Impact of Exercise Intervention Combined with Optimal Mediterranean Diet Adherence during Pregnancy on Postpartum Body Composition: A Quasi-Experimental Study—The GESTAFIT Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Randomization and Blinding
2.3. Exercise Intervention
2.4. Control Group
2.5. Sociodemographics
2.6. Maternal Anthropometry, Postpartum Weight Retention and Body Composition
2.7. Physical Activity
2.8. Dietary Assessment and Mediterranean Diet Adherence
2.9. Statistical Analysis
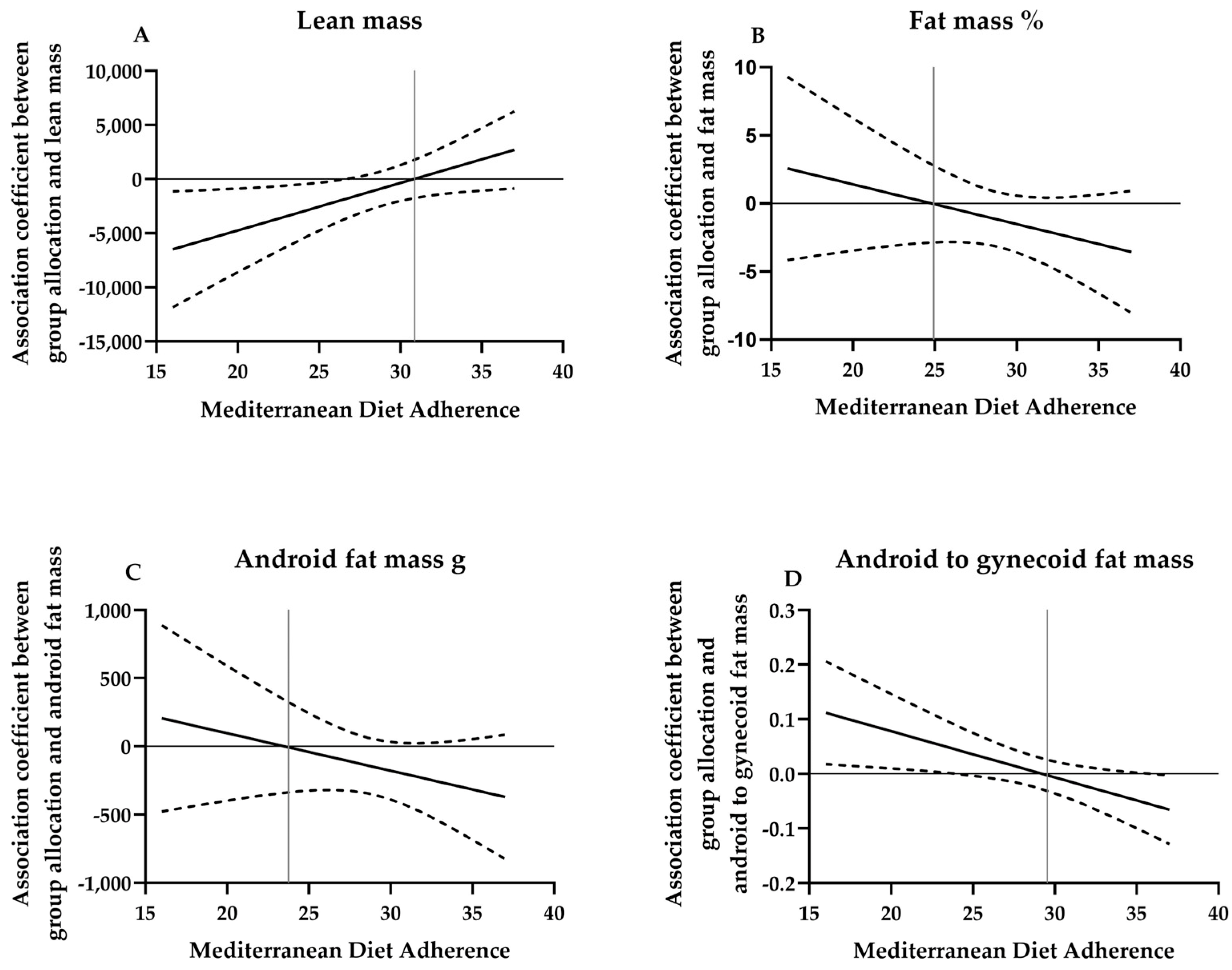
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gunderson, E.P.; Murtaugh, M.A.; Lewis, C.E.; Quesenberry, C.P.; West, D.S.; Sidney, S. Excess gains in weight and waist circumference associated with childbearing: The Coronary Artery Risk Development in Young Adults Study (CARDIA). Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2004, 28, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.L.; Boyle, J.A.; Harrison, C.L.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; et al. Gestational weight gain across continents and ethnicity: Systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Gore, S.A.; Brown, D.M.; West, D.S. The role of postpartum weight retention in obesity among women: A review of the evidence. Ann. Behav. Med. 2003, 26, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Boghossian, N.S.; Yeung, E.H.; Lipsky, L.M.; Poon, A.K.; Albert, P.S. Dietary patterns in association with postpartum weight retention. Am. J. Clin. Nutr. 2013, 97, 1338–1345. [Google Scholar] [CrossRef][Green Version]
- van der Pligt, P.; Ball, K.; Hesketh, K.D.; Teychenne, M.; Crawford, D.; Morgan, P.J.; Collins, C.E.; Campbell, K.J. A pilot intervention to reduce postpartum weight retention and central adiposity in first-time mothers: Results from the mums OnLiNE (Online, Lifestyle, Nutrition & Exercise) study. J. Hum. Nutr. Diet. 2018, 31, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Althuizen, E.; van Poppel, M.N.; de Vries, J.H.; Seidell, J.C.; van Mechelen, W. Postpartum behaviour as predictor of weight change from before pregnancy to one year postpartum. BMC Public Health 2011, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P.; Sternfeld, B.; Wellons, M.F.; Whitmer, R.A.; Chiang, V.; Quesenberry, C.P., Jr.; Lewis, C.E.; Sidney, S. Childbearing May Increase Visceral Adipose Tissue Independent of Overall Increase in Body Fat. Obesity 2008, 16, 1078–1084. [Google Scholar] [CrossRef]
- Smith, D.E.; Lewis, C.E.; Caveny, J.L.; Perkins, L.L.; Burke, G.L.; Bild, D.E. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. JAMA 1994, 271, 1747–1751. [Google Scholar] [CrossRef]
- Wiklund, P.; Toss, F.; Weinehall, L.; Hallmans, G.; Franks, P.W.; Nordström, A.; Nordström, P. Abdominal and gynoid fat mass are associated with cardiovascular risk factors in men and women. J. Clin. Endocrinol. Metab. 2008, 93, 4360–4366. [Google Scholar] [CrossRef]
