Diet Traps during Eating Disorders among Dentate Patients at an Oral Health Glance
Abstract
1. Introduction
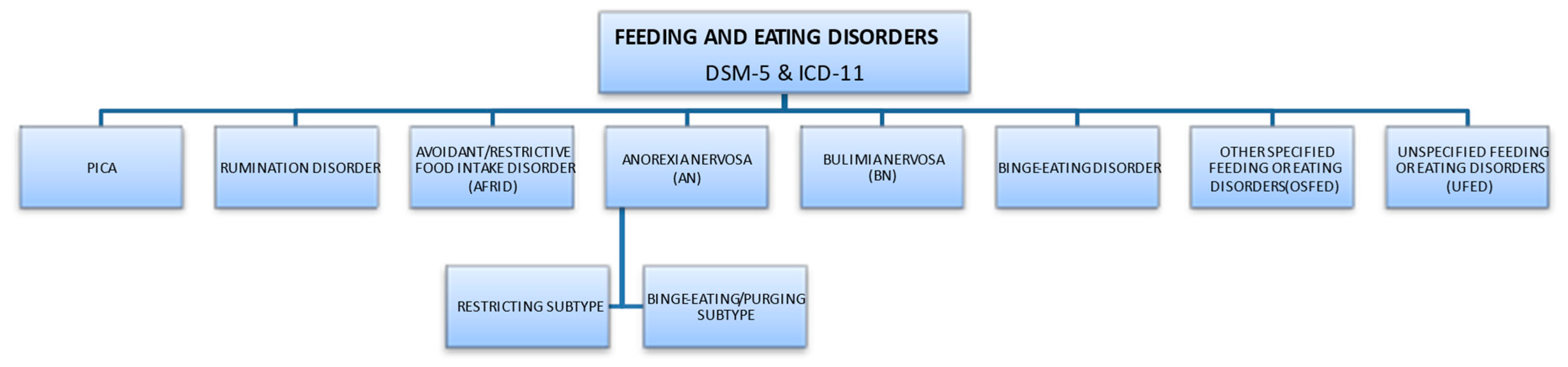
2. Eating Disorder Characteristics
2.1. Types of Eating Disorders
2.2. ED Risk Factors
2.3. Epidemiology
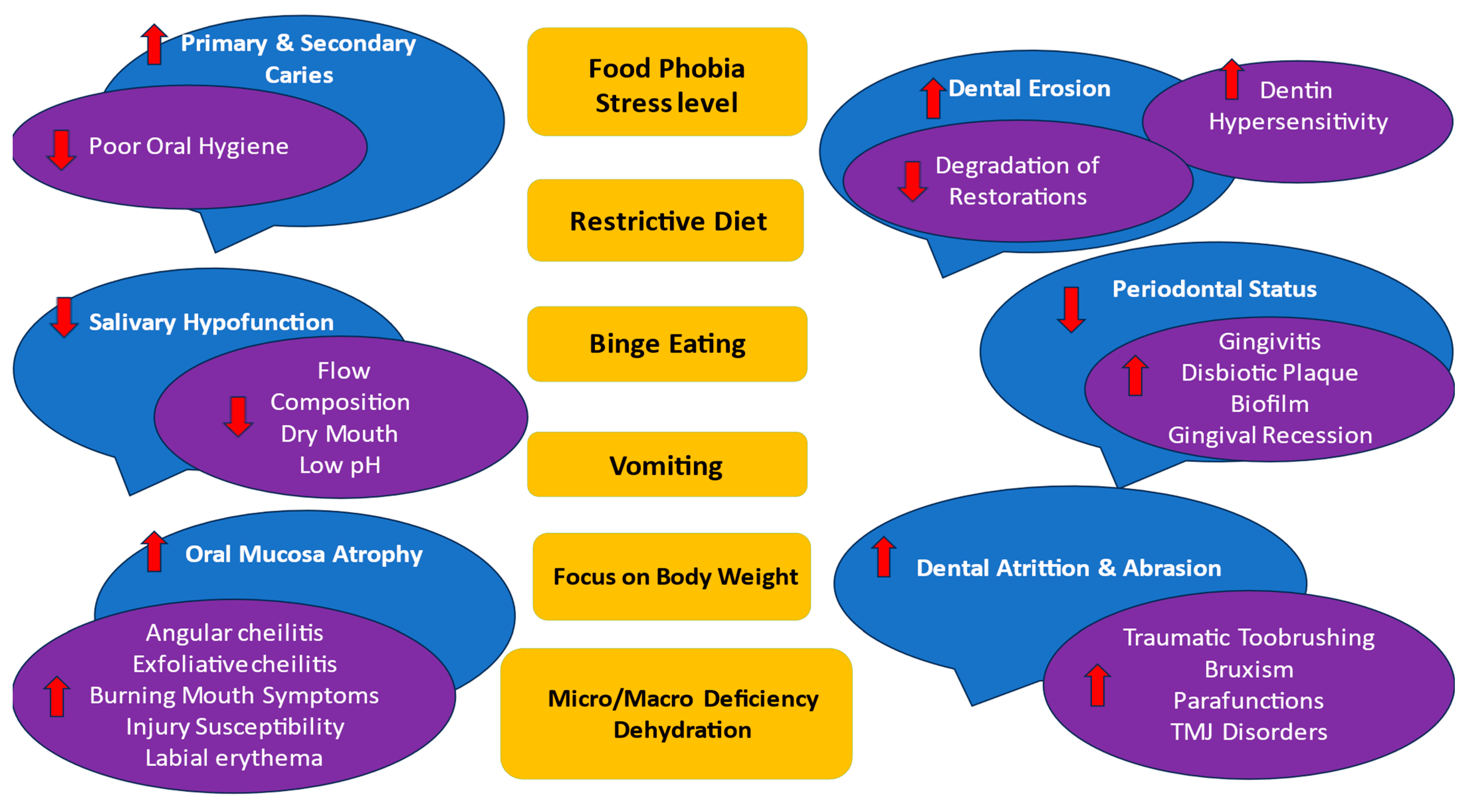
3. Common Dietary Intakes and Behavioral Habits Related to Oral Health in ED Patients
