Low Sodium Intake, Low Protein Intake, and Excess Mortality in an Older Dutch General Population Cohort: Findings in the Prospective Lifelines-MINUTHE Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Exposure Assessments
2.3. Ascertainment of Outcomes
2.4. Lifestyle Factors
2.5. Anthropometric Measurements and Comorbidities
2.6. Statistical Analyses
2.7. Declaration of Helsinki, Informed Consent, and Ethical Approval
2.8. Patient and Public Involvement
3. Results
3.1. Sodium intake and All-Cause Mortality
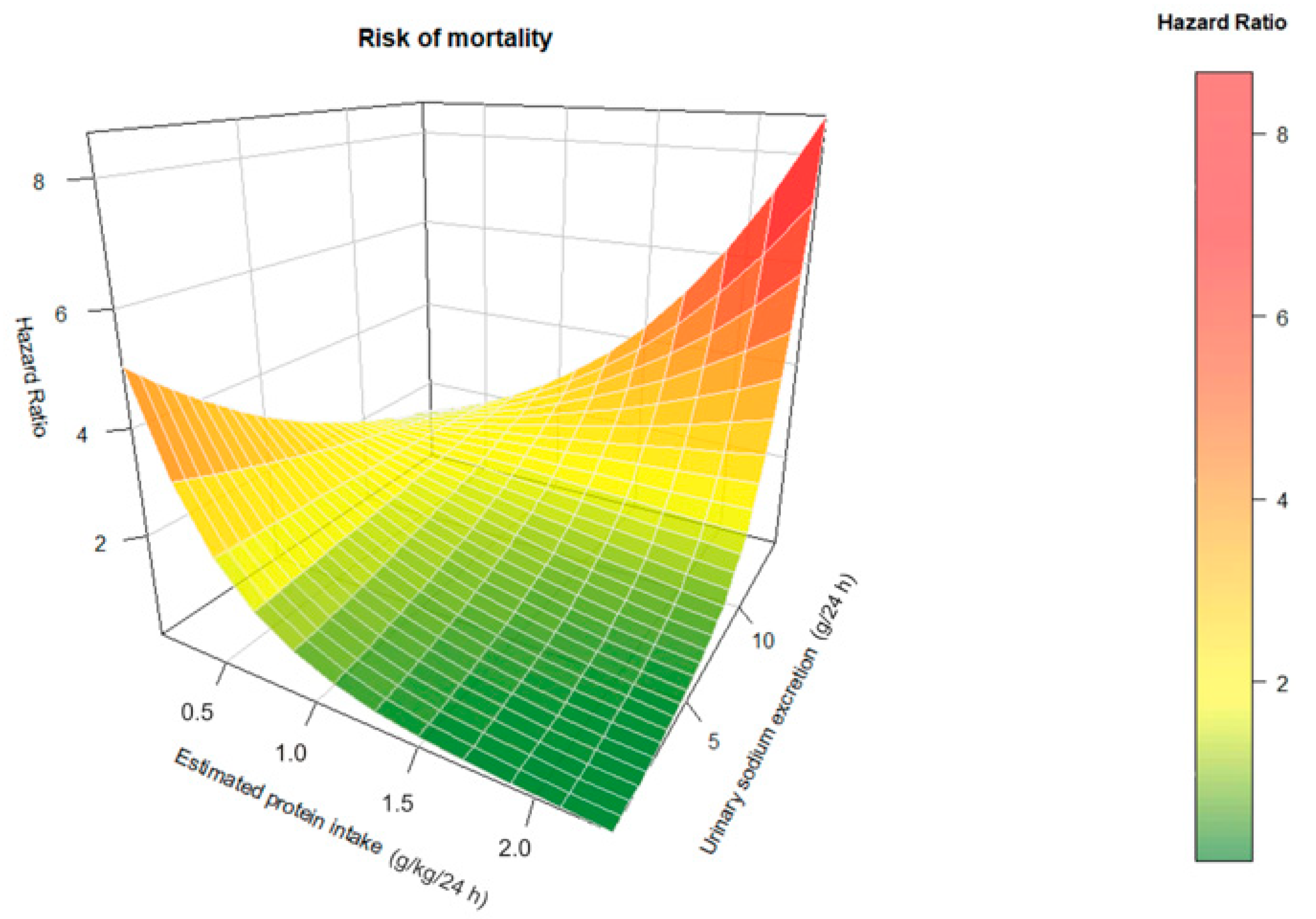
3.2. Effect of Interaction between Sodium Intake and Protein Intake on Mortality
3.3. Sensitivity Analyses
4. Discussion
4.1. Comparisons with Other Studies
4.2. Implications for Clinicians and Policymakers
4.3. Strengths and Weaknesses of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef] [PubMed]
- Thout, S.R.; Santos, J.A.; McKenzie, B.; Trieu, K.; Johnson, C.; McLean, R.; Arcand, J.A.; Campbell, N.R.C.; Webster, J. The Science of Salt: Updating the evidence on global estimates of salt intake. J. Clin. Hypertens. 2019, 21, 710–721. [Google Scholar] [CrossRef]
- Weinberger, M.H. Salt sensitivity of blood pressure in humans. Hypertens 1996, 27, 481–490. [Google Scholar] [CrossRef]
- Strazzullo, P.; D’Elia, L.; Kandala, N.-B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef]
- Olde Engberink, R.H.G.; Van Den Hoek, T.C.; Van Noordenne, N.D.; Van Den Born, B.J.H.; Peters-Sengers, H.; Vogt, L. Use of a single baseline versus multiyear 24-hour urine collection for estimation of long-term sodium intake and associated cardiovascular and renal risk. Circulation 2017, 136, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, M.E.; Mugavero, K.; Bowman, B.A.; Frieden, T.R. Dietary Sodium and Cardiovascular Disease Risk–Measurement Matters. N. Engl. J. Med. 2016, 375, 580–586. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Marciniak, M.; Visagie, E.; Markandu, N.D.; Anand, V.; Dalton, R.N.; MacGregor, G.A. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and asian mild hypertensives. Hypertension 2009, 54, 482–488. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Prevention of Recurrent Heart Attacks and Strokes in Low and Middle Income Populations: Evidence-Based Recommendations for Policy Makers and Health Professionals; WHO Publications: Geneva, Switzerland, 2013. [Google Scholar]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Pelaez, C.; et al. Dietary reference values for sodium. EFSA J. 2019, 17, e05778. [Google Scholar] [CrossRef]
- Strohm, D.; Bechthold, A.; Ellinger, S.; Leschik-Bonnet, E.; Stehle, P.; Heseker, H. Revised reference values for the intake of sodium and chloride. Ann. Nutr. Metab. 2018, 72, 12–17. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; USDA: Washington, DC, USA, 2015. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 10 September 2022).
- Nordic Nutrition Recommendations. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity; Nordic Council of Ministers: Copenhagen, Denmark, 2014. [Google Scholar] [CrossRef]
