Advanced Glycation End-Products and Their Effects on Gut Health
Abstract
1. Introduction
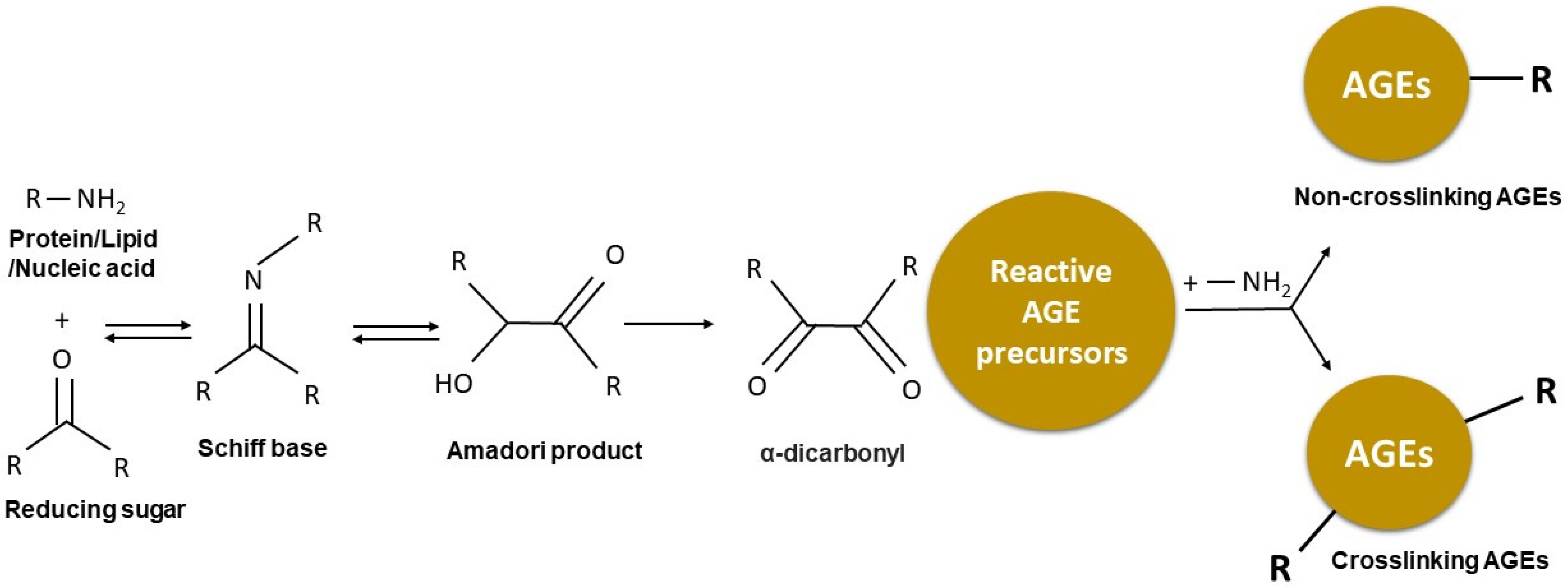
2. Formation of Advanced Glycation End-Products (AGEs)
3. Sources of AGEs
3.1. Endogenous AGEs
3.2. Dietary-Derived (Exogenous) AGEs
4. AGEs, Oxidative Stress, and Inflammation in the Gut
4.1. The Role of AGEs in the Pathophysiology of IBD
4.2. Dietary Approaches to Reducing AGE-Related Inflammation in the Gut
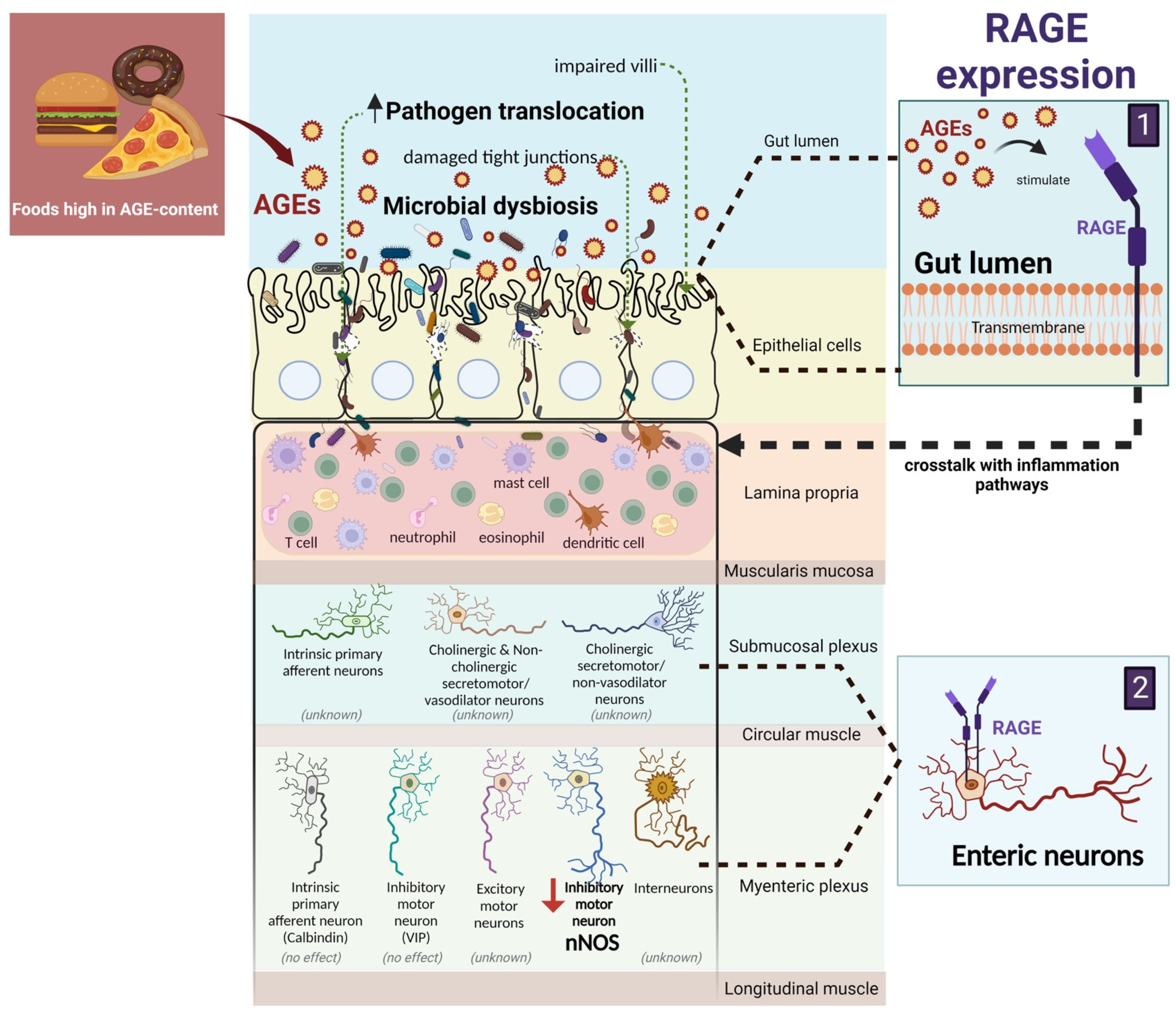
5. AGEs and the Gut Barrier
6. AGEs and Enteric Neurons
7. AGEs and the Gut Microbiota
7.1. Human Studies
7.2. Animal Studies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Jie, J.; Yao, J.; Li, W.; Cheng, Y.; Lu, W. Advanced glycation end products promote osteoporosis by inducing ferroptosis in osteoblasts. Mol. Med. Rep. 2022, 25, 140. [Google Scholar] [CrossRef] [PubMed]
- Galliera, E.; Marazzi, M.G.; Gazzaruso, C.; Gallotti, P.; Coppola, A.; Montalcini, T.; Pujia, A.; Romanelli, M.M.C. Evaluation of circulating sRAGE in osteoporosis according to BMI, adipokines and fracture risk: A pilot observational study. Immun. Ageing 2017, 14, 13. [Google Scholar] [CrossRef]
- Nakano, M.; Nakamura, Y.; Suzuki, T.; Miyazaki, A.; Takahashi, J.; Saito, M.; Shiraki, M. Pentosidine and carboxymethyl-lysine associate differently with prevalent osteoporotic vertebral fracture and various bone markers. Sci. Rep. 2020, 10, 22090. [Google Scholar] [CrossRef]
- Sun, K.; Semba, R.D.; Fried, L.P.; Schaumberg, D.A.; Ferrucci, L.; Varadhan, R. Elevated Serum Carboxymethyl-Lysine, an Advanced Glycation End Product, Predicts Severe Walking Disability in Older Women: The Women’s Health and Aging Study I. J. Aging Res. 2012, 2012, 586385. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Toyoguchi, T.; Inage, K.; Fujimoto, K.; Orita, S.; Suzuki, M.; Kanamoto, H.; Abe, K.; Norimoto, M.; Umimura, T.; et al. Advanced glycation end products are associated with sarcopenia in older women: Aging marker dynamics. J. Women Aging 2021, 33, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, J.; Ueda, S.; Yamagishi, S.-I.; Nohara, N.; Nagasawa, H.; Wakabayashi, K.; Matsui, T.; Yuichiro, H.; Kadoguchi, T.; Otsuka, T.; et al. Association of advanced glycation end products with sarcopenia and frailty in chronic kidney disease. Sci. Rep. 2020, 10, 17467. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, Y.; Dai, F.; Liu, C.; Hu, H.; Zhang, Q. Advanced glycation end products and diabetes and other metabolic indicators. Diabetol. Metab. Syndr. 2022, 14, 104. [Google Scholar] [CrossRef]
- Indyk, D.; Bronowicka-Szydełko, A.; Gamian, A.; Kuzan, A. Advanced glycation end products and their receptors in serum of patients with type 2 diabetes. Sci. Rep. 2021, 11, 13264. [Google Scholar] [CrossRef]
- Koska, J.; Gerstein, H.C.; Beisswenger, P.J.; Reaven, P.D. Advanced Glycation End Products Predict Loss of Renal Function and High-Risk Chronic Kidney Disease in Type 2 Diabetes. Diabetes Care 2022, 45, 684–691. [Google Scholar] [CrossRef]
- Vlassara, H.; Uribarri, J. Advanced Glycation End Products (AGE) and Diabetes: Cause, Effect, or Both? Curr. Diabetes Rep. 2014, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Nishad, R.; Tahaseen, V.; Kavvuri, R.; Motrapu, M.; Singh, A.K.; Peddi, K.; Pasupulati, A.K. Advanced-Glycation End-Products Induce Podocyte Injury and Contribute to Proteinuria. Front. Med. 2021, 8, 685447. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Yamasaki, S.; Ando, S.; Suzuki, K.; Toriumi, K.; Horiuchi, Y.; Yoshikawa, A.; Imai, A.; Nagase, Y.; Miyano, Y.; et al. Fingertip advanced glycation end products and psychotic symptoms among adolescents. Schizophrenia 2021, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Kobori, A.; Miyashita, M.; Miyano, Y.; Suzuki, K.; Toriumi, K.; Niizato, K.; Oshima, K.; Imai, A.; Nagase, Y.; Yoshikawa, A.; et al. Advanced glycation end products and cognitive impairment in schizophrenia. PLoS ONE 2021, 16, e0251283. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mooldijk, S.S.; Licher, S.; Waqas, K.; Ikram, M.K.; Uitterlinden, A.G.; Zillikens, M.C. Assessment of Advanced Glycation End Products and Receptors and the Risk of Dementia. JAMA Netw. Open 2021, 4, e2033012. [Google Scholar] [CrossRef]
- van Dooren, F.E.P.; Pouwer, F.; Schalkwijk, C.G.; Sep, S.J.S.; Stehouwer, C.D.A.; Henry, R.M.A.; Dagnelie, P.C.; Schaper, N.C.; van der Kallen, C.J.H.; Koster, A.; et al. Advanced Glycation End Product (AGE) Accumulation in the Skin is Associated with Depression: The Maastricht Study. Depress. Anxiety 2016, 34, 59–67. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Du Yan, S.; Wautier, J.-L.; Stern, D. Activation of Receptor for Advanced Glycation End Products. Circ. Res. 1999, 84, 489–497. [Google Scholar] [CrossRef]