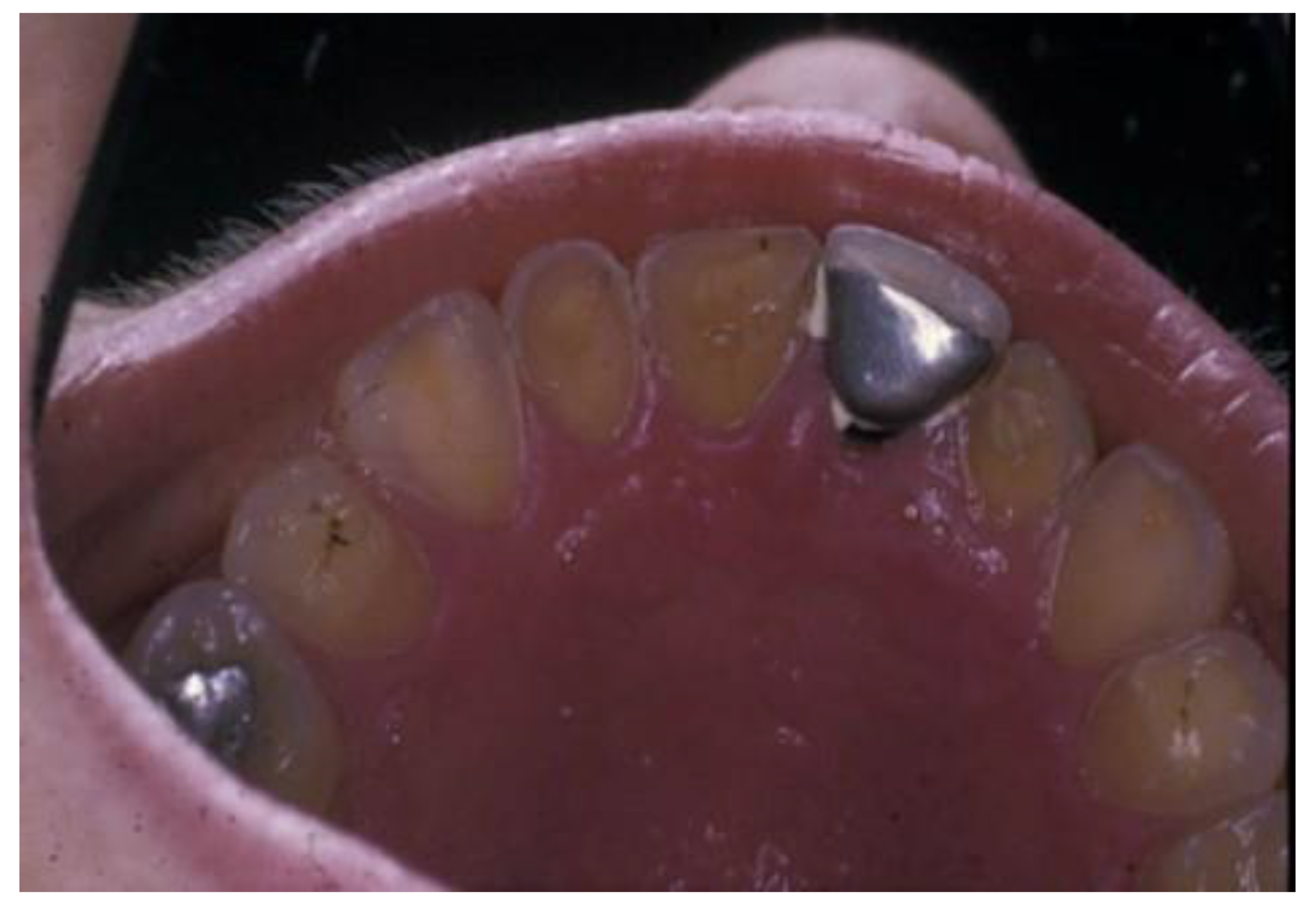
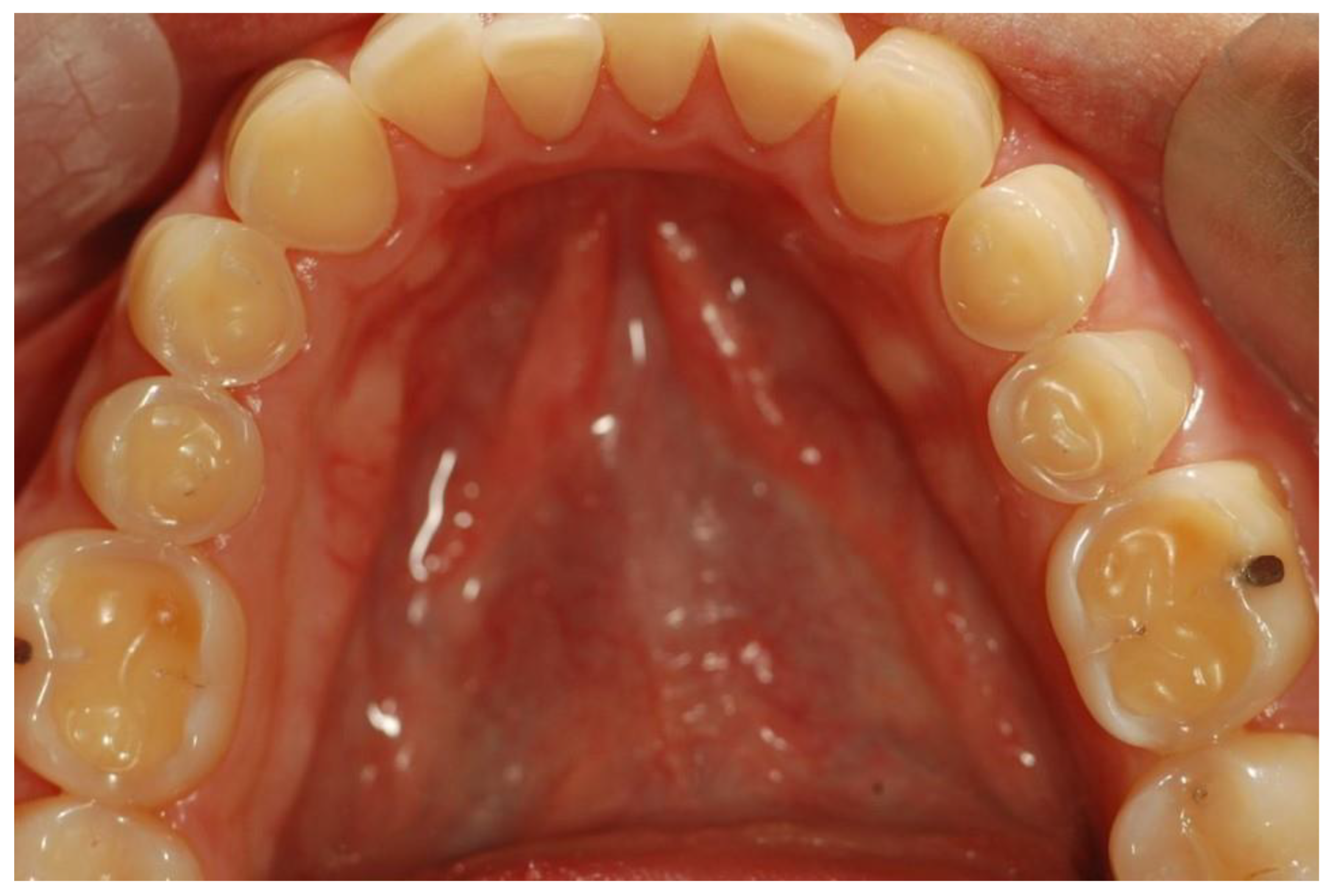
4. Oral Complications of ED
4.1. Oral Hygiene
4.2. Periodontal Health
4.3. Oral Mucosal Health
4.4. Dental Health
4.4.1. Dental Caries
4.4.2. Erosive Tooth Wear
5. Current Approaches to Managing ED
5.1. Counselling Relating to Dietary Habits
- Proteins: Total 5 oz/day
- Grains: 6 servings/day
- Dairy or alternative to dairy: 3 servings/day