- McGuire, S. Institute of Medicine. 2013. “Sodium intake in populations: Assessment of evidence.” The National Academies Press: Washington, DC, USA, 2013. Adv. Nutr. 2014, 5, 19–20. [Google Scholar] [CrossRef]
- O’Donnell, M.; Mente, A.; Rangarajan, S.; McQueen, M.J.; Wang, X.; Liu, L.; Yan, H.; Lee, S.F.; Mony, P.; Devanath, A.; et al. Urinary Sodium and Potassium Excretion, Mortality, and Cardiovascular Events. N. Engl. J. Med. 2014, 371, 612–623. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.; Mente, A.; Rangarajan, S.; McQueen, M.J.; O’Leary, N.; Yin, L.; Liu, X.; Swaminathan, S.; Khatib, R.; Rosengren, A.; et al. Joint association of urinary sodium and potassium excretion with cardiovascular events and mortality: Prospective cohort study. BMJ 2019, 364, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Graudal, N.; Jürgens, G.; Baslund, B.; Alderman, M.H. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: A meta-analysis. Am. J. Hypertens. 2014, 27, 1129–1137. [Google Scholar] [CrossRef]
- Pfister, R.; Michels, G.; Sharp, S.J.; Luben, R.; Wareham, N.J.; Khaw, K.T. Estimated urinary sodium excretion and risk of heart failure in men and women in the EPIC-Norfolk study. Eur. J. Heart Fail. 2014, 16, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, E.I.; Clarke, S.; Thomas, M.C.; Moran, J.L.; Cheong, K.; MacIsaac, R.J.; Jerums, G. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care 2011, 34, 703–709. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Yeh, T.-L.; Shih, M.-C.; Tu, Y.-K.; Chien, K.-L. Dietary Sodium Intake and Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-Analysis. Nutrients 2020, 12, 2934. [Google Scholar] [CrossRef]
- Meier, T.; Gräfe, K.; Senn, F.; Sur, P.; Stangl, G.I.; Dawczynski, C.; März, W.; Kleber, M.E.; Lorkowski, S. Cardiovascular mortality attributable to dietary risk factors in 51 countries in the WHO European Region from 1990 to 2016: A systematic analysis of the Global Burden of Disease Study. Eur. J. Epidemiol. 2019, 34, 37–55. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Mente, A.; Smyth, A.; Yusuf, S. Salt intake and cardiovascular disease: Why are the data inconsistent? Eur. Heart J. 2013, 34, 1034–1040. [Google Scholar] [CrossRef]
- O’Donnell, M.; Mente, A.; Alderman, M.H.; Brady, A.J.B.; Diaz, R.; Gupta, R.; López-Jaramillo, P.; Luft, F.C.; Lüscher, T.F.; Mancia, G.; et al. Salt and cardiovascular disease: Insufficient evidence to recommend low sodium intake. Eur. Heart J. 2020, 41, 3363–3373. [Google Scholar] [CrossRef]
- Slagman, M.C.J.; Waanders, F.; Hemmelder, M.H.; Woittiez, A.-J.; Janssen, W.M.T.; Lambers Heerspink, H.J.; Navis, G.; Laverman, G.D. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: Randomised controlled trial. BMJ 2011, 343, d4366. [Google Scholar] [CrossRef]
- Odermatt, A. The western-style diet: A major risk factor for impaired kidney function and chronic kidney disease. Am. J. Physiol.-Ren. Physiol. 2011, 301, G919–G928. [Google Scholar] [CrossRef] [PubMed]
