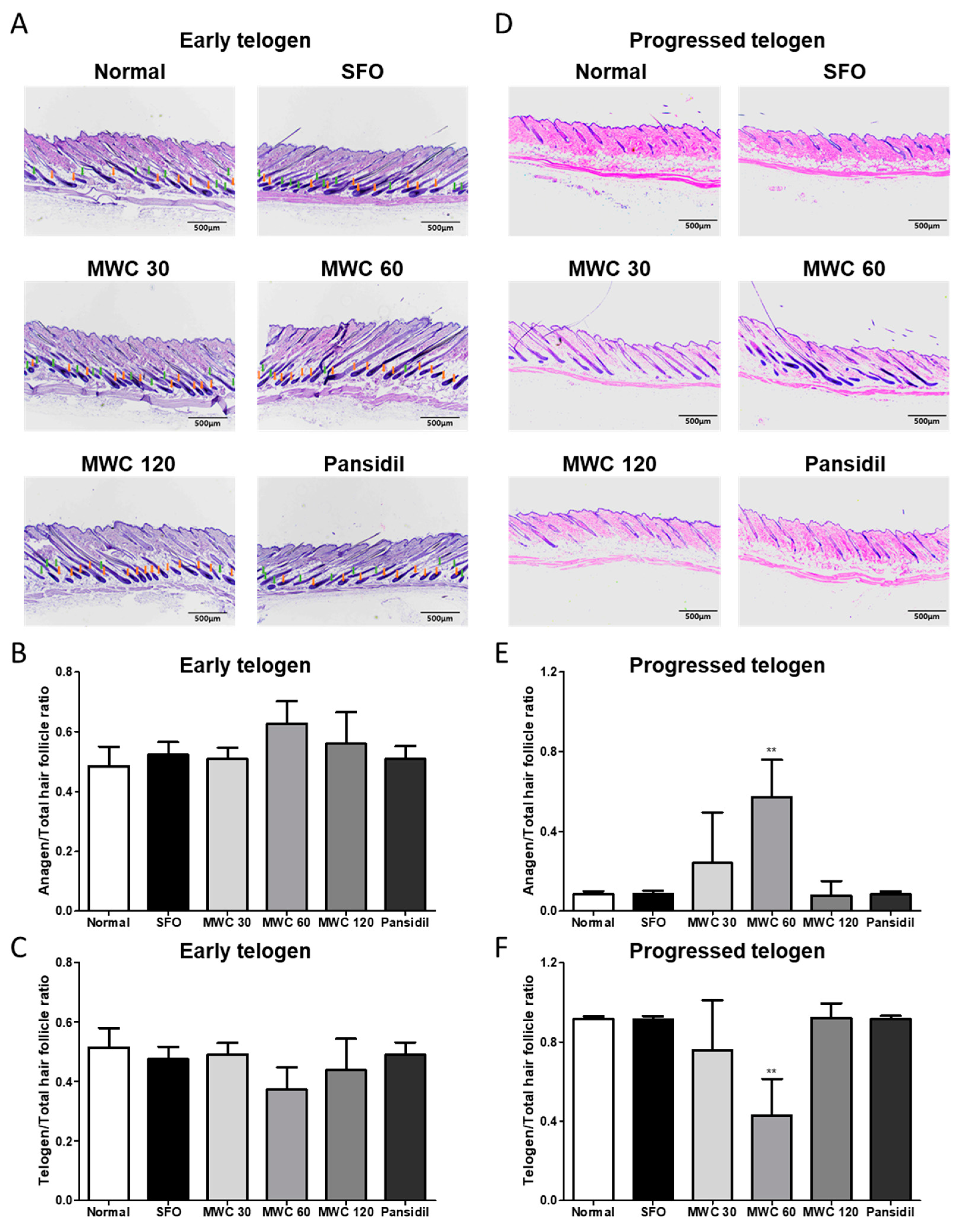
3.6. Effects of MWC on the Elongation of Hair during the Anagen Phase in the Anagen-Synchronized Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yesudian, P. Proceedings of the 7th World Congress of Hair Research. Int. J. Trichol. 2013, 5, 61–62. [Google Scholar] [CrossRef]
- Santos, Z.; Avci, P.; Hamblin, M.R. Drug discovery for alopecia: Gone today, hair tomorrow. Expert Opin. Drug Discov. 2015, 10, 269–292. [Google Scholar] [CrossRef]
- Trüeb, R.M. Oxidative stress in ageing of hair. Int. J. Trichol. 2009, 1, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhao, Y.; Cai, Z.; Caulloo, S.; McElwee, K.J.; Li, Y.; Chen, X.; Yu, M.; Yang, J.; Chen, W.; et al. Early stage alopecia areata is associated with inflammation in the upper dermis and damage to the hair follicle infundibulum. Aust. J. Dermatol. 2013, 54, 184–191. [Google Scholar] [CrossRef]
- Arck, P.C.; Handjiski, B.; Peters, E.M.J.; Peter, A.S.; Hagen, E.; Fischer, A.; Klapp, B.F.; Paus, R. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am. J. Pathol. 2003, 162, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, T.; Wang, J.; Jia, J.; Yi, Y.-H.; Chen, Y.-X.; Miao, Y.; Hu, Z.-Q. Hydroxytyrosol prevents dermal papilla cells inflammation under oxidative stress by inducing autophagy. J. Biochem. Mol. Toxicol. 2019, 33, e22377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ni, C.; Huang, Y.; Tang, Y.; Yang, K.; Shi, X.; Zhang, Y.; Li, Z.; Wang, J.; Zhu, Y.; et al. Hair growth-promoting effect of resveratrol in mice, human hair follicles and dermal papilla cells. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1805–1814. [Google Scholar] [CrossRef]
- Kim, K.-S.; Han, S.H.; An, I.-S.; Ahn, K.J. Protective effects of ellagic acid against UVA-induced oxidative stress in human dermal papilla. Asian J. Beauty Cosmetol. 2016, 14, 191–200. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of hair follicle dermal papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef]
- Stenn, K.S.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, J.S.; Lee, J.H. Antioxidative and antimutagenic effects of Panicum miliaceum L. Korean J. Food Preserv. 2020, 27, 261–269. [Google Scholar] [CrossRef]
- Park, M.-Y.; Jang, H.-H.; Kim, J.B.; Yoon, H.N.; Lee, J.-Y.; Lee, Y.-M.; Kim, J.-H.; Park, D.-S. Hog millet (Panicum miliaceum L.)-supplemented diet ameliorates hyperlipidemia and hepatic lipid accumulation in C57BL/6J-ob/ob mice. Nutr. Res. Pract. 2011, 5, 511–519. [Google Scholar] [CrossRef][Green Version]
