Abstract
(1) Background: For the storage of human milk (HM), freezing, thawing, and/or pasteurization are routinely used in neonatal intensive care units. We aimed to analyze the effects of different HM processing types on the nutritional contents in HM, adipose tissue, and the neuroprotection markers leptin and adiponectin. (2) Methods: HM samples from 136 mothers of preterm and term infants (gestational age 23 + 0 to 41 + 6) were collected and divided into four groups: (i) fresh HM, (ii) fresh pasteurized HM, (iii) thawed HM, and (iv) thawed pasteurized HM. The macronutrients were analyzed by mid-infrared transmission spectroscopy and the adiponectin and leptin were analyzed by high-sensitivity adiponectin and leptin enzyme-linked immunosorbent assay (ELISA). (3) Results: No significant differences were observed in the protein, carbohydrate, or fat concentrations between the HM processing types. The leptin levels were significantly lower after pasteurization in comparison to HM without pasteurization (p < 0.001). The protein levels in extremely preterm HM were significantly lower compared to those in moderate/late preterm HM and term HM (p < 0.05). (4) Conclusions: HM processing had an impact on leptin concentrations but no effect on the protein level. These data support the use of unpasteurized human milk for preterm infants’ nutrition and normal brain development. The protein levels of the milk of mothers from preterm compared to full-term infants differed, underlining the importance of individualized target fortification.
1. Introduction
Human milk (HM) is recommended for all infants and is the best source of nutrition for preterm and term infants. In particular, it is essential for preterm infants and supports the transition from intrauterine to extrauterine life. HM provides all the necessary nutrients the infant needs during the first months of life and encourages infants’ optimal growth and the development of organs and the immune system. The unique properties of HM, such as immunomodulatory, bioactive, and growth factors, digestive enzymes, hormones, prebiotics, and non-antibody mediated immune factors, enhance growth and metabolic maturation [1,2,3,4,5,6,7].
The macronutrient contents of HM are highly variable between mothers and maternal factors during pregnancy and after birth over the course of lactation, and these factors might affect the nutrient composition. HM contents are different from those in colostrum and differ between transitional and mature HM and between hindmilk and foremilk. Infant characteristics, such as birth weight, height, and length, gender of the infant, and gestational and postnatal age might play a role in the high variability of HM contents [3,8,9,10,11,12]. The comparison of preterm and term HM, and therefore, gestational age (GA), might be an important predictor of HM composition. However, previous studies have evaluated the composition of HM in preterm and term infants with inconsistent results. In a systematic review and meta-analysis, Gidrewicz and Fenton showed that gestational age was a major predictor of HM composition [8]. However, the impact of gestational age at birth on leptin and adiponectin concentrations has not yet been investigated and is a secondary aim of the current study. Detailed information on the composition of HM is of high interest in the care of preterm neonates because this would allow targeted nutritional management and individualized fortification concepts. These concepts in neonatal intensive care medicine are focused on optimizing nutritional management to improve long-term health outcomes in this vulnerable group of patients.
Studies have shown that the implementation of individualized target fortification is feasible and safe in clinical routines and has improved growth in preterm infants [13,14]. To implement individualized target fortification concepts in clinical routines, neonatologists need to know the daily nutritional contents of HM. The macronutrient contents, especially the protein levels, are substantial, as the requirements for preterm infants are higher compared to term infants [15]. Moreover, protein is the key nutrient for infant development, growth, and neurodevelopment [16,17,18]. In particular, in infants with extremely low birth weight, attention must be paid to their protein intake.
HM contains the hormones leptin and adiponectin. Adiponectin and leptin are primarily produced in the white adipose tissue, and serum leptin correlates with total body fat mass [19,20]. Adiponectin is highly concentrated in HM [21], and higher adiponectin levels in HM are associated with a higher fat mass [22]. The peptide hormone leptin regulates energy balance and suppresses food intake in adults [20,23]. Furthermore, it has been shown to play an important role in preterm infants’ growth and brain development [24,25]. In utero, high amounts of leptin are transferred from the placenta to the fetus in the last trimester [23]. Studies have demonstrated that the neurotrophic factor leptin dramatically decreases after preterm delivery [23,26]. Additionally, preterm infants have reduced leptin production, and, therefore, extremely preterm infants are at especially high risk for leptin deficiency [19,23]. Leptin receptors are widely distributed in the brain and affect brain development [26,27]. Several animal studies have demonstrated that leptin administration was associated with hypothalamic enlargement and improved neurodevelopment [24,25,28]. However, the mother’s own milk provides the optimum nutrition for preterm infants, and if it is not available, pasteurized donor HM is the best alternative. A previous study found that HM leptin concentrations were correlated with maternal serum concentrations [29]. An exploratory study with a small sample size showed that holder pasteurization had an impact on leptin concentrations in a combined study cohort with human breast milk and term donor HM samples [30]. However, the effect of HM processing (freezing, thawing, and/or pasteurization) on preterm mothers’ own breast milk alone has not thus far been investigated. The aim of the study was to investigate the effect of different human milk processing types on adiponectin and leptin concentrations in the mother’s own HM.