- Swift, P.A.; Markandu, N.D.; Sagnella, G.A.; He, F.J.; MacGregor, G.A. Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives: A randomized control trial. Hypertension 2005, 46, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Suckling, R.J.; He, F.J.; Markandu, N.D.; Macgregor, G.A. Modest salt reduction lowers blood pressure and albumin excretion in impaired glucose tolerance and type 2 diabetes mellitus: A randomized double-blind trial. Hypertension 2016, 67, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- McMahon, E.J.; Campbell, K.L.; Bauer, J.D.; Mudge, D.W. Altered dietary salt intake for people with chronic kidney disease. Cochrane database Syst. Rev. 2015, 2, CD010070. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-López, L.; Maseda, A.; De Labra, C.; Regueiro-Folgueira, L.; Rodríguez-Villamil, J.L.; Millán-Calenti, J.C. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr. 2017, 17, 108. [Google Scholar] [CrossRef]
- Lambers Heerspink, H.J.; Kwakernaak, A.; De Zeeuw, D.; Navis, G. Comment on: Ekinci et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care 2011;34: 703–709. Diabetes Care 2011, 34, 2011. [Google Scholar] [CrossRef]
- Klijs, B.; Scholtens, S.; Mandemakers, J.J.; Snieder, H.; Stolk, R.P.; Smidt, N. Representativeness of the LifeLines cohort study. PLoS ONE 2015, 10, 1–12. [Google Scholar] [CrossRef]
- van der Ende, M.Y.; Hartman, M.H.T.; Hagemeijer, Y.; Meems, L.M.G.; de Vries, H.S.; Stolk, R.P.; de Boer, R.A.; Sijtsma, A.; van der Meer, P.; Rienstra, M.; et al. The LifeLines Cohort Study: Prevalence and treatment of cardiovascular disease and risk factors. Int. J. Cardiol. 2017, 228, 495–500. [Google Scholar] [CrossRef]
- Scholtens, S.; Smidt, N.; Swertz, M.A.; Bakker, S.J.L.; Dotinga, A.; Vonk, J.M.; Van Dijk, F.; Van Zon, S.K.R.; Wijmenga, C.; Wolffenbuttel, B.H.R.; et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int. J. Epidemiol. 2015, 44, 1172–1180. [Google Scholar] [CrossRef]
- Vart, P.; Gansevoort, R.T.; Coresh, J.; Reijneveld, S.A.; Bültmann, U. Socioeconomic measures and CKD in the United States and The Netherlands. Clin. J. Am. Soc. Nephrol. 2013, 8, 1685–1693. [Google Scholar] [CrossRef]
- Riphagen, I.J.; Minović, I.; Groothof, D.; Post, A.; Eggersdorfer, M.L.; Kootstra-Ros, J.E.; de Borst, M.H.; Navis, G.; Muskiet, F.A.J.; Kema, I.P.; et al. Methylmalonic acid, vitamin B12, renal function, and risk of all-cause mortality in the general population: Results from the prospective Lifelines-MINUTHE study. BMC Med. 2020, 18, 380. [Google Scholar] [CrossRef] [PubMed]
- Maroni, B.J.; Steinman, T.I.; Mitch, W.E. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985, 27, 58–65. [Google Scholar] [CrossRef] [PubMed]
- RIVM Dutch food composition table (NEVO). 2011. Available online: https://www.rivm.nl/nieuws/nieuwe-nevo-tabel-2011-beschikbaar (accessed on 1 September 2022).
- Molag, M.L.; De Vries, J.H.M.; Duif, N.; Ocké, M.C.; Dagnelie, P.C.; Goldbohm, R.A.; Van’T Veer, P. Selecting informative food items for compiling food-frequency questionnaires: Comparison of procedures. Br. J. Nutr. 2010, 104, 446–456. [Google Scholar] [CrossRef]