- Park, M.-Y.; Kim, J.-H.; Park, D.-S. Anti-inflammatory activities of hog millet (Panicum miliaceum L.) in murine macrophages through IRAK-4 signaling. Korean J. Food Nutr. 2011, 24, 268–272. [Google Scholar] [CrossRef][Green Version]
- Keophiphath, M.; Courbière, C.; Manzato, L.; Lamour, I.; Gaillard, E. Miliacin encapsulated by polar lipids stimulates cell proliferation in hair bulb and improves telogen effluvium in women. J. Cosmet. Dermatol. 2020, 19, 485–493. [Google Scholar] [CrossRef]
- Boisnic, S.; Branchet, M.C.; Gaillard, E.; Lamour, I. Miliacin associated with polar lipids: Effect on growth factors excretion and extracellular matrix of the dermal papilla hair follicle model maintained in survival conditions. Hair Ther. Transplant 2016, 6, 1000143. [Google Scholar]
- Panfilova, T.V.; Shtil, A.A.; Frolov, B.A. Triterpenoid miliacin inhibits stress-induced lipid peroxidation. Bull. Exp. Biol. Med. 2006, 141, 685–687. [Google Scholar] [CrossRef]
- Krasikov, S.I.; Sharapova, N.V.; Karmanova, D.S. Vegetable-based triterpenoid miliacin limits insulin resistance in rats in experiment. Sys. Rev. Pharm. 2020, 11, 1830–1832. [Google Scholar]
- Nuzov, B.G. Effect of miliacin oil in the treatment of trophic ulcers. Patol. Fiziol. Eksp. Ter. 1991, 1, 34–35. [Google Scholar]
- Zheleznova, A.D.; Zheleznov, L.M.; Shtil, A.A.; Frolov, B.A. Morphological manifestations for the protective effect of miliacin in organs of immunogenesis after treatment with methotrexate. Bull. Exp. Biol. Med. 2007, 144, 575–579. [Google Scholar] [CrossRef]
- Panfilova, T.V.; Shtil, A.A.; Polosukhina, E.R.; Baryshnikov, A.Y.; Frolov, B.A. Effect of the triterpenoid miliacin on the sensitivity of lymphocytes in the thymus and spleen to dexamethasone-induced apoptosis. Bull. Exp. Biol. Med. 2003, 136, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-H.; Lee, M.Y.; Yang, S.-H.; Shin, H.-J.; Jeon, Y.J. Hydrophobic fractions of Triticum aestivum L. extracts contain polyphenols and alleviate inflammation by regulating nuclear factor-kappa B. Biotechnol. Bioprocess Eng. 2021, 26, 93–106. [Google Scholar] [CrossRef]
- Sahu, R.; Dua, T.K.; Das, S.; De Feo, V.; Dewanjee, S. Wheat phenolics suppress doxorubicin-induced cardiotoxicity via inhibition of oxidative stress, MAP kinase activation, NF-κB pathway, PI3K/Akt/mTOR impairment, and cardiac apoptosis. Food Chem. Toxicol. 2019, 125, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Jung, J.C.; Choi, Y.M.; Ryu, H.Y.; Lee, S.; Davis, B.A. Wheat extract oil (WEO) attenuates UVB-induced photoaging via collagen synthesis in human keratinocytes and hairless mice. Nutrients 2020, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Leipelt, M.; Warnecke, D.; Zähringer, U.; Ott, C.; Muller, F.; Hube, B.; Heinz, E. Glucosylceramide synthases, a gene family responsible for the biosynthesis of glucosphingolipids in animals, plants, and fungi. J. Biol. Chem. 2001, 276, 33621–33629. [Google Scholar] [CrossRef]