- Lee, M.-J.; Wu, Y.; Fried, S.K. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol. Asp. Med. 2013, 34, 1–11. [Google Scholar] [CrossRef]
- Muktabhant, B.; Lawrie, T.A.; Lumbiganon, P.; Laopaiboon, M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst. Rev. 2015, 6, CD007145. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.R.; Perales, M.; Pelaez, M.; Lopez, C.; Lucia, A.; Barakat, R. Supervised Exercise–Based Intervention to Prevent Excessive Gestational Weight Gain: A Randomized Controlled Trial. Mayo Clin. Proc. 2013, 88, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wei, Y.; Zhang, X.; Zhang, Y.; Xu, Q.; Sun, Y.; Su, S.; Zhang, L.; Liu, C.; Feng, Y.; et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. Obstet. Gynecol. 2017, 216, 340–351. [Google Scholar] [CrossRef]
- Hui, A.L.; Back, L.; Ludwig, S.; Gardiner, P.; Sevenhuysen, G.; Dean, H.J.; Sellers, E.; McGavock, J.; Morris, M.; Jiang, D.; et al. Effects of lifestyle intervention on dietary intake, physical activity level, and gestational weight gain in pregnant women with different pre-pregnancy Body Mass Index in a randomized control trial. BMC Pregnancy Childbirth 2014, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Lovelady, C.A.; Nommsen-Rivers, L.A.; McCrory, M.A.; Dewey, K.G. Effects of exercise on plasma lipids and metabolism of lactating women. Med. Sci. Sports Exerc. 1995, 27, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Lovelady, C.A.; Nommsen-Rivers, L.A.; McCrory, M.A.; Lönnerdal, B. A randomized study of the effects of aerobic exercise by lactating women on breast-milk volume and composition. N. Engl. J. Med. 1994, 330, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Taveras, E.M.; Popoola, F.A.; Rich-Edwards, J.W.; Gillman, M.W. Television, walking, and diet: Associations with postpartum weight retention. Am. J. Prev. Med. 2007, 32, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2013, 17, 2769–2782. [Google Scholar] [CrossRef]
- Flor-Alemany, M.; Acosta, P.; Marín-Jiménez, N.; Baena-García, L.; Aranda, P.; Aparicio, V.A. Influence of the degree of adherence to the Mediterranean Diet and its components on cardiometabolic risk during pregnancy. The GESTAFIT project. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2311–2318. [Google Scholar] [CrossRef] [PubMed]
- Kelaiditi, E.; Jennings, A.; Steves, C.J.; Skinner, J.; Cassidy, A.; MacGregor, A.J.; Welch, A.A. Measurements of skeletal muscle mass and power are positively related to a Mediterranean dietary pattern in women. Osteoporos. Int. 2016, 27, 3251–3260. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Corella, D.; Sánchez-Villegas, A.; Estruch, R.; Álvarez-Pérez, J.; Díaz-Benítez, E.M.; Martínez-González, M.Á.; Ruano-Rodríguez, C.; Salas-Salvadó, J. Influence of a Mediterranean Dietary Pattern on Body Fat Distribution: Results of the PREDIMED–Canarias Intervention Randomized Trial. J. Am. Coll. Nutr. 2016, 35, 568–580. [Google Scholar] [CrossRef]