- Siebelink, E.; Geelen, A.; De Vries, J.H.M. Self-reported energy intake by FFQ compared with actual energy intake to maintain body weight in 516 adults. Br. J. Nutr. 2011, 106, 274–281. [Google Scholar] [CrossRef]
- Vinke, P.C.; Corpeleijn, E.; Dekker, L.H.; Jacobs, D.R.; Navis, G.; Kromhout, D. Development of the food-based Lifelines Diet Score (LLDS) and its application in 129,369 Lifelines participants. Eur. J. Clin. Nutr. 2018, 72, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Wendel-Vos, G.C.W.; Schuit, A.J.; Saris, W.H.M.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th ed.; World Health Organization: Geneva, Switzerland, 2010; ISBN 9789241549165. Available online: http://apps.who.int/classifications/icd10/browse/2010/en (accessed on 1 September 2022).
- Marathe, P.H.; Gao, H.X.; Close, K.L. American Diabetes Association Standards of Medical Care in Diabetes 2017. J. Diabetes 2017, 9, 320–324. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Richter, M.; Baerlocher, K.; Bauer, J.M.; Elmadfa, I.; Heseker, H.; Leschik-Bonnet, E.; Stangl, G.; Volkert, D.; Stehle, P. Revised Reference Values for the Intake of Protein. Ann. Nutr. Metab. 2019, 74, 242–250. [Google Scholar] [CrossRef]
- Gomes Neto, A.W.; Boslooper-Meulenbelt, K.; Geelink, M.; van Vliet, I.M.Y.; Post, A.; Joustra, M.L.; Knoop, H.; Berger, S.P.; Navis, G.J.; Bakker, S.J.L. Protein intake, fatigue and quality of life in stable outpatient kidney transplant recipients. Nutrients 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Cook, N.R.; Appel, L.J.; Whelton, P.K. Sodium Intake and All-Cause Mortality Over 20 Years in the Trials of Hypertension Prevention. J. Am. Coll. Cardiol. 2016, 68, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, F.J.; Sun, Q.; Yuan, C.; Kieneker, L.M.; Curhan, G.C.; MacGregor, G.A.; Bakker, S.J.L.; Campbell, N.R.C.; Wang, M.; et al. 24-Hour Urinary Sodium and Potassium Excretion and Cardiovascular Risk. N. Engl. J. Med. 2021, 386, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; Campbell, N.R.C.; He, F.J.; Jacobson, M.F.; MacGregor, G.A.; Antman, E.; Appel, L.J.; Arcand, J.A.; Blanco-Metzler, A.; Cook, N.R.; et al. Sodium and Health: Old Myths and a Controversy Based on Denial. Curr. Nutr. Rep. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Food Based Dietary Guidelines in the WHO European Region, Eur/03/5045414; World Health Organization: Geneva, Switzerland, 2003; pp. 1–37. [Google Scholar]
- EFSA. Scientific Opinion on establishing Food-Based Dietary Guidelines. EFSA J. 2010, 8, 1460. [Google Scholar] [CrossRef]
- World Health Organization. Promoting a Healthy Diet for the WHO Eastern Mediterranean Region: User-Friendly Guide; World Health Organization: Geneva, Switzerland, 2012; Available online: http://applications.emro.who.int/dsaf/emropub_2011_1274.pdf%0Ahttp://www.who.int/nutrition/publications/nutrientrequirements/healtydietguide2012_emro/en/ (accessed on 5 October 2022).