- Vegetables and fruits: 5 servings/day
- Fats and oils: 4 servings/day
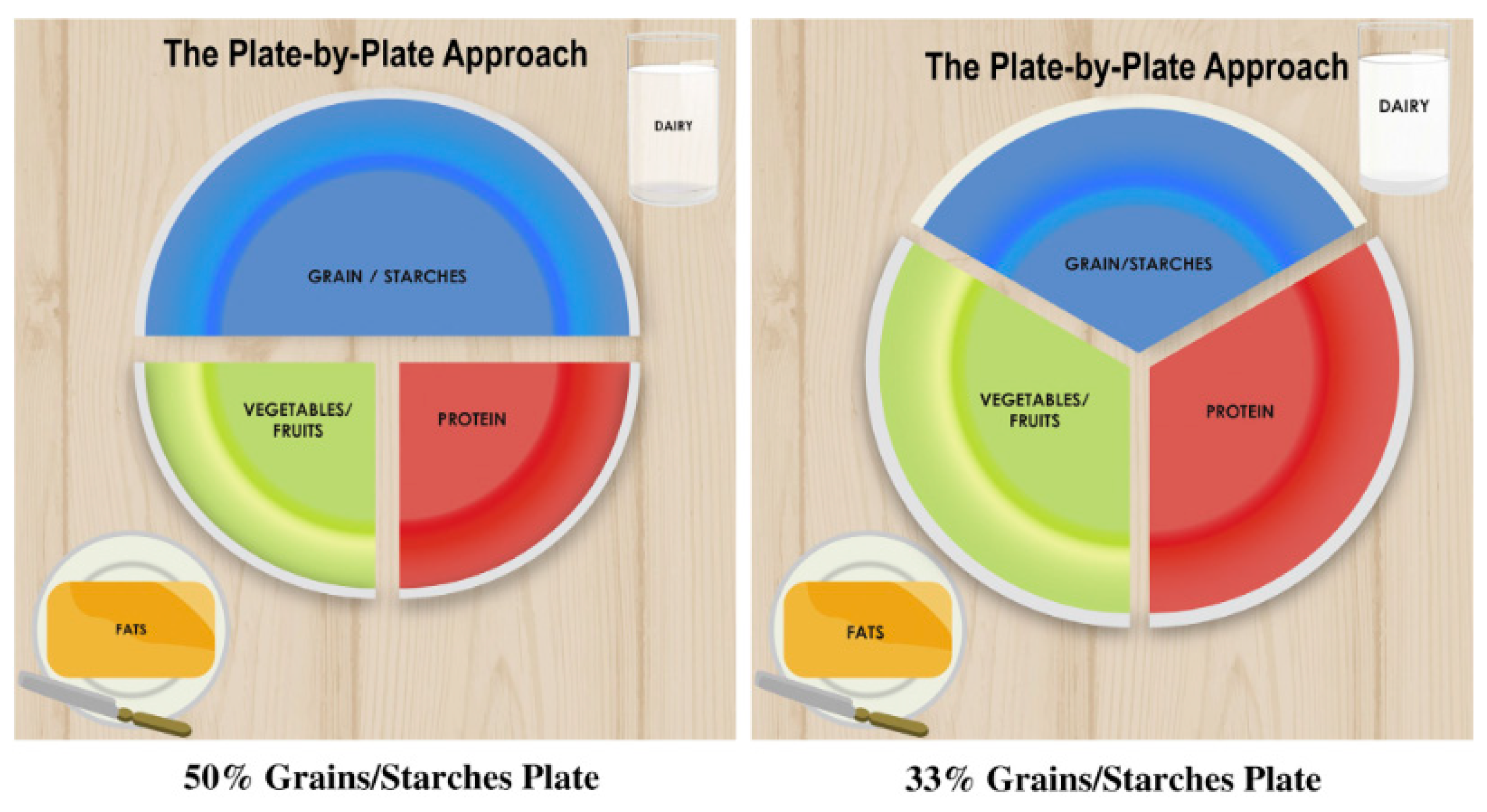
| STEP 1 | CHOOSE A 10-inch PLATE |
| STEP 2 | PLATE ALL FOOD GROUPS |
| STEP 3 | FILL THE PLATE UP |
| STEP 4 | DECIDE HOW MANY MEALS AND SNACKS |
| STEP 5 | INCLUDE VARIETY |
| STEP 6 | DOES THE MEAL MAKE SENSE? |
| STEP 7 | THE FINAL REVIEW: HOW DOES THE PLATE LOOK?