- Issa, C.; Lairon, D.; Batal, M.; Salameh, P.; Maillot, M.; Darmon, N. A Mediterranean diet pattern with low consumption of liquid sweets and refined cereals is negatively associated with adiposity in adults from rural Lebanon. Int. J. Obes. 2010, 35, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Lovelady, C.A.; Garner, K.E.; Moreno, K.L.; Williams, J.P. The effect of weight loss in overweight, lactating women on the growth of their infants. N. Engl. J. Med. 2000, 342, 449–453. [Google Scholar] [CrossRef]
- Amorim Adegboye, A.R.; Linne, Y.M. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst. Rev. 2013, 7, CD005627. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Manzano, P.; Acosta, F.M.; Coll-Risco, I.; Romero-Gallardo, L.; Flor-Alemany, M.; Martínez-González, L.J.; Alvarez-Cubero, M.J.; Segura-Jiménez, V.; Aparicio, V.A. The Influence of Exercise, Lifestyle Behavior Components, and Physical Fitness on Maternal Weight Gain, Postpartum Weight Retention, and Excessive Gestational Weight Gain. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 425–438. [Google Scholar] [CrossRef]
- Aparicio, V.A.; Ocon, O.; Padilla-Vinuesa, C.; Soriano-Maldonado, A.; Romero-Gallardo, L.; Borges-Cosic, M.; Coll-Risco, I.; Ruiz-Cabello, P.; Acosta-Manzano, P.; Estevez-Lopez, F.; et al. Effects of supervised aerobic and strength training in overweight and grade I obese pregnant women on maternal and foetal health markers: The GESTAFIT randomized controlled trial. BMC Pregnancy Childbirth 2016, 16, 290. [Google Scholar] [CrossRef]
- Selya, A.S.; Rose, J.S.; Dierker, L.C.; Hedeker, D.; Mermelstein, R.J. A Practical Guide to Calculating Cohen’s f(2), a Measure of Local Effect Size, from PROC MIXED. Front. Psychol. 2012, 3, 111. [Google Scholar] [CrossRef]
- Kehler, A.K.; Heinrich, K.M. A selective review of prenatal exercise guidelines since the 1950s until present: Written for women, health care professionals, and female athletes. Women Birth 2015, 28, e93–e98. [Google Scholar] [CrossRef]
- ACOG committee opinion. Exercise during pregnancy and the postpartum period. Number 267, January 2002. American College of Obstetricians and Gynecologists. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2002, 77, 79–81. [Google Scholar] [CrossRef]
- Barakat, R.; Pelaez, M.; Lopez, C.; Lucia, A.; Ruiz, J.R. Exercise during pregnancy and gestational diabetes-related adverse effects: A randomised controlled trial. Br. J. Sports Med. 2013, 47, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Zavorsky, G.S.; Longo, L.D. Exercise guidelines in pregnancy: New perspectives. Sports Med. 2011, 41, 345–360. [Google Scholar] [CrossRef]