- Schleicher, E.D.; Wagner, E.; Nerlich, A.G. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J. Clin. Investig. 1997, 99, 457–468. [Google Scholar] [CrossRef]
- Scheijen, J.L.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef]
- A Finot, P.; Magnenat, E. Metabolic transit of early and advanced Maillard products. Prog. Food Nutr. Sci. 1981, 5, 193–207. [Google Scholar]
- Hodge, J.E. Dehydrated Foods, Chemistry of Browning Reactions in Model Systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Zhang, Q.; Ames, J.M.; Smith, R.; Baynes, J.W.; Metz, T.O. A Perspective on the Maillard Reaction and the Analysis of Protein Glycation by Mass Spectrometry: Probing the Pathogenesis of Chronic Disease. J. Proteome Res. 2008, 8, 754–769. [Google Scholar] [CrossRef] [PubMed]
- Harohally, N.V.; Srinivas, S.M.; Umesh, S. ZnCl2-mediated practical protocol for the synthesis of Amadori ketoses. Food Chem. 2014, 158, 340–344. [Google Scholar] [CrossRef]
- Wu, C.-H.; Huang, S.-M.; Lin, J.-A.; Yen, G.-C. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct. 2011, 2, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Yurek-George, A.; Argirov, O.K. Kinetics and mechanism of the reaction of aminoguanidine with the α-oxoaldehydes glyoxal, methylglyoxal, and 3-deoxyglucosone under physiological conditions. Biochem. Pharmacol. 2000, 60, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Jacobs, K.; Haucke, E.; Santos, A.N.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Deluyker, D.; Evens, L.; Bito, V. Advanced glycation end products (AGEs) and cardiovascular dysfunction: Focus on high molecular weight AGEs. Amino Acids 2017, 49, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.-X.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. The Advanced Glycation End Product, Nepsilon-(Carboxymethyl)lysine, Is a Product of both Lipid Peroxidation and Glycoxidation Reactions. J. Biol. Chem. 1996, 271, 9982–9986. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T.; Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Dhar, I.; Prasad, K. Oxidative Stress as a Mechanism of Added Sugar-Induced Cardiovascular Disease. Int. J. Angiol. 2014, 23, 217–226. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Wells-Knecht, K.J.; Brinkmann, E.; Baynes, J.W. Characterization of an Imidazolium Salt Formed from Glyoxal and N.alpha.-Hippuryllysine: A Model for Maillard Reaction Crosslinks in Proteins. J. Org. Chem. 1995, 60, 6246–6247. [Google Scholar] [CrossRef]
- Lorenzi, M. The Polyol Pathway as a Mechanism for Diabetic Retinopathy: Attractive, Elusive, and Resilient. Exp. Diabetes Res. 2007, 2007, 61038. [Google Scholar] [CrossRef] [PubMed]
- Kellow, N.J.; Savige, G.S. Dietary advanced glycation end-product restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: A systematic review. Eur. J. Clin. Nutr. 2013, 67, 239–248. [Google Scholar] [CrossRef]
- Leslie, R.D.G.; Beyan, H.; Sawtell, P.; Boehm, B.O.; Spector, T.D.; Snieder, H. Level of an Advanced Glycated End Product Is Genetically Determined. Diabetes 2003, 52, 2441–2444. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Pricci, F.; Iacobini, C.; Leto, G.; Amadio, L.; Barsotti, P.; Frigeri, L.; Hsu, D.K.; Vlassara, H.; Liu, F.-T.; et al. Accelerated diabetic glomerulopathy in galectin-3/AGE receptor 3 knockout mice. FASEB J. 2001, 15, 2471–2479. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products. Dermato-Endocrinol. 2012, 4, 259–270. [Google Scholar] [CrossRef]
- Much, G.; Thome, J.; Foley, P.; Schinzel, R.; Riederer, P. AGEs in aging and Alzheimers disease. Brain Res. Rev. 1997, 23, 6. [Google Scholar]
- Khan, N.; Bakshi, K.S.; Jaggi, A.S.; Singh, N. Ameliorative Potential of Spironolactone in Diabetes Induced Hyperalgesia in Mice. YAKUGAKU ZASSHI 2009, 129, 593–599. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced Glycation End Products in Foods and a Practical Guide to Their Reduction in the Diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef]
- Uribarri, J.; Del Castillo, M.D.; De La Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Bastos, D.H.M.; Medrano, A.; Menini, T.; et al. Dietary Advanced Glycation End Products and Their Role in Health and Disease. Adv. Nutr. Int. Rev. J. 2015, 6, 461–473. [Google Scholar] [CrossRef]
- Hechtman, L. 209—Polycystic Ovary Syndrome (PCOS). In Textbook of Natural Medicine, 5th ed.; Pizzorno, J.E., Murray, M.T., Eds.; Churchill Livingstone: St. Louis, MO, USA, 2020; pp. 1694–1706.e7. [Google Scholar]
- Delgado-Andrade, C. Carboxymethyl-lysine: Thirty years of investigation in the field of AGE formation. Food Funct. 2015, 7, 46–57. [Google Scholar] [CrossRef]