For the storage of HM samples, freezing, thawing, and/or pasteurization are routinely used in neonatal intensive care units. In neonatology, the processing of HM is widely used to reduce microbiological germs. Pasteurization eliminates microorganisms and decreases the protein, immunoglobulin A (IgA), lactoferrin, and energy concentrations in HM. Freezing decreases viral and bacterial activity. The freezing step and subsequent thawing decrease digestive enzymes such as lipases, amylases, and proteases [31,32].
The primary aim of our study was to analyze the effects of different processing types on the nutritional contents in HM, with a special focus on protein. The secondary aim was to investigate the effects of different HM processing types on leptin and adiponectin concentrations. Moreover, we aimed to determine the differences between preterm and term HM composition.
2. Materials and Methods
2.1. Recruitment
All mothers aged between 18 and 50 years who were breastfeeding or pumping for their preterm or term infant at the General Hospital of Vienna, Medical University of Vienna, Vienna, Austria were eligible for the study. Mothers with mastitis and mothers with insufficient milk production caused by medication, maternal illness (e.g., Sheehan’s syndrome, hypothyroidism, prolactinoma, and polycystic ovarian syndrome), maternal stress, and substance abuse were excluded. In addition, mothers of infants with gastrointestinal diseases, such as Hirschsprung disease, chronic inflammatory bowel disease, congenital heart disease, major congenital birth defects, and chromosomal aberrations were not recruited.
2.2. Study Design
In this prospective, monocentric study, we aimed to analyze the effects of HM processing on the HM composition of preterm and term lactating mothers. A total of 136 HM samples were collected from lactating mothers of preterm and term infants from March 2016 to July 2022. In total, 544 HM samples were analyzed. The human milk samples were divided into four groups according to the type of processing performed:
- (i)
- Fresh HM;
- (ii)
- Fresh pasteurized HM;
- (iii)
- Thawed HM;
- (iv)
- Thawed pasteurized HM.
The following diagram summarizes all study procedures within the project (Figure 1).
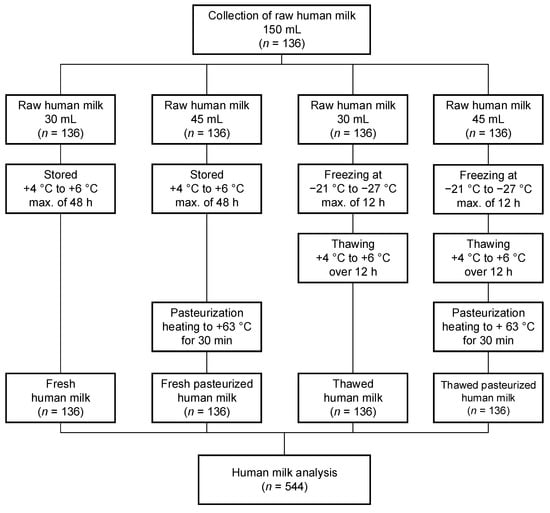
Figure 1.
Description of study design. max., maximum.
This study was approved by the local ethics committee (approval number 2022/2015, 05/FEB/2016) of the Medical University of Vienna. All lactating mothers gave their prior written informed consent.
2.3. Human Milk Collection
During this study, HM samples were collected in sterile feeding bottles from lactating mothers. Overall, an HM sample volume of approximately 150 mL was needed from each lactating mother. The volume of 150 mL was expressed within 24 h and was pooled into one sample per mother. The mothers expressed their HM samples directly in the ward in the hospital, and discharged mothers were advised to transport their HM samples in a cooling bag to ensure the cold chain continued at all times up to their transportation to the hospital. Each 150 mL HM sample was divided into four sterile bottles in the following volumes to determine the (i) fresh HM: 30 mL, (ii) fresh pasteurized HM: 45 mL, (iii) thawed HM: 30 mL, and (iv) thawed pasteurized HM: 45 mL (Figure 1). After the different processing types were carried out, only 3 mL was needed to conduct a measurement of an HM sample with the Miris Human Milk AnalyzerTM (Miris AB, Uppsala, Sweden). Duplicate measurements were carried out for each sample with this device; therefore, about 6 mL was needed. For the adiponectin and leptin measurements, only a volume of 1–2 mL HM was required. For both parameters, a double measurement was performed.