- Lee, Y.; Oliynyk, S.; Jung, J.C.; Han, J.J.; Oh, S. Administration of glucosylceramide ameliorated the memory impairment in aged mice. Evid.-Based Complement. Altern. Med. 2013, 2013, 824120. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, C.; Shapiro, J. Hair care products: Waving, straightening, conditioning, and coloring. Clin. Dermatol. 2001, 19, 431–436. [Google Scholar] [CrossRef]
- Müller-Röver, S.; Foitzik, K.; Paus, R.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef]
- Trüeb, R.M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef]
- Rogers, N.E.; Avram, M.R. Medical treatments for male and female pattern hair loss. J. Am. Acad. Dermatol. 2008, 59, 547–566. [Google Scholar] [CrossRef]
- Dorrington-Ward, P.; McCartney, A.C.E.; Holland, S.; Scully, J.; Carter, G.; Alaghband-Zadeh, J.; Wise, P. The effect of spironolactone on hirsutism and female androgen metabolism. Clin. Endocrinol. 1985, 23, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Rückert, R.; Lindner, G.; Bulfone-Paus, S.; Paus, R. High-dose proinflammatory cytokines induce apoptosis of hair bulb keratinocytes in vivo. Br. J. Dermatol. 2000, 143, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. The impact of oxidative stress on hair. Int. J. Cosmet. Sci. 2015, 37, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tannous, E.; Zheng, J.J. Oxidative stress upregulates Wnt signaling in human retinal microvascular endothelial cells through activation of disheveled. J. Cell. Biochem. 2019, 120, 14044–14054. [Google Scholar] [CrossRef]
- Gómez-Sámano, M.Á.; Grajales-Gómez, M.; Zuarth-Vázquez, J.M.; Navarro-Flores, M.F.; Martínez-Saavedra, M.; Juárez-León, Ó.A.; Morales-García, M.G.; Enríquez-Estrada, V.M.; Gómez-Pérez, F.J.; Cuevas-Ramos, D. Fibroblast growth factor 21 and its novel association with oxidative stress. Redox Biol. 2017, 11, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; McCullough, K.D.; Franke, T.F.; Holbrook, N.J. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J. Biol. Chem. 2000, 275, 14624–14631. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, H.; Chen, Y.; Li, G.; Bin, Y.; Zhou, X. Moderate oxidative stress promotes epithelial–mesenchymal transition in the lens epithelial cells via the TGF-β/Smad and Wnt/β-catenin pathways. Mol. Cell. Biochem. 2021, 476, 1631–1642. [Google Scholar] [CrossRef]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative stress–associated senescence in dermal papilla cells of men with androgenetic alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef]