- Bettiga, A.; Fiorio, F.; Di Marco, F.; Trevisani, F.; Romani, A.; Porrini, E.; Salonia, A.; Montorsi, F.; Vago, R. The Modern Western Diet Rich in Advanced Glycation End-Products (AGEs): An Overview of Its Impact on Obesity and Early Progression of Renal Pathology. Nutrients 2019, 11, 1748. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Helou, C.; Gadonna-Wideham, P.; Robert, N.; Branlard, G.; Thebault, J.; Librere, S.; Jacquot, S.; Mardon, J.; Piquet-Pissaloux, A.; Chapron, S.; et al. The impact of raw materials and baking conditions on Maillard reaction products, thiamine, folate, phytic acid and minerals in white bread. Food Funct. 2016, 7, 2498–2507. [Google Scholar] [CrossRef] [PubMed]
- Hull, G.L.; Woodside, J.V.; Ames, J.M.; Cuskelly, G.J. Nε-(carboxymethyl)lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012, 131, 170–174. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Rajakaruna, S.; Pastoriza, S.; Paliy, O.; Rufián-Henares, J. Bioactivity of food melanoidins is mediated by gut microbiota. Food Chem. 2020, 316, 126309. [Google Scholar] [CrossRef]
- Cerami, C.; Founds, H.; Nicholl, I.; Mitsuhashi, T.; Giordano, D.; Vanpatten, S.; Lee, A.; Al-Abed, Y.; Vlassara, H.; Bucala, R.; et al. Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl. Acad. Sci. USA 1997, 94, 13915–13920. [Google Scholar] [CrossRef]
- Berg, T.J.; Snorgaard, O.; Faber, J.; A Torjesen, P.; Hildebrandt, P.; Mehlsen, J.; Hanssen, K.F. Serum levels of advanced glycation end products are associated with left ventricular diastolic function in patients with type 1 diabetes. Diabetes Care 1999, 22, 1186–1190. [Google Scholar] [CrossRef]
- Gopal, P.; Reynaert, N.L.; Scheijen, J.L.J.M.; Engelen, L.; Schalkwijk, C.G.; Franssen, F.M.; Wouters, E.F.; Rutten, E.P. Plasma advanced glycation end-products and skin autofluorescence are increased in COPD. Eur. Respir. J. 2013, 43, 430–438. [Google Scholar] [CrossRef]
- Nicholl, I.D.; Stitt, A.W.; Moore, J.E.; Ritchie, A.J.; Archer, D.B.; Bucala, R. Increased Levels of Advanced Glycation Endproducts in the Lenses and Blood Vessels of Cigarette Smokers. Mol. Med. 1998, 4, 594–601. [Google Scholar] [CrossRef]
- Kehm, R.; Rückriemen, J.; Weber, D.; Deubel, S.; Grune, T.; Höhn, A. Endogenous advanced glycation end products in pancreatic islets after short-term carbohydrate intervention in obese, diabetes-prone mice. Nutr. Diabetes 2019, 9, 9. [Google Scholar] [CrossRef]
- Snelson, M.; Tan, S.M.; Clarke, R.E.; de Pasquale, C.; Thallas-Bonke, V.; Nguyen, T.-V.; Penfold, S.A.; Harcourt, B.E.; Sourris, K.C.; Lindblom, R.S.; et al. Processed foods drive intestinal barrier permeability and microvascular diseases. Sci. Adv. 2021, 7, eabe4841. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, B.A.; Gokhale, R.; Cho, J.H. Clinical Aspects and Pathophysiology of Inflammatory Bowel Disease. Clin. Microbiol. Rev. 2002, 15, 79–94. [Google Scholar] [CrossRef]
- Loddo, I.; Romano, C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front. Immunol. 2015, 6, 551. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhao, J.; Gregersen, H. Up-Regulated Expression of Advanced Glycation End-Products and Their Receptor in the Small Intestine and Colon of Diabetic Rats. Dig. Dis. Sci. 2011, 57, 48–57. [Google Scholar] [CrossRef]
- Chen, P.-M.; Gregersen, H.; Zhao, J.-B. Advanced glycation end-product expression is upregulated in the gastrointestinal tract of type 2 diabetic rats. World J. Diabetes 2015, 6, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Nagai, R.; Sakashita, N.; Takeya, M.; Horiuchi, S.; Takahashi, K. Immunohistochemical Distribution and Quantitative Biochemical Detection of Advanced Glycation End Products in Fetal to Adult Rats and in Rats with Streptozotocin-Induced Diabetes. Lab. Investig. 2001, 81, 845–861. [Google Scholar] [CrossRef]
- Sparvero, L.J.; Asafu-Adjei, D.; Kang, R.; Tang, D.; Amin, N.; Im, J.; Rutledge, R.; Lin, B.; A Amoscato, A.; Zeh, H.J.; et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE Ligands, and their role in Cancer and Inflammation. J. Transl. Med. 2009, 7, 17. [Google Scholar] [CrossRef]
- Kato, S.; Itoh, K.; Ochiai, M.; Iwai, A.; Park, Y.; Hata, S.; Takeuchi, K.; Ito, M.; Imaki, J.; Miura, S.; et al. Increased pentosidine, an advanced glycation end-product, in urine and tissue reflects disease activity in inflammatory bowel diseases. J. Gastroenterol. Hepatol. 2008, 23, S140–S145. [Google Scholar] [CrossRef]
- Andrassy, M.; Igwe, J.; Autschbach, F.; Volz, C.; Remppis, A.; Neurath, M.F.; Schleicher, E.; Humpert, P.M.; Wendt, T.; Liliensiek, B.; et al. Posttranslationally Modified Proteins as Mediators of Sustained Intestinal Inflammation. Am. J. Pathol. 2006, 169, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R. Role of the advanced glycation end products receptor in Crohn’s disease inflammation. World J. Gastroenterol. 2013, 19, 8269–8281. [Google Scholar] [CrossRef]