2.4. Human Milk Processing
The donated HM samples of each mother underwent the same processing steps in the milk kitchen at the Department of Pediatrics and Adolescent Medicine at the Medical University of Vienna. Each HM sample was divided into four groups according to the different processing types:
- (i).
- Fresh HM: the expressed raw HM samples were stored at +4 to +6 degrees Celsius in the refrigerator for a maximum of 48 h;
- (ii).
- Fresh pasteurized HM: the expressed raw HM samples were stored at +4 to +6 degrees Celsius in the refrigerator for a maximum of 48 h and were pasteurized by heating the samples to +63 degrees Celsius for thirty minutes with the Barkey clinitherm Pasteur XPT (Barkey GmbH & Co. KG, Leopoldshöhe, Germany);
- (iii).
- Thawed HM: the expressed raw HM samples were frozen at −21 to −27 degrees Celsius for a maximum of 12 h, followed by thawing at +4 to +6 degrees Celsius in the refrigerator over twelve hours;
- (iv).
- Thawed pasteurized HM: the expressed raw HM samples were frozen at −21 to −27 degrees Celsius for a maximum of 12 h, followed by thawing at +4 to +6 degrees Celsius in the refrigerator over 12 h, and additionally followed by pasteurization by heating the samples to +63 degrees Celsius for thirty minutes with the Barkey clinitherm Pasteur XPT (Barkey GmbH & Co. KG, Leopoldshöhe, Germany).
2.5. Human Milk Analysis
The Miris Ultrasonic ProcessorTM, the Miris HeaterTM, and the Miris Human Milk AnalyzerTM (Miris AB, Uppsala, Sweden) were used for the HM analysis according to the manufacturer’s operating instructions (MIRIS AB, Uppsala, Sweden) [33,34,35]. The Miris Ultrasonic ProcessorTM was utilized for the homogenization of the milk samples based on high-frequency ultrasonic waves [35]. The Miris HeaterTM is a water-free system and was applied to heat the milk samples to the optimum temperature for analyses [34]. The HM composition was evaluated with the Miris Human Milk AnalyzerTM (HMA). The HMA is an analytical instrument and is designed for the bedside analysis of HM based on a combination of mid-infrared (IR) transmission spectroscopy for the direct determination of the nutritional contents of HM [33]. The HM composition was calculated on the basis of spectral content. The HMA analyzed the true protein (g/100 mL), crude protein (g/100 mL), fat (g/100 mL), and carbohydrate (g/100 mL) levels in the HM samples. Further, the total solids (g/100 mL) and energy (kcal/100 mL) were calculated. For one measurement, only 3 mL was needed. The HM samples were measured twice, and the average value was taken.
The leptin and adiponectin concentrations were analyzed in the HM samples using the respective leptin and high-sensitivity adiponectin enzyme-linked immunosorbent assay (ELISA) kits purchased from BioVendor (Brno, Czech Republic). An application protocol was used, which was proven for leptin and adiponectin measurements in HM. Assay procedures were performed as recommended by the manufacturer, and the samples were analyzed in duplicate. For the leptin assay, the samples used were undiluted, whereas the adiponectin was analyzed in HM samples diluted 3× in dilution buffer [36,37,38].
2.6. Statistical Methods
The primary objective of the present study was to determine the protein contents of HM under different types of HM processing. In addition, the secondary objectives were related to the fat, carbohydrate, energy, total solids, adiponectin, and leptin contents of HM under different types of HM processing. The results of the primary and secondary objectives are expressed as the median and range or mean and standard deviation (SD) in the tables and the text. Given the non-normal distribution of the data, all comparisons were performed using nonparametric tests. The Kruskal-Wallis ANOVA was followed by a Mann-Whitney U-test for a comparison of the protein, fat, carbohydrate, energy, total solids, adiponectin, and leptin levels under different HM processing methods. The nutritional milk compositions of the four different processing types were compared by a Friedman test. The Mann-Whitney U-test was used to compare differences in the HM composition between the gestational age (GA) groups. Data analysis was carried out using the Statistical Package for Social Science (SPSS) for Windows (version 26.0, SPSS Inc., Chicago, IL, USA). A p-value of < 0.05 was considered statistically significant.
2.7. Sample Size Calculation
The primary question was whether there was a distinction between different processing groups of HM samples of preterm and term lactating mothers in the means of protein. A sample size of 136 in each group had 80% power to detect a difference in means of 0.13 assuming that the common standard deviation was 0.38 using a two-group t-test with a 0.050 two-sided significance level. Therefore, we aimed to enroll at least 136 lactating mothers.
3. Results
3.1. Participant Characteristics
A total of 136 lactating mothers were included in this study; 91% had a cesarean delivery, and a high percentage (93%) gave birth to a preterm infant. The maternal and infant characteristics are summarized in Table 1.