|
5.2. Counseling Relating to the Prevention of Effects on Oral Soft Tissues
5.3. Counseling Relating to the Prevention of Effects on Oral Hard Tissues (Dental Caries)
| Control the bacterial biofilm | via physical methods (toothbrush, interdental and tongue brushes, dental floss, irrigators) along with antibacterial preparations if temporarily needed (toothpaste or mouthwashes containing active agents, e.g., chlorhexidine, stannous salts, or zinc salts) |
| Increase in the resistance of hard dental tissues to the demineralizing effect of bacterial acids | via the topical use of remineralizing agents (at home and in-office application) |
| Modification of the diet | limiting the consumption of fermentable carbohydrates; avoiding sticky/acid products and replacing them with caries-protective foods such as raw vegetables, nuts, and cheese |
| Stimulation of salivary flow | with consistent food and sugar-free chewing gums containing sucrose substitutes i.e., xylitol |
| Altering medication-induced hyposalivation if needed [101,102,103] | |
| Regular dental check-ups every 3 months to monitor the oral condition and motivate the patient | |
5.4. Counseling Relating to the Prevention of Effects on Oral Hard Tissues (Erosive Tooth Wear)
5.5. Steps to Protect Affected Oral Soft and Hard Tissues
5.6. Recall Visits for Continued Care to Maintain Compliance and Oral Health
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romanos, G.E.; Javed, F.; Romanos, E.B.; Williams, R.C. Oro-facial manifestations in patients with eating disorders. Appetite 2012, 59, 499–504. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, TX, USA, 2013. [Google Scholar]
- International Classification of Diseases, Eleventh Revision (ICD-11), World Health Organization (WHO) 2019/2021. Licensed under Creative Commons Attribution-NoDerivatives 3.0 IGO Licence (CC BY-ND 3.0 IGO). Available online: https://icd.who.int/browse11 (accessed on 1 July 2023).
- Reed, G.M.; First, M.B.; Kogan, C.S.; Hyman, S.E.; Gureje, O.; Gaebel, W.; Maj, M.; Stein, D.J.; Maercker, A.; Tyrer, P.; et al. Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders. World Psychiatry 2019, 18, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Özbaran, N.B.; Yılancıoğlu, H.Y.; Tokmak, S.H.; YuluğTaş, B.; Çek, D.; Bildik, T. Changes in the psychosocial and clinical profiles of anorexia nervosa patients during the pandemic. Front. Psychiatry 2023, 14, 1207526. [Google Scholar] [CrossRef] [PubMed]
- Jagielska, G.; Kacperska, I. Outcome, comorbidity and prognosis in anorexia nervosa. Psychiatr. Pol. 2017, 51, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, T.; Dutkiewicz, A.; Paszynska, E.; Dmitrzak-Weglarz, M.; Slopien, A.; Tyszkiewicz-Nwafor, M. Neurobiochemical and psychological factors influencing the eating behaviors and attitudes in anorexia nervosa. J. Physiol. Biochem. 2017, 73, 297–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bardone-Cone, A.M.; Fitzsimmons-Craft, E.E.; Harney, M.B.; Maldonado, C.R.; Lawson, M.A.; Smith, R.; Robinson, D.P. The inter-relationships between vegetarianism and eating disorders among females. J. Acad. Nutr. Diet. 2012, 112, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.J.; Muñoz, M.E.; Garza, A.; Galindo, M. Concurrent and prospective analyses of peer, television and social media influences on body dissatisfaction, eating disorder symptoms and life satisfaction in adolescent girls. J. Youth Adolesc. 2014, 43, 1–14. [Google Scholar] [CrossRef]
- Antczak, A.J.; Brininger, T.L. Diagnosed eating disorders in the U.S. Military: A nine year review. Eat. Disord. 2008, 16, 363–377. [Google Scholar] [CrossRef]
- Filaire, E.; Rouveix, M.; Pannafieux, C.; Ferrand, C. Eating Attitudes, Perfectionism and Body-esteem of Elite Male Judoists and Cyclists. J. Sports Sci. Med. 2007, 6, 50–57. [Google Scholar]
- Penniment, K.J.; Egan, S.J. Perfectionism and learning experiences in dance class as risk factors for eating disorders in dancers. Eur. Eat. Disord. Rev. 2012, 20, 13–22. [Google Scholar] [CrossRef]
- Lindberg, L.; Hjern, A. Risk factors for anorexia nervosa: A national cohort study. Int. J. Eat. Disord. 2003, 34, 397–408. [Google Scholar] [CrossRef] [PubMed]
- McClelland, L.; Crisp, A. Anorexia nervosa and social class. Int. J. Eat. Disord. 2001, 29, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Dmitrzak-Weglarz, M.; Moczko, J.; Skibinska, M.; Slopien, A.; Tyszkiewicz, M.; Pawlak, J.; Zaremba, D.; Szczepankiewicz, A.; Rajewski, A.; Hauser, J. The study of candidate genes related to the neurodevelopmental hypothesis of anorexia nervosa: Classical association study versus decision tree. Psychiatry Res. 2013, 30, 117–121. [Google Scholar] [CrossRef]
- Dmitrzak-Weglarz, M.; Szczepankiewicz, A.; Slopien, A.; Tyszkiewicz, M.; Maciukiewicz, M.; Zaremba, D.; Twarowska-Hauser, J. Association of the glucocorticoid receptor gene polymorphisms and their interaction with stressful life events in Polish adolescent girls with anorexia nervosa. Psychiatr. Danub. 2016, 28, 51–57. [Google Scholar]
- Baker, J.H.; Schaumberg, K.; Munn-Chernoff, M.A. Genetics of Anorexia Nervosa. Curr. Psychiatry Rep. 2017, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Boraska, V.; Franklin, C.S.; Floyd, J.A.; Thornton, L.M.; Huckins, L.M.; Southam, L.; Rayner, N.W.; Tachmazidou, I.; Klump, K.L.; Treasure, J. A genome-wide association study of anorexia nervosa. Mol. Psychiatry 2014, 19, 1085–1094. [Google Scholar] [CrossRef]