- van der Lugt, T.; Weseler, A.R.; Gebbink, W.A.; Vrolijk, M.F.; Opperhuizen, A.; Bast, A. Dietary Advanced Glycation Endproducts Induce an Inflammatory Response in Human Macrophages in Vitro. Nutrients 2018, 10, 1868. [Google Scholar] [CrossRef] [PubMed]
- Tessier, F. The Maillard reaction in the human body. The main discoveries and factors that affect glycation. Pathol. Biol. 2010, 58, 214–219. [Google Scholar] [CrossRef]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Yang, H.; Qiu, P.; Wang, H.; Wang, F.; Zhao, Q.; Fang, J.; Nie, J. Consumption of ultra-processed foods and health outcomes: A systematic review of epidemiological studies. Nutr. J. 2020, 19, 86. [Google Scholar] [CrossRef]
- Ottum, M.S.; Mistry, A.M. Advanced glycation end-products: Modifiable environmental factors profoundly mediate insulin resistance. J. Clin. Biochem. Nutr. 2015, 57, 1–12. [Google Scholar] [CrossRef]
- Boșca, A.B.; Mihu, C.M.; Ilea, A. Advanced Glycation End Products as Biomarkers in Nutrition; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–23. [Google Scholar] [CrossRef]
- Bramhall, M.; Rich, K.; Chakraborty, A.; Logunova, L.; Han, N.; Wilson, J.; McLaughlin, J.; Brass, A.; Cruickshank, S.M. Differential Expression of Soluble Receptor for Advanced Glycation End-products in Mice Susceptible or Resistant to Chronic Colitis. Inflamm. Bowel Dis. 2019, 26, 360–368. [Google Scholar] [CrossRef]
- Body-Malapel, M.; Djouina, M.; Waxin, C.; Langlois, A.; Gower-Rousseau, C.; Zerbib, P.; Schmidt, A.-M.; Desreumaux, P.; Boulanger, E.; Vignal, C. The RAGE signaling pathway is involved in intestinal inflammation and represents a promising therapeutic target for Inflammatory Bowel Diseases. Mucosal Immunol. 2019, 12, 468–478. [Google Scholar] [CrossRef]
- McDaniel, D.; Eden, K.; Ringel, V.M.; Allen, I.C. Emerging Roles for Noncanonical NF-κB Signaling in the Modulation of Inflammatory Bowel Disease Pathobiology. Inflamm. Bowel Dis. 2016, 22, 2265–2279. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle∥, P.A.; Schölmerich, J.; Gross, V. Nuclear factor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998, 115, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Nikolaus, S.; Hampe, J. Activation of nuclear factor κB in inflammatory bowel disease. Gut 1998, 42, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ouyang, Y.; Lu, N.; Li, N. The NF-κB Signaling Pathway, the Microbiota, and Gastrointestinal Tumorigenesis: Recent Advances. Front. Immunol. 2020, 11, 1387. [Google Scholar] [CrossRef]
- Nass, N.; Bayreuther, K.; Simm, A. Systemic activation of NF-κB driven luciferase activity in transgenic mice fed advanced glycation end products modified albumin. Glycoconj. J. 2017, 34, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.; Fritz, G.; Vetter, S.W.; Heizmann, C.W. Binding of S100 proteins to RAGE: An update. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2009, 1793, 993–1007. [Google Scholar] [CrossRef]
- Zwadlo, G.; Brüggen, J.; Gerhards, G.; Schlegel, R.; Sorg, C. Two calcium-binding proteins associated with specific stages of myeloid cell differentiation are expressed by subsets of macrophages in inflammatory tissues. Clin. Exp. Immunol. 1988, 72, 510–515. [Google Scholar]
- Xia, C.; Braunstein, Z.; Toomey, A.C.; Zhong, J.; Rao, X. S100 Proteins As an Important Regulator of Macrophage Inflammation. Front. Immunol. 2018, 8, 1908. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Ren, Z.; Turton, J.; Pang, G.; Daebritz, J.; Ehrchen, J.; Heidemann, J.; Borody, T.; Roth, J.; et al. Phagocyte-specific S100 proteins are released from affected mucosa and promote immune responses during inflammatory bowel disease. J. Pathol. 2008, 216, 183–192. [Google Scholar] [CrossRef]
- Leach, S.T.; Yang, Z.; Messina, I.; Song, C.; Geczy, C.L.; Cunningham, A.M.; Day, A.S. Serum and mucosal S100 proteins, calprotectin (S100A8/S100A9) and S100A12, are elevated at diagnosis in children with inflammatory bowel disease. Scand. J. Gastroenterol. 2007, 42, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Grosso, M.; Petruzzelli, R.; Izzo, P.; Calì, G.; D’Armiento, F.P.; Rocco, A.; Naradone, G.; et al. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol. Motil. 2009, 21, 1209-e112. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Cirillo, C.; Sarnelli, G.; De Filippis, D.; D’Armiento, F.P.; Rocco, A.; Nardone, G.; Petruzzelli, R.; Grosso, M.; Izzo, P.; et al. Enteric Glial-Derived S100B Protein Stimulates Nitric Oxide Production in Celiac Disease. Gastroenterology 2007, 133, 918–925. [Google Scholar] [CrossRef]