- Sattler, E.L.P.; Ishikawa, Y.; Trivedi-Kapoor, R.; Zhang, D.; Quyyumi, A.A.; Dunbar, S.B. Association between the prognostic nutritional index and dietary intake in community-dwelling older adults with heart failure: Findings from NHANES III. Nutrients 2019, 11, 2608. [Google Scholar] [CrossRef]
- Streng, K.W.; Hillege, H.L.; Maaten, J.M.; Veldhuisen, D.J.; Dickstein, K.; Ng, L.L.; Samani, N.J.; Metra, M.; Ponikowski, P.; Cleland, J.G.; et al. Clinical implications of low estimated protein intake in patients with heart failure. J. Cachexia. Sarcopenia Muscle 2022, 13, 1762–1770. [Google Scholar] [CrossRef]
- Saka, B.; Kaya, O.; Ozturk, G.B.; Erten, N.; Karan, M.A. Malnutrition in the elderly and its relationship with other geriatric syndromes. Clin. Nutr. 2010, 29, 745–748. [Google Scholar] [CrossRef]
- Rand, W.M.; Pellett, P.L.; Young, V.R. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am. J. Clin. Nutr. 2003, 77, 109–127. [Google Scholar] [CrossRef]
- Ji, C.; Sykes, L.; Paul, C.; Dary, O.; Legetic, B.; Campbell, N.R.; Cappuccio, F.P. Systematic review of studies comparing 24-hour and spot urine collections for estimating population salt intake. Rev. Panam. Salud Publica/Pan Am. J. Public Heal. 2012, 32, 307–315. [Google Scholar] [CrossRef]
- Mitch, W.E. Dietary protein restriction in patients with chronic renal failure. Kidney Int. 1991, 40, 326–341. [Google Scholar] [CrossRef]
- Matsuda, T.; Kato, H.; Suzuki, H.; Mizugaki, A.; Ezaki, T.; Ogita, F. Within-Day Amino Acid Intakes and Nitrogen Balance in Male Collegiate Swimmers during the General Preparation Phase. Nutrients 2018, 10, 1809. [Google Scholar] [CrossRef] [PubMed]
- Cobb, L.K.; Anderson, C.A.M.; Elliott, P.; Hu, F.B.; Liu, K.; Neaton, J.D.; Whelton, P.K.; Woodward, M.; Appel, L.J. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: A science advisory from the American Heart Association. Circulation 2014, 129, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- Dekker, L.H.; De Borst, M.H.; Meems, L.M.G.; De Boer, R.A.; Bakker, S.J.L.; Navis, G.J. The association of multimorbidity within cardio-metabolic disease domains with dietary patterns: A cross-sectional study in 129 369 men and women from the Lifelines cohort. PLoS ONE 2019, 14, e0220368. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 1603) | Quartiles of Sodium Intake | |||||
|---|---|---|---|---|---|---|
| Q1 (n = 401) | Q2 (n = 401) | Q3 (n = 401) | Q4 (n = 400) | p | ||