- Headen, I.; Cohen, A.K.; Mujahid, M.; Abrams, B. The accuracy of self-reported pregnancy-related weight: A systematic review. Obes. Rev. 2017, 18, 350–369. [Google Scholar] [CrossRef] [PubMed]
- Mataix, J.L.; Martinez de Victoria, E.; Montellano, M.A.; Lopez, M.; Aranda, P.L. Valoración del Estado Nutricional de la Comunidad Autónoma de Andalucía; Consejeria de salud: Madrid, Spain, 2000. [Google Scholar]
- Huang, W.-Q.; Lu, Y.; Xu, M.; Huang, J.; Su, Y.-X.; Zhang, C.-X. Excessive fruit consumption during the second trimester is associated with increased likelihood of gestational diabetes mellitus: A prospective study. Sci. Rep. 2017, 7, 43620. [Google Scholar] [CrossRef]
- Flor-Alemany, M.; Nestares, T.; Alemany-Arrebola, I.; Marín-Jiménez, N.; Aparicio, V.A. Influence of Dietary Habits and Mediterranean Diet Adherence on Sleep Quality during Pregnancy. The GESTAFIT Project. Nutrients 2020, 12, 3569. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Manzano, P.; Coll-Risco, I.; Van Poppel, M.N.M.; Segura-Jiménez, V.; Femia, P.; Romero-Gallardo, L.; Borges-Cosic, M.; Díaz-Castro, J.; Moreno-Fernández, J.; Ochoa-Herrera, J.J.; et al. Influence of a Concurrent Exercise Training Intervention during Pregnancy on Maternal and Arterial and Venous Cord Serum Cytokines: The GESTAFIT Project. J. Clin. Med. 2019, 8, 1862. [Google Scholar] [CrossRef]
- Hayes, A. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. J. Educ. Meas. 2013, 51, 335–337. [Google Scholar]
- Rooney, B.L.; Schauberger, C.W. Excess pregnancy weight gain and long-term obesity: One decade later. Obstet. Gynecol. 2002, 100, 245–252. [Google Scholar] [CrossRef]
- Linné, Y. Effects of obesity on women’s reproduction and complications during pregnancy. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2004, 5, 137–143. [Google Scholar] [CrossRef]
- Walker, L.O.; Sterling, B.S.; Timmerman, G.M. Retention of Pregnancy-Related Weight in the Early Postpartum Period: Implications for Women’s Health Services. J. Obstet. Gynecol. Neonatal Nurs. 2005, 34, 418–427. [Google Scholar] [CrossRef]
- Schauberger, C.W.; Rooney, B.L.; Brimer, L.M. Factors that influence weight loss in the puerperium. Obstet. Gynecol. 1992, 79, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Claesson, I.-M.; Klein, S.; Sydsjö, G.; Josefsson, A. Physical activity and psychological well-being in obese pregnant and postpartum women attending a weight-gain restriction programme. Midwifery 2014, 30, 11–16. [Google Scholar] [CrossRef]