- Cirillo, C. S100B protein in the gut: The evidence for enteroglial-sustained intestinal inflammation. World J. Gastroenterol. 2011, 17, 1261–1266. [Google Scholar] [CrossRef]
- Srikrishna, G.; Turovskaya, O.; Shaikh, R.; Newlin, R.; Foell, D.; Murch, S.; Kronenberg, M.; Freeze, H.H. Carboxylated glycans mediate colitis through activation of NF-κB. J. Immunol. 2005, 175, 5412–5422. [Google Scholar] [CrossRef]
- Palone, F.; Vitali, R.; Cucchiara, S.; Pierdomenico, M.; Negroni, A.; Aloi, M.; Nuti, F.; Felice, C.; Armuzzi, A.; Stronati, L. Role of HMGB1 as a Suitable Biomarker of Subclinical Intestinal Inflammation and Mucosal Healing in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2014, 20, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, L.; Khan, M.N.; Shi, L.; Wang, Z.; Zheng, F.; Gong, F.; Fang, M. HMGB1 exacerbates experimental mouse colitis by enhancing innate lymphoid cells 3 inflammatory responses via promoted IL-23 production. J. Endotoxin Res. 2016, 22, 696–705. [Google Scholar] [CrossRef]
- Stavely, R.; Sahakian, L.; Filippone, R.T.; Stojanovska, V.; Bornstein, J.C.; Sakkal, S.; Nurgali, K. Oxidative Stress-Induced HMGB1 Translocation in Myenteric Neurons Contributes to Neuropathy in Colitis. Biomolecules 2022, 12, 1831. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, D.; Sun, L. Clinical Significance of High-Mobility Group Box 1 Protein (HMGB1) and Nod-Like Receptor Protein 3 (NLRP3) in Patients with Ulcerative Colitis. J. Pharmacol. Exp. Ther. 2020, 26, e919530. [Google Scholar] [CrossRef] [PubMed]
- Palone, F.; Vitali, R.; Cucchiara, S.; Mennini, M.; Armuzzi, A.; Pugliese, D.; D’incà, R.; Barberio, B.; Stronati, L. Fecal HMGB1 Reveals Microscopic Inflammation in Adult and Pediatric Patients with Inflammatory Bowel Disease in Clinical and Endoscopic Remission. Inflamm. Bowel Dis. 2016, 22, 2886–2893. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Inagaki, Y.; Kido, J.; Nagata, T. Advanced glycation end products increase expression of S100A8 and A9viaRAGE-MAPK in rat dental pulp cells. Oral Dis. 2014, 21, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Toyomura, T.; Tomiyama, M.; Wake, H.; Liu, K.; Teshigawara, K.; Takahashi, H.; Nishibori, M.; Mori, S. Advanced glycation end products (AGEs) synergistically potentiated the proinflammatory action of lipopolysaccharide (LPS) and high mobility group box-1 (HMGB1) through their direct interactions. Mol. Biol. Rep. 2020, 47, 7153–7159. [Google Scholar] [CrossRef]
- Shangari, N.; Depeint, F.; Furrer, R.; Bruce, W.R.; Popovic, M.; Zheng, F.; O’Brien, P.J. A thermolyzed diet increases oxidative stress, plasma α-aldehydes and colonic inflammation in the rat. Chem.-Biol. Interact. 2007, 169, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Šebeková, K.; Saavedra, G.; Zumpe, C.; Somoza, V.; Klenovicsová, K.; Birlouez-Aragon, I. Plasma Concentration and Urinary Excretion of Nɛ-(Carboxymethyl)lysine in Breast Milk- and Formula-fed Infants. Ann. N. Y. Acad. Sci. 2008, 1126, 177–180. [Google Scholar] [CrossRef]
- Siljander, H.; Jason, E.; Ruohtula, T.; Selvenius, J.; Koivusaari, K.; Salonen, M.; Ahonen, S.; Honkanen, J.; Ilonen, J.; Vaarala, O.; et al. Effect of Early Feeding on Intestinal Permeability and Inflammation Markers in Infants with Genetic Susceptibility to Type 1 Diabetes: A Randomized Clinical Trial. J. Pediatr. 2021, 238, 305–311.e3. [Google Scholar] [CrossRef]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferruci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Cai, W.; Tripp, E.; Pyzik, R.; Yee, K.; Goldberg, L.; Tansman, L.; Chen, X.; Mani, V.; Fayad, Z.A.; et al. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: A randomised controlled trial. Diabetologia 2016, 59, 2181–2192. [Google Scholar] [CrossRef]
- Uribarri, J.; Cai, W.; Ramdas, M.; Goodman, S.; Pyzik, R.; Chen, X.; Zhu, L.; Striker, G.E.; Vlassara, H. Restriction of Advanced Glycation End Products Improves Insulin Resistance in Human Type 2 Diabetes. Diabetes Care 2011, 34, 1610–1616. [Google Scholar] [CrossRef]
- Yacoub, R.; Nugent, M.; Cai, W.; Nadkarni, G.N.; Chaves, L.D.; Abyad, S.; Honan, A.M.; Thomas, S.A.; Zheng, W.; Valiyaparambil, S.A.; et al. Advanced glycation end products dietary restriction effects on bacterial gut microbiota in peritoneal dialysis patients; a randomized open label controlled trial. PLoS ONE 2017, 12, e0184789. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, W.; Yu, J.; Liu, H.; He, S.; Zhu, L.; Xu, J. Dietary Advanced Glycation End Products Shift the Gut Microbiota Composition and Induce Insulin Resistance in Mice. Diabetes Metab. Syndr. Obesity Targets Ther. 2022, 2022, 427–437. [Google Scholar] [CrossRef]