| Na, g/day (range) | 0.7–2.8 | 2.8–3.6 | 3.6–4.7 | 4.7–14.3 | ||
| Demographics | ||||||
| Male, % | 50 | 24.9 | 42.4 | 55.9 | 76.7 | <0.001 |
| Age, years | 66 ± 4 | 66 ± 4 | 66 ± 4 | 66 ± 4 | 65 ± 4 | 0.7 |
| Low SES, % | 50 | 45.1 | 45.1 | 50.6 | 59.2 | <0.001 |
| Smoking | ||||||
| Current | 12 | 12.9 | 10.8 | 13 | 11.4 | <0.001 |
| Former | 53.6 | 45.1 | 53.9 | 53.4 | 62 | |
| Never | 34.4 | 42.1 | 35.3 | 33.6 | 26.6 | |
| Alcohol, g/day | 6.4 (1.2–16) | 3.8 (0.8–12) | 6.4 (0.8–17) | 6.8 (1.7–17) | 6.6 (1.6–17) | 0.008 |
| Energy intake, kcal/day | 1909 ± 518 | 1777 ± 450 | 1861 ± 467 | 1987 ± 549 | 2011 ± 565 | <0.001 |
| MVPA, min/week | 260 (110–523) | 270 (120–510) | 240 (100–500) | 300 (120–535) | 240 (90–540) | 0.6 |
| LLDS | 23.9 ± 6.2 | 25.4 ± 6.2 | 24.1 ± 6.2 | 23.3 ± 6.0 | 22.9 ± 6.0 | <0.001 |
| TV watching, h/day | 2.9 ± 1.6 | 2.8 ± 1.5 | 2.8 ± 1.5 | 3.1 ± 2 | 3.0 ± 1.5 | 0.02 |
| Sleeping, h/day | 7.5 ± 1.0 | 7.6 ± 1.0 | 7.5 ± 0.9 | 7.6 ± 1.1 | 7.4 ± 1.0 | 0.08 |
| Morbidities | ||||||
| T2D, % | 29 | 29.4 | 28.2 | 28.4 | 30 | 0.9 |
| CVD, % | 40.9 | 37.9 | 38.2 | 42.9 | 44.5 | 0.1 |
| Renal disease, % | 5.2 | 7.2 | 5.5 | 4.5 | 3.5 | 0.1 |
| Protein intake, g/day | 71.8 ± 18.3 | 66.0 ± 16.0 | 70.0 ± 17.3 | 74.2 ± 18.7 | 77.2 ± 19.4 | <0.001 |
| Protein intake, g/kg/day | 1.1 ± 0.3 | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.3 | <0.001 |
| <0.8 | 10.3 | 23.2 | 10.0 | 5.0 | 2.8 | <0.001 |
| 0.8–1.2 | 58.6 | 60.5 | 64.7 | 61.3 | 47.9 | |
| >1.2 | 31.1 | 16.2 | 25.2 | 33.7 | 49.4 | |
| BMI, kg/m2 | 26.9 ± 4.1 | 26.1 ± 4.3 | 26.5 ± 3.9 | 26.7 ± 4.0 | 28.4 ± 3.8 | <0.001 |
| SBP, mmHg | 134 ± 17 | 132 ± 18 | 134 ± 18 | 135 ± 17 | 135 ± 16 | 0.06 |
| DBP, mmHg | 75 ± 9 | 74 ± 9 | 75 ± 9 | 76 ± 9 | 76 ± 9 | 0.004 |
| Urinary parameters | ||||||
| Na intake, g/day | 3.9 ± 1.6 | 2.2 ± 0.5 | 3.2 ± 0.2 | 4.1 ± 0.3 | 6.0 ± 1.3 | <0.001 |
| K excretion, g/day | 3.5 ± 1.1 | 2.9 ± 0.9 | 3.3 ± 0.9 | 3.6 ± 1.0 | 4.2 ± 1.1 | <0.001 |
| Na/K ratio | 1.8 (1.4–2.3) | 1.3 (1.0–1.6) | 1.6 (1.3–2.0) | 1.9 (1.6–2.3) | 2.3 (2.0–2.8) | <0.001 |
| Total protein excretion, g/24 h | 0.11 (0.10–0.15) | 0.1 (0.09–0.12) | 0.11 (0.10–0.14) | 0.12 (0.11–0.14) | 0.14 (0.12–0.17) | <0.001 |
| Urea, mmol/24 h | 399 ± 129 | 302 ± 89 | 372 ± 94 | 408 ± 95 | 515 ± 131 | <0.001 |
| Creatinine, mmol/24 h | 12.0 ± 3.8 | 9.4 ± 2.8 | 11.2 ± 2.9 | 12.4 ± 3.0 | 15.2 ± 3.8 | <0.001 |