- Janas-Kozik, M.; Stachowicz, M.; Krupka-Matuszczyk, I.; Szymszal, J.; Krysta, K.; Janas, A.; Rybakowski, J.K. Plasma levels of leptin and orexin a in the restrictive type of anorexia nervosa. Regul. Pept. 2011, 168, 5–9. [Google Scholar] [CrossRef]
- Pałasz, A.; Tyszkiewicz-Nwafor, M.; Suszka-Świtek, A.; Bacopoulou, F.; Dmitrzak-Węglarz, M.; Dutkiewicz, A.; Słopień, A.; Janas-Kozik, M.; Wilczyński, K.M.; Filipczyk, Ł.; et al. Longitudinal study on novel neuropeptides phoenixin, spexin and kisspeptin in adolescent inpatients with anorexia nervosa—Association with psychiatric symptoms. Nutr. Neurosci. 2021, 24, 896–906. [Google Scholar] [CrossRef]
- Grzelak, T.; Tyszkiewicz-Nwafor, M.; Dutkiewicz, A.; Mikulska, A.A.; Dmitrzak-Weglarz, M.; Slopien, A.; Czyzewska, K.; Paszynska, E. Neuropeptide B and Vaspinas New Biomarkers in Anorexia Nervosa. Biomed. Res. Int. 2018, 10, 9727509. [Google Scholar]
- Tyszkiewicz-Nwafor, M.; Jowik, K.; Paszynska, E.; Dutkiewicz, A.; Słopien, A.; Dmitrzak-Weglarz, M. Expression of immune-related proteins and their association with neuropeptides in adolescent patients with anorexia nervosa. Neuropeptides 2022, 91, 102214. [Google Scholar] [CrossRef]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [PubMed]
- Keski-Rahkonen, A.; Mustelin, L. Epidemiology of eating disorders in Europe: Prevalence, incidence, comorbidity, course, consequences, and risk factors. Curr. Opin. Psychiatry 2016, 29, 340–345. [Google Scholar] [CrossRef] [PubMed]
- van Eeden, A.E.; van Hoeken, D.; Hoek, H.W. Incidence, prevalence and mortality of anorexia nervosa and bulimia nervosa. Curr. Opin. Psychiatry 2021, 34, 515–524. [Google Scholar] [CrossRef]
- van Noort, B.M.; Lohmar, S.K.; Pfeiffer, E.; Lehmkuhl, U.; Winter, S.M.; Kappel, V. Clinical characteristics of early onset anorexia nervosa. Eur. Eat. Disord. Rev. 2018, 26, 519–525. [Google Scholar] [CrossRef]
- Bryant-Waugh, R. Feeding and Eating Disorders in Children. Psychiatr. Clin. N. Am. 2019, 42, 157–167. [Google Scholar] [CrossRef]
- Johansson, A.K.; Norring, C.; Unell, L.; Johansson, A. Eating disorders and oral health: A matched case-control study. Eur. J. Oral Sci. 2012, 120, 61. [Google Scholar] [CrossRef]
- Schebendach, J.E.; Mayer, L.E.; Devlin, M.J.; Attia, E.; Contento, I.R.; Wolf, R.L.; Walsh, B.T. Dietary energy density and diet variety as predictors of outcome in anorexia nervosa. Am. J. Clin. Nutr. 2008, 87, 810–816, Erratum in Am. J. Clin. Nutr. 2012, 96, 222. [Google Scholar] [CrossRef]
- Raatz, S.K.; Jahns, L.; Johnson, L.K.; Crosby, R.; Mitchell, J.E.; Crow, S.; Peterson, C.; Le Grange, D.; Wonderlich, S.A. Nutritional adequacy of dietary intake in women with anorexia nervosa. Nutrients 2015, 15, 3652–3665. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Costa Louzada, M.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Casarin, M.; da Silveira, T.M.; Bezerra, B.; Pirih, F.Q.; Pola, N.M. Association between different dietary patterns and eating disorders and periodontal diseases. Front. Oral Health 2023, 22, 1152031. [Google Scholar] [CrossRef]
- Dommisch, H.; Kuzmanova, D.; Jönsson, D.; Grant, M.; Chapple, I. Effect of micronutrient malnutrition on periodontal disease and periodontal therapy. Periodontol. 2000 2018, 78, 129–153. [Google Scholar] [CrossRef]
- Kumar, P.S. Interventions to prevent periodontal disease in tobacco-, alcohol-, and drug-dependent individuals. Periodontol. 2000 2020, 84, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.; Gupta, J.; Kaur, R.K.; Bansal, D.; Jain, A. Effect of anxiety and psychologic stress on periodontal health: A systematic review and meta-analysis. Quintessence Int. 2022, 53, 144–154. [Google Scholar] [PubMed]
- Liew, V.P.; Frisken, K.W.; Touyz, S.W.; Beumont, P.J.; Williams, H. Clinical and microbiological investigations of anorexia nervosa. Aust. Dent. J. 1991, 36, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Hellström, I. Oral complications in anorexia nervosa. Scand. J. Dent. Res. 1977, 85, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Hanachi, M.; Dicembre, M.; Rives-Lange, C.; Ropers, J.; Bemer, P.; Zazzo, J.F.; Poupon, J.; Dauvergne, A.; Melchior, J.C. Micronutrients Deficiencies in 374 Severely Malnourished Anorexia Nervosa Inpatients. Nutrients 2019, 5, 792. [Google Scholar] [CrossRef]
- Godart, N.T.; Flament, M.F.; Perdereau, F.; Jeammet, P. Comorbidity between eating disorders and anxiety disorders: A review. Int. J. Eat. Disord. 2002, 32, 253–270. [Google Scholar] [CrossRef]
- Godart, N.T.; Perdereau, F.; Rein, Z.; Berthoz, S.; Wallier, J.; Jeammet, P.H.; Flament, M.F. Comorbidity studies of eating disorders and mood disorders. Critical review of the literature. J. Affect. Disord. 2007, 97, 37–49. [Google Scholar] [CrossRef]
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L.C. Dental plaque-induced gingival conditions. J. Periodontol. 2018, 89, 17–27. [Google Scholar] [CrossRef]
- Papapanou, P.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, 162–170. [Google Scholar] [CrossRef]