- Luévano-Contreras, C.; Garay-Sevilla, M.E.; Wrobel, K.; Malacara, J.M.; Wrobel, K. Dietary advanced glycation end products restriction diminishes inflammation markers and oxidative stress in patients with type 2 diabetes mellitus. J. Clin. Biochem. Nutr. 2013, 52, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Linkens, A.M.; Houben, A.J.; Niessen, P.M.; Wijckmans, N.E.; de Goei, E.E.; Eynde, M.D.V.D.; Scheijen, J.L.; van den Waarenburg, M.P.; Mari, A.; Berendschot, T.T.; et al. A 4-week high-AGE diet does not impair glucose metabolism and vascular function in obese individuals. J. Clin. Investig. 2022, 7, e156950. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.B.; Poulsen, M.W.; Andersen, S.; Andersen, J.M.; Bak, M.J.; Ritz, C.; Holst, J.J.; Nielsen, J.; de Courten, B.; Dragsted, L.O.; et al. Consumption of a Diet Low in Advanced Glycation End Products for 4 Weeks Improves Insulin Sensitivity in Overweight Women. Diabetes Care 2013, 37, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ott, B.; Skurk, T.; Hastreiter, L.; Lagkouvardos, I.; Fischer, S.; Büttner, J.; Kellerer, T.; Clavel, T.; Rychlik, M.; Haller, D.; et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci. Rep. 2017, 7, 11955. [Google Scholar] [CrossRef] [PubMed]
- Adolph, T.E.; Zhang, J. Diet fuelling inflammatory bowel diseases: Preclinical and clinical concepts. Gut 2022, 71, 2574–2586. [Google Scholar] [CrossRef]
- Sbierski-Kind, J.; Grenkowitz, S.; Schlickeiser, S.; Sandforth, A.; Friedrich, M.; Kunkel, D.; Glauben, R.; Brachs, S.; Mai, K.; Thürmer, A.; et al. Effects of caloric restriction on the gut microbiome are linked with immune senescence. Microbiome 2022, 10, 57. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Snelson, M.; Lucut, E.; Coughlan, M.T. The Role of AGE-RAGE Signalling as a Modulator of Gut Permeability in Diabetes. Int. J. Mol. Sci. 2022, 23, 1766. [Google Scholar] [CrossRef]
- Foell, D.; Kucharzik, T.; Kraft, M.; Vogl, T.; Sorg, C.; Domschke, W.; Roth, J. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut 2003, 52, 847–853. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef]
- Baye, E.; Kiriakova, V.; Uribarri, J.; Moran, L.J.; de Courten, B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: Meta-analysis of randomised controlled trials. Sci. Rep. 2017, 7, 2266. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, R.; Dragonas, C.; Kannenkeril, D.; Hoffmann, I.; Mueller, A.; Beckmann, M.W.; Pischetsrieder, M. A diet rich in Maillard reaction products protects LDL against copper induced oxidation ex vivo, a human intervention trial. Food Res. Int. 2009, 42, 1315–1322. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Buckley, A.; Turner, J.R. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harb. Perspect. Biol. 2017, 10, a029314. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Papageorgiou, I.; Charonis, A. Enterocytes’ tight junctions: From molecules to diseases. World J. Gastrointest. Pathophysiol. 2011, 2, 123–137. [Google Scholar] [CrossRef]
- Bhor, V.; Sivakami, S. Regional variations in intestinal brush border membrane fluidity and function during diabetes and the role of oxidative stress and non-enzymatic glycation. Mol. Cell. Biochem. 2003, 252, 125–132. [Google Scholar] [CrossRef]
- Kim, K.A.; Jung, J.H.; Kang, I.G.; Choi, Y.S.; Kim, S.T. ROS Is Involved in Disruption of Tight Junctions of Human Nasal Epithelial Cells Induced by HRV16. Laryngoscope 2018, 128, E393–E401. [Google Scholar] [CrossRef] [PubMed]
- Kellow, N.; Coughlan, M.T. Effect of diet-derived advanced glycation end products on inflammation. Nutr. Rev. 2015, 73, 737–759. [Google Scholar] [CrossRef]
- Guibourdenche, M.; Haug, J.; Chevalier, N.; Spatz, M.; Barbezier, N.; Gay-Quéheillard, J.; Anton, P.M. Food Contaminants Effects on an In Vitro Model of Human Intestinal Epithelium. Toxics 2021, 9, 135. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, X.-H. Influence of the Maillard-type caseinate glycation with lactose on the intestinal barrier activity of the caseinate digest in IEC-6 cells. Food Funct. 2019, 10, 2010–2021. [Google Scholar] [CrossRef]
- Shi, J.; Fu, Y.; Zhao, X.; Lametsch, R. Glycation sites and bioactivity of lactose-glycated caseinate hydrolysate in lipopolysaccharide-injured IEC-6 cells. J. Dairy Sci. 2021, 104, 1351–1363. [Google Scholar] [CrossRef]
- Qu, W.; Yuan, X.; Zhao, J.; Zhang, Y.; Hu, J.; Wang, J.; Li, J. Dietary advanced glycation end products modify gut microbial composition and partially increase colon permeability in rats. Mol. Nutr. Food Res. 2017, 61, 1700118. [Google Scholar] [CrossRef]
- Pendyala, S.; Walker, J.M.; Holt, P.R. A High-Fat Diet Is Associated With Endotoxemia That Originates From the Gut. Gastroenterology 2012, 142, 1100–1101.e2. [Google Scholar] [CrossRef]