| Q1 (0.7–2.8 g/day) | Q2 (2.8–3.6 g/day) | Q3 (3.6–4.7 g/day) | Q4 (4.7–14.3 g/day) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | Reference | HR (95% CI) | p-Value | p-Trend | |
| Model 1 | 2.05 (1.16–3.62) | 0.01 | 1.85 (1.08–3.20) | 0.03 | 1 | 1.74 (1.03–2.95) | 0.04 | 0.07 |
| Model 2 | 2.12 (1.20–3.74) | 0.01 | 1.89 (1.09–3.25) | 0.02 | 1 | 1.68 (0.99–2.84) | 0.06 | 0.09 |
| Model 3 | 2.05 (1.16–3.62) | 0.01 | 1.85 (1.08–3.19) | 0.03 | 1 | 1.75 (1.03–2.98) | 0.04 | 0.06 |
| Model 4 | 1.97 (1.11–3.48) | 0.02 | 1.82 (1.06–3.14) | 0.03 | 1 | 1.76 (1.03–3.00) | 0.04 | 0.05 |
| Model 5 | 1.94 (1.09–3.47) | 0.03 | 1.79 (1.03–3.09) | 0.04 | 1 | 1.70 (1.00–2.91) | 0.05 | 0.07 |
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Sodium intake, g/day | 0.71 (0.54–0.95) | 0.02 | 0.70 (0.52–0.93) | 0.02 | 0.72 (0.53–0.98) | 0.04 | 0.74 (0.54–1.01) | 0.05 | 0.71 (0.52–0.97) | 0.04 |
| Protein intake, g/day | 0.10 (0.03–0.32) | <0.001 | 0.11 (0.03–0.37) | <0.001 | 0.09 (0.02–0.32) | <0.001 | 0.10 (0.03–0.37) | 0.001 | 0.10 (0.02–0.39) | 0.001 |
| Sodium intake × protein intake | 1.34 (1.09–1.65) | 0.006 | 1.34 (1.09–1.64) | 0.007 | 1.34 (1.08–1.68) | 0.01 | 1.33 (1.06–1.66) | 0.02 | 1.35 (1.07–1.69) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hessels, N.R.; Zhu, Y.; Bakker, S.J.L.; de Borst, M.H.; Navis, G.J.; Riphagen, I.J. Low Sodium Intake, Low Protein Intake, and Excess Mortality in an Older Dutch General Population Cohort: Findings in the Prospective Lifelines-MINUTHE Study. Nutrients 2023, 15, 428. https://doi.org/10.3390/nu15020428
Hessels NR, Zhu Y, Bakker SJL, de Borst MH, Navis GJ, Riphagen IJ. Low Sodium Intake, Low Protein Intake, and Excess Mortality in an Older Dutch General Population Cohort: Findings in the Prospective Lifelines-MINUTHE Study. Nutrients. 2023; 15(2):428. https://doi.org/10.3390/nu15020428
Chicago/Turabian StyleHessels, Niek R., Yinjie Zhu, Stephan J. L. Bakker, Martin H. de Borst, Gerjan J. Navis, and Ineke J. Riphagen. 2023. "Low Sodium Intake, Low Protein Intake, and Excess Mortality in an Older Dutch General Population Cohort: Findings in the Prospective Lifelines-MINUTHE Study" Nutrients 15, no. 2: 428. https://doi.org/10.3390/nu15020428
APA StyleHessels, N. R., Zhu, Y., Bakker, S. J. L., de Borst, M. H., Navis, G. J., & Riphagen, I. J. (2023). Low Sodium Intake, Low Protein Intake, and Excess Mortality in an Older Dutch General Population Cohort: Findings in the Prospective Lifelines-MINUTHE Study. Nutrients, 15(2), 428. https://doi.org/10.3390/nu15020428