- Silness, J.; Loe, H. Periodontal Disease in Pregnancy. II. Correlation between Oral Hygiene and Periodontal Condtion. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Altschuler, B.D.; Dechow, P.C.; Waller, D.A.; Hardy, B.W. An investigation of the oral pathologies occurring in bulimia nervosa. Int. J. Eat. Disord. 1990, 9, 191–199. [Google Scholar] [CrossRef]
- Milosevic, A.; Slade, P.D. The orodental status of anorexics and bulimics. Br. Dent. J. 1989, 22, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Philipp, E.; Willershausen-Zönnchen, B.; Hamm, G.; Pirke, K.M. Oral and dental characteristics in bulimic and anorectic patients. Int. J. Eat. Disord. 1991, 10, 423–431. [Google Scholar] [CrossRef]
- Pallier, A.; Karimovaa, A.; Boillota, A.; Colon, P.; Ringuenete, D.; Bouchard, P. Dental and periodontal health in adults with eating disorders: A case-control study. J. Dent. 2019, 84, 55–59. [Google Scholar] [CrossRef]
- Touyz, S.; Liew, V.; Tseng, P.; Frisken, K.; Williams, H.; Beumont, P. Oral and Dental Complications in Dieting Disorders. Int. J. Eat. Disord. 1993, 14, 341–347. [Google Scholar] [CrossRef]
- Kisely, S.; Baghaie, H.; Lalloo, R.; Johnson, N. Association between poor oral health and eating disorders: Systematic review and meta-analysis. Br. J. Psychiatry 2015, 207, 299–305. [Google Scholar] [CrossRef]
- Chapple, I.L.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.l.; Lingstrom, P.; et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44, 39–51. [Google Scholar] [CrossRef]
- Chapple, I.L.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, 68–77. [Google Scholar] [CrossRef]
- Garrido-Martínez, P.; Domínguez-Gordillo, A.; Cerero-Lapiedra, R.; Burgueño-García, M.; Martínez-Ramírez, M.J.; Gómez-Candela, C.; Cebrián-Carretero, J.L.; Esparza-Gómez, G.G. Oral and dental health status in patients with eating disorders in Madrid, Spain. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, 595–602. [Google Scholar] [CrossRef]
- Lourenço, M.; Azevedo, A.; Brandão, I.; Gomes, P. Orofacial manifestations in outpatients with anorexia nervosa and bulimia nervosa focusing on the vomiting behaviour. Clin. Oral Investig. 2018, 22, 1915–1922. [Google Scholar] [CrossRef]
- Loe, H.; Silness, J. Periodontal Disease in Pregnancy. I. Prevalence and Severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Cutress, T.W.; Ainamo, J.; Sardo-Infirri, J. The community periodontal index of treatment needs (CPITN) procedure for population groups and individuals. Int. Dent. J. 1987, 37, 222–333. [Google Scholar]
- Chiba, F.Y.; Sumida, D.H.; Moimaz, S.A.S.; Chaves Neto, A.H.; Nakamune, A.; Garbin, A.J.I.; Garbin, C.A.S. Periodontal condition, changes in salivary biochemical parameters, and oral health-related quality of life in patients with anorexia and bulimia nervosa. J. Periodontol. 2019, 90, 1423–1430. [Google Scholar] [CrossRef]
- Cortellini, P.; Bissada, N.F. Mucogingival conditions in the natural dentition: Narrative review, case definitions, and diagnostic considerations. J. Periodontol. 2018, 45, 190–198. [Google Scholar]
- Range, H.; Colon, P.; Godart, N.; Kapila, Y.; Bouchard, P. Eating disorders through the periodontal lens. Periodontol. 2000 2021, 87, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Stanislav, N.; Tolkachjov, M.D.; Bruce, A.J. Oral manifestations of nutritional disorders. Clin. Dermatol. 2017, 35, 441–452. [Google Scholar]
- Panico, R.; Piemonte, E.; Lazos, J.; Gilligan, G.; Zampini, A.; Lanfranchi, H. Oral mucosal lesions in Anorexia Nervosa, Bulimia Nervosa and EDNOS. J. Psychiatr. Res. 2018, 96, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Lesar, T.; Vidović Juras, D.; Tomić, M.; Čimić, S.; Kraljević Šimunković, S. Oral Changes in Pediatric Patients with Eating Disorders. Acta Clin. Croat. 2022, 61, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Mascitti, M.; Coccia, E.; Vignini, A.; Aquilanti, L.; Santarelli, A.; Salvolini, E.; Sabbatinelli, J.; Mazzanti, L.; Procaccini, M.; Rappelli, G. Anorexia, Oral Health and Antioxidant Salivary System: A Clinical Study on Adult Female Subjects. Dent. J. 2019, 7, 60. [Google Scholar] [CrossRef]
- Paszyńska, E.; Słopień, A.; Slebioda, Z.S.; Dyszkiewicz-Konwińska, M.; Monika Weglarz, M.; Rajewski, A. Macroscopic evaluation of the oral mucosa and analysis of salivary pH in patients with anorexia nervosa. Psychiatr. Pol. 2014, 48, 453–464. [Google Scholar]
- Back-Brito, G.N.; da Mota, A.J.; de Souza Bernardes, L.Â.; Takamune, S.S.; Prado Ede, F.; Cordás, T.A.; Balducci, I.; da Nobrega, F.G.; Koga-Ito, C.Y. Effects of eating disorders on oral fungal diversity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Hedman, A.; Breithaupt, L.; Hübel, C.; Thornton, L.M.; Tillander, A.; Norring, C.; Birgegård, A.; Larsson, H.; Ludvigsson, J.F.; Sävendahl, L.; et al. Bidirectional relationship between eating disorders and autoimmune diseases. J. Child Psychol. Psychiatry 2019, 60, 803–812. [Google Scholar] [CrossRef]
- Yadav, K.; Prakash, S. Dental Caries: A review. Asian J. Biomed. Pharm. Sci. 2016, 6, 1–7. [Google Scholar]
- Moynihan, P.; Petersen, P.E. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004, 7, 201–226. [Google Scholar] [CrossRef]
- Johansson, A.K.; Norring, C.; Unell, L.; Johansson, A. Diet and behavioral habits related to oral health in eating disorder patients: A matched case-control study. J. Eat. Disord. 2020, 8, 7. [Google Scholar] [CrossRef]