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- Kitaura, A.; Nishinaka, T.; Hamasaki, S.; Hatipoglu, O.F.; Wake, H.; Nishibori, M.; Mori, S.; Nakao, S.; Takahashi, H. Advanced glycation end-products reduce lipopolysaccharide uptake by macrophages. PLoS ONE 2021, 16, e0245957. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Furness, J.B.; Nguyen, T.V.; Nurgali, K.; Shimizu, Y. The Enteric Nervous System and Its Extrinsic Connections. In Textbook of Gastroenrerology; Wiley: Hoboken, NJ, USA, 2008; pp. 15–39. [Google Scholar] [CrossRef]
- Furness, J.B. The Enteric Nervous System; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Furness, J.B. Enteric Nervous System. In Encyclopedia of Neuroscience; Binder, M.D., Hirokawa, N., Windhorst, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1122–1125. [Google Scholar]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.-J. The Enteric Nervous System and Gastrointestinal Innervation: Integrated Local and Central Control. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Springer: Berlin, Germany, 2014; Volume 817, pp. 39–71. [Google Scholar] [CrossRef]
- Aube, A.-C.; Cabarrocas, J.; Bauer, J.; Philippe, D.; Aubert, P.; Doulay, F.; Liblau, R.; Galmiche, J.P.; Neunlist, M. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut 2006, 55, 630–637. [Google Scholar] [CrossRef]
- Schemann, M.; Michel, K.; Ceregrzyn, M.; Zeller, F.; Seidl, S.; Bischoff, S.C. Human mast cell mediator cocktail excites neurons in human and guinea-pig enteric nervous system. Neurogastroenterol. Motil. 2004, 17, 281–289. [Google Scholar] [CrossRef]
- De Giorgio, R.; Bianco, F.; Latorre, R.; Caio, G.; Clavenzani, P.; Bonora, E. Enteric neuropathies: Yesterday, Today and Tomorrow. Enteric Nerv. Syst. 2016, 891, 123–133. [Google Scholar] [CrossRef]
- Korenaga, K.; Micci, M.-A.; Taglialatela, G.; Pasricha, P.J. Suppression of nNOS expression in rat enteric neurones by the receptor for advanced glycation end-products. Neurogastroenterol. Motil. 2006, 18, 392–400. [Google Scholar] [CrossRef]
- Moro, M.; Cárdenas, A.; Hurtado, O.; Leza, J.; Lizasoain, I. Role of nitric oxide after brain ischaemia. Cell Calcium 2004, 36, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Love, S. Oxidative Stress in Brain Ischemia. Brain Pathol. 2006, 9, 119–131. [Google Scholar] [CrossRef]
- Mallick, I.H.; Yang, W.; Winslet, M.C.; Seifalian, A.M. REVIEW: Ischemia–Reperfusion Injury of the Intestine and Protective Strategies Against Injury. Dig. Dis. Sci. 2004, 49, 1359–1377. [Google Scholar] [CrossRef]
- Jeyabal, P.V.S.; Kumar, R.; Gangula, P.R.R.; Micci, M.-A.; Pasricha, P.J. Inhibitors of advanced glycation end-products prevent loss of enteric neuronal nitric oxide synthase in diabetic rats. Neurogastroenterol. Motil. 2008, 20, 253–261. [Google Scholar] [CrossRef]
- Voukali, E.; Shotton, H.R.; Lincoln, J. Selective responses of myenteric neurons to oxidative stress and diabetic stimuli. Neurogastroenterol. Motil. 2011, 23, 964-e411. [Google Scholar] [CrossRef]
- Sharkey, K.A. Emerging roles for enteric glia in gastrointestinal disorders. J. Clin. Investig. 2015, 125, 918–925. [Google Scholar] [CrossRef]
- Hellwig, M.; Bunzel, D.; Huch, M.; Franz, C.M.A.P.; Kulling, S.E.; Henle, T. Stability of Individual Maillard Reaction Products in the Presence of the Human Colonic Microbiota. J. Agric. Food Chem. 2015, 63, 6723–6730. [Google Scholar] [CrossRef]
- Seiquer, I.; Rubio, L.A.; Peinado, M.J.; Delgado-Andrade, C.; Navarro, M.P. Maillard reaction products modulate gut microbiota composition in adolescents. Mol. Nutr. Food Res. 2014, 58, 1552–1560. [Google Scholar] [CrossRef]
- Tannock, G.W. A Special Fondness for Lactobacilli. Appl. Environ. Microbiol. 2004, 70, 3189–3194. [Google Scholar] [CrossRef]
- Qin, D.; Ma, Y.; Wang, Y.; Hou, X.; Yu, L. Contribution of Lactobacilli on Intestinal Mucosal Barrier and Diseases: Perspectives and Challenges of Lactobacillus casei. Life 2022, 12, 1910. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, Y.K.; Sun, Y.; Ip, L.Y.T.; Wang, L.; Chan, F.K.L.; Miao, Y.; Ng, S.C. Prevotella species in the human gut is primarily comprised of Prevotella copri, Prevotella stercorea and related lineages. Sci. Rep. 2022, 12, 9055. [Google Scholar] [CrossRef]
- Qu, W.; Nie, C.; Zhao, J.; Ou, X.; Zhang, Y.; Yang, S.; Bai, X.; Wang, Y.; Wang, J.; Li, J. Microbiome–Metabolomics Analysis of the Impacts of Long-Term Dietary Advanced-Glycation-End-Product Consumption on C57BL/6 Mouse Fecal Microbiota and Metabolites. J. Agric. Food Chem. 2018, 66, 8864–8875. [Google Scholar] [CrossRef]
- Marungruang, N.; Fåk, F.; Tareke, E. Heat-treated high-fat diet modifies gut microbiota and metabolic markers in apoe−/− mice. Nutr. Metab. 2016, 13, 22. [Google Scholar] [CrossRef]