- Cavalcante, S.R.; Cecatti, J.G.; Pereira, R.I.; Baciuk, E.P.; Bernardo, A.L.; Silveira, C. Water aerobics II: Maternal body composition and perinatal outcomes after a program for low risk pregnant women. Reprod. Health 2009, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Ruchat, S.-M.; Davenport, M.H.; Giroux, I.; Hillier, M.; Batada, A.; Sopper, M.M.; Hammond, J.M.S.; Mottola, M.F. Nutrition and exercise reduce excessive weight gain in normal-weight pregnant women. Med. Sci. Sports Exerc. 2012, 44, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Haakstad, L.A.H.; Bø, K. Exercise in pregnant women and birth weight: A randomized controlled trial. BMC Pregnancy Childbirth 2011, 11, 66. [Google Scholar] [CrossRef]
- Caudwell, P.; Hopkins, M.; King, N.A.; Stubbs, R.J.; Blundell, J.E. Exercise alone is not enough: Weight loss also needs a healthy (Mediterranean) diet? Public Health Nutr. 2009, 12, 1663–1666. [Google Scholar] [CrossRef]
- Barakat, R.; Perales, M.; Garatachea, N.; Ruiz, J.R.; Lucia, A. Exercise during pregnancy. A narrative review asking: What do we know? Br. J. Sports Med. 2015, 49, 1377–1381. [Google Scholar] [CrossRef]

| Variable | Total Women (n = 83) | Control (n = 40) | Exercise (n = 43) |
|---|---|---|---|
| Age (years) | 33.5 (4.3) | 33.8 (4.3) | 33.3 (4.3) |
| Pre-pregnancy body mass index (kg/m2) | 23.7 (3.9) | 23.2 (3.3) | 24.4 (4.0) |
| Total physical activity (min/day) (n = 36 vs. 41) | 427.0 (94.2) | 437.6 (96.8) | 417.7 (92.0) |
| Moderate–vigorous physical activity (min/day) (n = 36 vs. 41) | 39.2 (21.7) | 37.8 (23.4) | 40.5 (20.3) |
| Percentage of attendance * | 86.3 (6.5) | ||
| Postpartum body composition | |||
| Body weight (kg) (n = 37 vs. 42) | 68.2 (10.7) | 68.0 (70.8) | 68.4 (10.7) |
| Height (cm) (n = 37 vs. 42) | 163.6 (5.6) | 163.5 (5.5) | 163.7 (5.7) |
| Postpartum weight retention (kg) (pp-pre) (n = 37 vs. 42) | 4.1 (5.6) | 5.8 (4.6) | 2.6 (5.9) |
| Body mass index (kg/m2) (n = 37 vs. 42) | 25.4 (4.2) | 25.5 (3.99) | 24.4 (4.0) |
| Total lean mass (g) | 38,665.1 (4514.6) | 38,544.8 (4780.2) | 38,776.9 (4306.8) |
| Total fat mass (g) | 26,138.6 (7257.6) | 25,993.0 (6916.4) | 26,273.9 (7640.5) |
| Total fat mass (%) | 38.5 (5.5) | 38.5 (5.3) | 38.5 (5.8) |
| Total android fat mass (g) | 1855.1 (701.0) | 1834.4 (637.8) | 1874.5 (762.1) |
| Total gynecoid fat mass (g) | 5218.7 (1280.9) | 5287.8 (1215.6) | 5154.3 (1350.0) |
| Visceral fat (g) | 365.3 (152.7) | 363.3 (152.3) | 367.1 (154.8) |
| Gynecoid to total fat mass ratio | 0.202 (0.02) | 0.206 (0.02) | 0.198 (0.02) |
| Android to gynecoid fat mass | 0.350 (0.08) | 0.343 (0.08) | 0.357 (0.08) |
| Mediterranean Diet adherence (0–50) | 29.1 (3.9) | 28.4 (4.0) | 29.7 (3.7) |
| Educational status | |||
| University degree | 55 (66.3) | 29 (72.5) | 26 (60.5) |
| No university degree | 28 (33.7) | 11 (27.5) | 17 (39.5) |
| Marital status | |||
| Married | 49 (59.0) | 24 (60.0) | 25 (58.1) |
| Single/divorced/widow | 34 (41.0) | 16 (40.0) | 18 (41.9) |
| Type of breastfeeding (n = 81) | |||
| Exclusive (only breast) | 55 (67.9) | 23 (60.5) | 32 (74.4) |
| Mixed (breast and formula milk) | 16 (19.8) | 10 (26.3) | 6 (14.0) |