- Jung, E.H.; Jun, M.K. Relationship between risk factors related to eating disorders and subjective health and oral health. Children 2022, 9, 786. [Google Scholar] [CrossRef] [PubMed]
- Sheetal, A.; Hiremath, V.K.; Patil, A.G.; Sajjansetty, S.; Kumar, S.R. Malnutrition and its oral outcome—A review. J. Clin. Diagn. Res. 2013, 7, 178–180. [Google Scholar] [CrossRef]
- Johansson, A.K.; Norring, C.; Unell, L.; Johansson, A. Eating disorders and biochemical composition of saliva: A retrospective matched case–control study. Eur. J. Oral Sci. 2015, 123, 158–164. [Google Scholar] [CrossRef][Green Version]
- Paszynska, E.; Schlueter, N.; Slopien, A.; Dmitrzak-Weglarz, M.; Dyszkiewicz-Konwinska, M.; Hannig, C. Salivary enzyme activity in anorexic persons-a controlled clinical trial. Clin. Oral Investig. 2015, 19, 1981–1989. [Google Scholar]
- Dynesen, A.W.; Bardow, A.; Petersson, B.; Nielsen, L.R.; Nauntofte, B. Salivary changes and dental erosion in bulimia nervosa. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 106, 696–707. [Google Scholar] [CrossRef]
- Ximenes, R.; Couto, G.; Sougey, E. Eating disorders in adolescents and their repercussions in oral health. Int. J. Eat. Disord. 2010, 43, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Paszynska, E.; Hernik, A.; Slopien, A.; Roszak, M.; Jowik, K.; Dmitrzak-Weglarz, M.; Tyszkiewicz-Nwafor, M. Risk of dental caries and erosive tooth wear in 117 children and adolescents anorexia nervosa population-a case-control study. Front. Psychiatry 2022, 13, 874263. [Google Scholar] [CrossRef] [PubMed]
- Szupiany-Janeczek, T.; Rutkowski, K.; Pytko-Polończyk, J. Oral cavity clinical evaluation in psychiatric patients with eating disorders: A case-control study. Int. J. Environ. Res. Public Health 2023, 20, 4792. [Google Scholar] [CrossRef] [PubMed]
- Manevski, J.; Stojšin, I.; Vukoje, K.; Janković, O. Dental aspects of purging bulimia. Vojnosanit. Pregl. 2020, 77, 300–307. [Google Scholar] [CrossRef]
- Shaughnessy, B.F.; Feldman, H.A.; Cleveland, R.; Sonis, A.; Brown, J.N.; Gordon, C.M. Oral health and bone density in adolescents and young women with anorexia nervosa. J. Clin. Pediatr. Dent. 2008, 33, 87–92. [Google Scholar] [CrossRef][Green Version]
- Hermont, A.P.; Pordeus, I.A.; Paiva, S.M.; Abreu, M.H.; Auad, S.M. Eating disorder risk behavior and dental implications among adolescents. Int. J. Eat. Disord. 2013, 46, 677–683. [Google Scholar] [CrossRef]
- Brandt, L.M.T.; Freitas Fernandes, L.H.; Silva Aragão, A.; Costa Aguiar, Y.P.; Auad, S.M.; Dias de Castro, R.; D’Ávila Lins Bezerra, B.; Leite Cavalcanti, A. Relationship between risk behavior for eating disorders and dental caries and dental erosion. Sci. World J. 2017, 2017, 1656417. [Google Scholar] [CrossRef]
- Jugale, P.V.; Pramila, M.; Murthy, A.K.; Rangath, S. Oral manifestations of suspected eating disorders among women of 20–25 years in Bangalore City, India. J. Health Popul. Nutr. 2014, 32, 46–50. [Google Scholar]
- De Moor, R.J.G. Eating disorder-induced dental complications: A case report. J. Oral Rehabil. 2004, 31, 725–732. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Higham, S.M. Dental erosion: Possible approaches to prevention and control. J. Dent. 2005, 33, 243–252. [Google Scholar] [CrossRef]
- The Erosive Tooth Wear Foundation. What Is Erosive Toothwear? Available online: www.erosivetoothwear.com (accessed on 13 August 2023).
- Schlueter, N.; Luka, B. Risk groups. Br. Dent. J. 2018, 224, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, N.; Tveit, A.B. Prevalence of erosive tooth wear in risk groups. Monogr. Oral Sci. 2014, 25, 74–98. [Google Scholar] [PubMed]
- Petersson, C.; Svedlund, A.; Wallengren, O.; Swolin-Eide, D.; Paulson Karlssonet, G.; Ellegard, L. Dietary Intake and Nutritional States in Adolescents & Young Adults with Anorexia Nervosa: A 3-Year Follow-Up Study. Clin. Nutr. 2021, 40, 5391–5398. [Google Scholar]
- McMaster, C.M.; Fong, M.; Franklin, J.; Hart, S. Dietetic Intervention for Adult Outpatients with Eating Disorders: A Systemic Review and Assessment of Evidence Quality. Nutr. Rev. 2021, 79, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Conti, J.; McMaster, C.M.; Hay, P. Beyond Refeeding: The Effects of Including a Dietitian in Eating Disorder Treatment. A Systemic Review. Nutrients 2021, 13, 4490. [Google Scholar] [CrossRef]
- Sterling, W.; Crosbie, C.; Shaw, N.; Martin, S. The Use of the Plate-by-Plate Approach for Adolescents Undergoing Family Based Treatment. J. Acad. Nutr. Diet. 2019, 119, 1075–1084. [Google Scholar] [CrossRef]
- Heruc, G.; Hart, S.; Stiles, G.; Fleming, K.; Casey, A.; Sutherland, F.; Jeffrey, S.; Roberton, M.; Hurst, K. ANZAED practice and training standards for dietitians providing eating disorder treatment. J. Eat. Disord. 2018, 8, 77. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Trombini, J.; Scribante, A. Evaluation of Children Caries Risk Factors: A Narrative Review of Nutritional Aspects, Oral Hygiene Habits, and Bacterial Alterations. Children 2022, 9, 262. [Google Scholar] [CrossRef]
- Pitts, N.B.; Ismail, A.I.; Martignon, S.; Ekstrand, K.; Douglas, G.V.A.; Longbottom, C. International Caries Classification and Management System (ICCMS™) Guide for Practitioners and Educators. 2014. Available online: www.iccms-web.com (accessed on 16 August 2023).