- Moschen, A.R.; Gerner, R.R.; Wang, J.; Klepsch, V.; E Adolph, T.; Reider, S.J.; Hackl, H.; Pfister, A.; Schilling, J.; Moser, P.L.; et al. Lipocalin 2 Protects from Inflammation and Tumorigenesis Associated with Gut Microbiota Alterations. Cell Host Mircrobe 2016, 19, 455–469. [Google Scholar] [CrossRef]
- Mao, Z.; Ren, Y.; Zhang, Q.; Dong, S.; Han, K.; Feng, G.; Wu, H.; Zhao, Y. Glycated fish protein supplementation modulated gut microbiota composition and reduced inflammation but increased accumulation of advanced glycation end products in high-fat diet fed rats. Food Funct. 2019, 10, 3439–3451. [Google Scholar] [CrossRef]
- Fung, K.Y.C.; Cosgrove, L.; Lockett, T.; Head, R.; Topping, D.L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br. J. Nutr. 2012, 108, 820–831. [Google Scholar] [CrossRef]
- Topping, D.L.; Fukushima, M.; Bird, A.R. Resistant starch as a prebiotic and synbiotic: State of the art. Proc. Nutr. Soc. 2003, 62, 171–176. [Google Scholar] [CrossRef]
- Mastrocola, R.; Collotta, D.; Gaudioso, G.; Le Berre, M.; Cento, A.; Ferreira, G.A.; Chiazza, F.; Verta, R.; Bertocchi, I.; Manig, F.; et al. Effects of Exogenous Dietary Advanced Glycation End Products on the Cross-Talk Mechanisms Linking Microbiota to Metabolic Inflammation. Nutrients 2020, 12, 2497. [Google Scholar] [CrossRef] [PubMed]
- Linkens, A.M.A.; van Best, N.; Niessen, P.M.; Wijckmans, N.E.G.; de Goei, E.E.C.; Scheijen, J.L.J.M.; van Dongen, M.C.J.M.; van Gool, C.C.J.A.W.; de Vos, W.M.; Houben, A.J.H.M.; et al. A 4-Week Diet Low or High in Advanced Glycation Endproducts Has Limited Impact on Gut Microbial Composition in Abdominally Obese Individuals: The deAGEing Trial. Int. J. Mol. Sci. 2022, 23, 5328. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Kim, M.; Bennett, B.J. Modulating the Microbiota as a Therapeutic Intervention for Type 2 Diabetes. Front. Endocrinol. 2021, 12, 632335. [Google Scholar] [CrossRef]
- Cui, J.; Ramesh, G.; Wu, M.; Jensen, E.T.; Crago, O.; Bertoni, A.G.; Gao, C.; Hoffman, K.L.; Sheridan, P.A.; Wong, K.E.; et al. Butyrate-Producing Bacteria and Insulin Homeostasis: The Microbiome and Insulin Longitudinal Evaluation Study (MILES). Diabetes 2022, 71, 2438–2446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, S.; Li, H.; Zhang, Z.; Zhang, Q.; Chen, L.; Zhao, Y.; Chen, Y.; Gu, J.; Min, L.; et al. Identification of Gut Microbiota and Metabolites Signature in Patients With Irritable Bowel Syndrome. Front. Cell. Infect. Microbiol. 2019, 9, 346. [Google Scholar] [CrossRef]
- Dai, L.; Tang, Y.; Zhou, W.; Dang, Y.; Sun, Q.; Tang, Z.; Zhu, M.; Ji, G. Gut Microbiota and Related Metabolites Were Disturbed in Ulcerative Colitis and Partly Restored After Mesalamine Treatment. Front. Pharmacol. 2021, 11, 620724. [Google Scholar] [CrossRef]
- Olaisen, M.; Flatberg, A.; Granlund, A.V.B.; Røyset, E.S.; Martinsen, T.C.; Sandvik, A.K.; Fossmark, R. Bacterial Mucosa-associated Microbiome in Inflamed and Proximal Noninflamed Ileum of Patients With Crohn’s Disease. Inflamm. Bowel Dis. 2020, 27, 12–24. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, L.; Song, Y.; Wu, Y.; Zhang, Y.; Wang, S. Preventive effects of polysaccharides from Physalis alkekengi L. on dietary advanced glycation end product-induced insulin resistance in mice associated with the modulation of gut microbiota. Int. J. Biol. Macromol. 2022, 204, 204–214. [Google Scholar] [CrossRef]
- van Dongen, K.C.; Linkens, A.M.; Wetzels, S.M.; Wouters, K.; Vanmierlo, T.; van de Waarenburg, M.P.; Scheijen, J.L.; de Vos, W.M.; Belzer, C.; Schalkwijk, C.G. Dietary advanced glycation endproducts (AGEs) increase their concentration in plasma and tissues, result in inflammation and modulate gut microbial composition in mice; evidence for reversibility. Food Res. Int. 2021, 147, 110547. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuong-Nguyen, K.; McNeill, B.A.; Aston-Mourney, K.; Rivera, L.R. Advanced Glycation End-Products and Their Effects on Gut Health. Nutrients 2023, 15, 405. https://doi.org/10.3390/nu15020405
Phuong-Nguyen K, McNeill BA, Aston-Mourney K, Rivera LR. Advanced Glycation End-Products and Their Effects on Gut Health. Nutrients. 2023; 15(2):405. https://doi.org/10.3390/nu15020405
Chicago/Turabian StylePhuong-Nguyen, Kate, Bryony A. McNeill, Kathryn Aston-Mourney, and Leni R. Rivera. 2023. "Advanced Glycation End-Products and Their Effects on Gut Health" Nutrients 15, no. 2: 405. https://doi.org/10.3390/nu15020405
APA StylePhuong-Nguyen, K., McNeill, B. A., Aston-Mourney, K., & Rivera, L. R. (2023). Advanced Glycation End-Products and Their Effects on Gut Health. Nutrients, 15(2), 405. https://doi.org/10.3390/nu15020405