| Artificial (only formula milk) | 10 (12.3) | 5 (13.2) | 5 (11.6) |
| Smoking habit (yes, n (%)) | 6 (7.2) | 5 (12.5) | 1 (2.3) |
| Alcohol intake (yes, n (%)) | 0 (0) | 0 (0) | 0 (0) |
| Women returning to pre-pregnancy weight (yes, n (%)) a (n = 37 vs. 42) | 16 (20.3) | 1 (2.7) | 15 (35.7) |
| Variable | Control (n = 40) | Exercise (n = 43) | Between-Group Difference (B) (95% CI) | p |
|---|---|---|---|---|
| Per-protocol basis * | ||||
| Body weight (kg) (n = 37 vs. 42) | 69.59 (1.05) | 67.33 (0.99) | 2.26 (−0.63 to 5.15) | 0.124 |
| Body mass index (kg/m2) (n = 37 vs. 42) | 26.07 (0.36) | 24.89 (0.35) | 1.18 (0.17–2.18) | 0.022 |
| Total lean mass (g) | 39,054.49 (568.19) | 38,302.81 (547.72) | 751.68 (−830.81 to 2334.17) | 0.347 |
| Total fat mass (g) | 27,031.30 (703.76) | 25,308.14 (678.40) | 1723.16 (−236.91 to 3683.22) | 0.084 |
| Total fat mass (%) | 39.14 (0.70) | 37.95 (0.67) | 1.19 (0–0.75 to 3.14) | 0.226 |
| Total Android fat mass (g) | 1931.42 (70.77) | 1784.20 (68.22) | 147.23 (−49.89 to 344.34) | 0.141 |
| Total gynecoid fat mass (g) | 5442.07 (153.56) | 5010.86 (148.03) | 431.21 (3.52–858.89) | 0.048 |
| Visceral fat (g) | 381.16 (18.1) | 350.5 (17.5) | 30.65 (−19.87 to 81.17) | 0.231 |
| Gynecoid to total fat mass | 0.204 (0.002) | 0.200 (0.002) | 0.004 (−0.002 to 0.010) | 0.196 |
| Android to gynecoid fat mass | 0.351 (0.010) | 0.349 (0.010) | 0.002 (−0.027 to 0.030) | 0.897 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flor-Alemany, M.; Acosta-Manzano, P.; Migueles, J.H.; Henriksson, P.; Löf, M.; Aparicio, V.A. Impact of Exercise Intervention Combined with Optimal Mediterranean Diet Adherence during Pregnancy on Postpartum Body Composition: A Quasi-Experimental Study—The GESTAFIT Project. Nutrients 2023, 15, 4413. https://doi.org/10.3390/nu15204413
Flor-Alemany M, Acosta-Manzano P, Migueles JH, Henriksson P, Löf M, Aparicio VA. Impact of Exercise Intervention Combined with Optimal Mediterranean Diet Adherence during Pregnancy on Postpartum Body Composition: A Quasi-Experimental Study—The GESTAFIT Project. Nutrients. 2023; 15(20):4413. https://doi.org/10.3390/nu15204413
Chicago/Turabian StyleFlor-Alemany, Marta, Pedro Acosta-Manzano, Jairo H. Migueles, Pontus Henriksson, Marie Löf, and Virginia A. Aparicio. 2023. "Impact of Exercise Intervention Combined with Optimal Mediterranean Diet Adherence during Pregnancy on Postpartum Body Composition: A Quasi-Experimental Study—The GESTAFIT Project" Nutrients 15, no. 20: 4413. https://doi.org/10.3390/nu15204413
APA StyleFlor-Alemany, M., Acosta-Manzano, P., Migueles, J. H., Henriksson, P., Löf, M., & Aparicio, V. A. (2023). Impact of Exercise Intervention Combined with Optimal Mediterranean Diet Adherence during Pregnancy on Postpartum Body Composition: A Quasi-Experimental Study—The GESTAFIT Project. Nutrients, 15(20), 4413. https://doi.org/10.3390/nu15204413