- Walsh, T.; Worthington, H.V.; Glenny, A.M.; Marinho, V.C.C.; Jeroncic, A. Fluoride toothpaste of different concentrations for preventing dental caries. Cochrane Database Syst. Rev. 2019, 3, CD007868. [Google Scholar] [CrossRef]
- Paszynska, E.; Pawinska, M.; Gawriolek, M.; Kaminska, I.; Otulakowska-Skrzynska, J.; Marczuk-Kolada, G.; Rzatowski, S.; Sokolowska, K.; Olszewska, A.; Schlagenhauf, U.; et al. Impact of toothpaste with microcrystalline hydroxyapatite on the occurrence of early childhood caries: A 1-year randomized clinical trial. Sci. Rep. 2021, 11, 2650. [Google Scholar] [CrossRef]
- Paszynska, E.; Pawinska, M.; Enax, J.; Meyer, F.; Schulze zur Wiesche, E.; May, T.W.; Amaechi, B.T.; Limeback, H.; Hernik, A.; Otulakowska-Skrzynska, J.; et al. Caries-preventing effect of a hydroxyapatite-toothpaste in adults: An 18-month double-blinded randomized clinical trial Front. Front. Public Health 2023, 11, 1199728. [Google Scholar] [CrossRef] [PubMed]
- Grocholewicz, K.; Matkowska-Cichocka, G.; Makowiecki, P.; Drozdzik, A.; Ey-Chmielewska, H.; Dziewulska, A.; Tomasik, M.; Trybek, G.; Janiszewska-Olszowska, J. Effect of nano-hydroxyapatite and ozone on approximal initial caries: A randomized clinical trial. Sci. Rep. 2020, 10, 11192. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.A.; Kau, C.H.; English, J.D.; Lee, R.P.; Powers, J.; Nguyen, J.T. MI Paste Plus to prevent demineralization in orthodontic patients: A prospective randomized controlled trial. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Karabekiroglu, S.; Ünlü, N.; Küçükyilmaz, E.; Sener, S.; Botsali, M.S.; Malkoç, S. Treatment of post-orthodontic white spot lesions with CPP-ACP paste: A three year follow up study. Dent. Mater. J. 2017, 36, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Limeback, H.; Enax, J.; Meyer, F. Biomimetic hydroxyapatite and caries prevention: A systematic review and meta-analysis. Can. J. Dent. Hyg. 2021, 55, 148–159. [Google Scholar] [PubMed]
- Paszyńska, E.; Jurga-Krokowicz, J.; Shaw, H. The use of parotid gland activity analysis in patients with gastroesophageal reflux disease (GERD) and bulimia nervosa. Adv. Med. Sci. 2006, 51, 208–213. [Google Scholar]
- Paszynska, E.; Linden, R.W.; Slopien, A.; Rajewski, A. Flow rates and inorganic composition of whole saliva in purging bulimic patients treated with a fluoxetine. World J. Biol. Psychiatry 2011, 12, 282–287. [Google Scholar] [CrossRef]
- Paszyńska, E.; Słopień, A.; Węglarz, M.; Linden, R.W. Parotid salivary parameters in bulimic patients—A controlled clinical trial. Psychiatr. Pol. 2015, 49, 709–720. [Google Scholar] [CrossRef]
- Nehme, M.; Parkinson, C.R.; Zero, D.T.; Hara, A.T. Randomised study of the effects of fluoride and time on in situ remineralisation of acid-softened enamel. Clin. Oral Investig. 2019, 23, 4455–4463. [Google Scholar] [CrossRef]
- Hughes, J.A.; West, N.X.; Parker, D.M.; Newcombe, R.G.; Addy, M. Development and evaluation of a low erosive blackcurrant juice drink 3. Final drink and concentrate, formulae comparisons in situ and overview of the concept. J. Dent. 1999, 27, 345–350. [Google Scholar] [CrossRef]
- Haute Autorité de Santé. Detection and Management of Dental Problems by Dentists. Available online: www.has-sante.fr (accessed on 16 August 2023).
- Schiff, T.; Wachs, G.N.; Petrone, D.M.; Chaknis, P.; Kemp, J.H.; DeVizio, W. The efficacy of a newly designed toothbrush to decrease tooth sensitivity. Compend. Contin. Educ. Dent. 2009, 30, 238–240. [Google Scholar]
- Antezack, A.; Ohanessian, R.; Sadowski, C.; Faure-Brac, M.; Brincat, A.; Etchecopar-Etchart, D.; Monnet-Corti, V. Effectiveness of surgical root coverage on dentin hypersensitivity: A systematic review and meta-analysis. J. Clin. Periodontol. 2022, 49, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Erley, K.J.; Swiec, G.D.; Herold, R.; Bisch, F.C.; Peacock, M.E. Gingival recession treatment with connective tissue grafts in smokers and non-smokers. J. Periodontol. 2006, 77, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, A.; Lechte, C.; Kanzow, P. Adhesion to eroded enamel and dentin: Systematic review and meta-analysis. Dent. Mater. 2021, 37, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- King, P.A. Tooth surface loss: Adhesive techniques. Br. Dent. J. 1999, 186, 371–376. [Google Scholar] [CrossRef]
- Milosevic, A. Use of porcelain veneers to restore palatal tooth loss. Restor. Dent. 1990, 6, 15–18. [Google Scholar]
- Carvalho, T.S.; Colon, P.; Ganss, C.; Huysmans, M.C.; Lussi, A.; Schlueter, N.; Schmalz, G.; Shellis, R.P.; Tveit, A.B.; Wiegand, A. Consensus report of the European Federation of Conservative Dentistry: Erosive tooth wear—Diagnosis and management. Clin. Oral. Investig. 2015, 19, 1557–1561. [Google Scholar] [CrossRef]
- Colon, P.; Lussi, A. Minimal intervention dentistry: Part 5. Ultra-conservative approach to the treatment of erosive and abrasive lesions. Br. Dent. J. 2014, 216, 463–468. [Google Scholar] [CrossRef]

| Carbohydrate % | Fat% | Protein% | NOVA Category | |
|---|---|---|---|---|
| Ice cream | 47 | 46 | 7 | 4 |
| Doughnut | 40–45 | 45–50 | 2–3 | 4 |
| Pita bread and hummus | 50 | 39 | 11 | 4 |
| Raisin bagels | 80 | 6 | 8 | 4 |
| Cookies | 60 | 38 | 2 | 4 |
| Nuts | 12 | 75 | 13 | 1 or 4 |
| Diet soda | Artificial sweetener | 0 | 0 | 4 |
| Potato chips | 35 | 60 | 5 | 4 |
| Chocolate cake | 57 | 40 | 3 | 4 |
| Cherry yoghurt | 75 | 10 | 15 | 4 |
| Pizza | 48 | 37 | 15 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paszynska, E.; Hernik, A.; Rangé, H.; Amaechi, B.T.; Gross, G.S.; Pawinska, M. Diet Traps during Eating Disorders among Dentate Patients at an Oral Health Glance. Nutrients 2023, 15, 4414. https://doi.org/10.3390/nu15204414
Paszynska E, Hernik A, Rangé H, Amaechi BT, Gross GS, Pawinska M. Diet Traps during Eating Disorders among Dentate Patients at an Oral Health Glance. Nutrients. 2023; 15(20):4414. https://doi.org/10.3390/nu15204414
Chicago/Turabian StylePaszynska, Elzbieta, Amadeusz Hernik, Hélène Rangé, Bennett T. Amaechi, Georgiana S. Gross, and Malgorzata Pawinska. 2023. "Diet Traps during Eating Disorders among Dentate Patients at an Oral Health Glance" Nutrients 15, no. 20: 4414. https://doi.org/10.3390/nu15204414
APA StylePaszynska, E., Hernik, A., Rangé, H., Amaechi, B. T., Gross, G. S., & Pawinska, M. (2023). Diet Traps during Eating Disorders among Dentate Patients at an Oral Health Glance. Nutrients, 15(20), 4414. https://doi.org/10.3390/nu15204414