| Primer | Sequence | |
|---|---|---|
| GAPDH | Forward | 5′-TGACCTCAACTACATGGTCTACA-3′ |
| Reverse | 5′-CTTCCCATTCTCGGCCTTG-3′ | |
| Catalase | Forward | 5′-AGCGACCAGATGAAGCAGTG-3′ |
| Reverse | 5′-TCCGCTCTCTGTCAAAGTGTG-3′ | |
| SOD1 | Forward | 5′-AACCAGTTGTGTTGTCAGGAC-3′ |
| Reverse | 5′-CCACCATGTTTCTTAGAGTGAGG-3′ | |
| IL-1β | Forward | 5′-TTCAGGCAGGCAGTATCACTC-3′ |
| Reverse | 5′-GAAGGTCCACGGAAAGACAC-3′ | |
| IL-6 | Forward | 5′-TGAACAACGATGATGCACTTG-3′ |
| Reverse | 5′-CTGAAGGACTCTGGCTTTGTC-3′ | |
| TNF-α | Forward | 5′-CAGGCGGTGCCTATGTCTC-3′ |
| Reverse | 5′-CGATCACCCCGAAGTTCAGTAG-3′ | |
| IGF-1 | Forward | 5′-AGGAAGTACATTTGAAGAACGCAAGT-3′ |
| Reverse | 5′-CCTGCGGTGGCATGTCA-3′ | |
| VEGF | Forward | 5′-GTGGACATCTTCCAGGAGTACC-3′ |
| Reverse | 5′-TGTTGTGCTGTAGGAAGCTCAT-3′ | |
| FGF-7 | Forward | 5′-CTGTCGAACACAGTGGTACCTG-3′ |
| Reverse | 5′-CCAACTGCCACTGTCCTGATTTC-3′ | |
| SOX-2 | Forward | 5′-GAGCTTTGCAGGAAGTTTGC-3′ |
| Reverse | 5′-GCAAGAAGCCTCTCCTTGAA-3′ | |
| β-catenin | Forward | 5′-AAAGCGGCTGTTAGTCACTGG-3′ |
| Reverse | 5′-CGAGTCATTGCATACTGTCCAT-3′ | |
| wnt10b | Forward | 5′-CTCGGGATTTCTTGGATTCCAGG-3′ |
| Reverse | 5′-GCCATGACACTTGCATTTCCGC-3′ | |
| TGFβ2 | Forward | 5′-AAGAAGCGTGCTTTGGATGCGG-3′ |
| Reverse | 5′-ATGCTCCAGCACAGAAGTTGGC-3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, N.; Kim, K.C.; Jeong, P.-Y.; Kim, B. Effects of the Complex of Panicum miliaceum Extract and Triticum aestivum Extract on Hair Condition. Nutrients 2023, 15, 4411. https://doi.org/10.3390/nu15204411
Choi N, Kim KC, Jeong P-Y, Kim B. Effects of the Complex of Panicum miliaceum Extract and Triticum aestivum Extract on Hair Condition. Nutrients. 2023; 15(20):4411. https://doi.org/10.3390/nu15204411
Chicago/Turabian StyleChoi, Nahyun, Ki Cheon Kim, Pan-Young Jeong, and Bumsik Kim. 2023. "Effects of the Complex of Panicum miliaceum Extract and Triticum aestivum Extract on Hair Condition" Nutrients 15, no. 20: 4411. https://doi.org/10.3390/nu15204411
APA StyleChoi, N., Kim, K. C., Jeong, P.-Y., & Kim, B. (2023). Effects of the Complex of Panicum miliaceum Extract and Triticum aestivum Extract on Hair Condition. Nutrients, 15(20), 4411. https://doi.org/10.3390/nu15204411