Table 1.
Demographic characteristics of mothers and infants.
3.2. Human Milk Processing and Human Milk Composition
The HM compositions of the different processing types are reported in Table 2. The protein contents—true as well as crude protein—in the HM did not differ between the types of processing. All types of processing significantly differed in the leptin concentrations (p < 0.001). Pasteurization decreased the nutrient value in the HM in all measured parameters (n.s.) and was significantly different in regard to leptin (p < 0.001).

Table 2.
Human milk nutrient compositions in different processing types.
3.3. Human Milk Composition of Mothers from Preterm and Term Infants
The 136 mothers and their infants were assigned to four groups according to the infants’ gestational age (GA). Out of the 136 born infants, a total of 126 (93%) were preterm infants, and thereof, 34 (25%) infants were born moderate to late preterm (GA: 32 to <37 weeks), 30 (22%) were born very preterm (GA: 28 to <32 weeks), and 62 (46%) were born extremely preterm (GA: <28 weeks). Table 3 shows the characteristics of the mothers and their infants grouped according to the GA.

Table 3.
Maternal and infant characteristics divided into different GAs.
Table 4 depicts the HM, which was divided into different groups according to the GA of the infants.

Table 4.
Human milk nutrient composition in different GAs.
Statistically significant differences were found between the groups of preterm and term lactating mothers with respect to protein. Mothers with extremely preterm infants showed significantly lower protein contents (both true and crude protein) (p = 0.001), energy levels (p = 0.022), and total solids (p = 0.023) compared to mothers of moderate to late preterm infants. Moreover, extremely preterm HM had significantly lower true protein (p = 0.009) and crude protein (p = 0.012) levels compared to term HM. The nutritional HM contents were significantly lower in mothers with very preterm infants compared to moderate/late preterm infants in regard to true protein (p = 0.030), crude protein (p = 0.037), carbohydrates (p = 0.032), energy (p = 0.017), and total solids (p = 0.006).
4. Discussion
In the present study, we examined the impact of processing HM from the mothers of preterm and term infants. The main observations from the present study highlight that processing had no impact on the protein content or the other macronutrients, fat, and carbohydrates. However, contrary to the protein concentrations, the leptin levels in HM were affected by processing. HM composition is associated with the prematurity of the HM. The protein levels were significantly lower in HM from preterm infants in comparison to term infants.
The outcomes of HM processing are not homogenous or even contradictory. The HM composition might affect the quality and quantity of HM components through different methods of storage, freezing, thawing, pasteurization, and HM analysis [39,40,41,42].
Studies have shown that pasteurization via Maillard reaction and lipid oxidation leads to protein carbonylation on amino acid residues [43,44]. This might be the reason why Vieira et al. indicated a 4% protein reduction after HM pasteurization [45]. However, the results of the current study show that pasteurization had no impact on the HM protein content, which is in line with results previously published in the literature [46,47,48]. Valentine et al. analyzed the amino acid levels in HM and did not find significant differences before and after pasteurization [49]. Kotrri et al. measured the total amount of nitrogen in HM with elemental analysis, and it remained stable after pasteurization and the chemical structure did not change significantly [47]. Espinosa-Martos et al. observed that the lactose contents in colostrum did not change significantly after pasteurization [50]. Peila et al. found a non-significant total protein decrease after HM pasteurization [48]. It can be concluded that HM pasteurization preserves the biological activity of proteins and amino acids remain stable, and therefore, are available for an infant’s enteral intake. Collectively, our data show that all types of HM processing did not affect the protein concentrations, including true and crude protein. Therefore, the protein levels in HM seemed to be stable, and heating and freezing had no impact on this macronutrient. This is in line with other studies and represents a benefit in the nutritional management of preterm infants [51,52].
Our method used in the milk kitchen seemed to not accelerate the heat-induced denaturation and aggregation of whey proteins. However, the following factors, such as temperature, duration, and the method of pasteurization, might have had an impact on the findings [46]. Moreover, another study assessed that HM processing did not affect protein concentrations or any other HM nutrient but had an impact on osmolarity and should be especially considered in combination with fortification steps performed in preterm infants [51].
Freezing and thawing especially interact with the macronutrient fat contents due to the changes in fat globules [53,54,55]. With regard to pasteurization, evidence has been accumulating over the last few years on the decreasing effect on fat [56]. We found that fat contents were reduced after pasteurization, but the results are not significantly different, and these findings are in line with the literature [57]. Previous studies have revealed that pasteurization decreased the activity of immune and bioactive components, such as growth factors and adipokines [39,58]. Our study corresponds to these previous studies, and we observed that the heating process decreased the level of adiponectin in HM, and leptin seemed to be almost completely inactivated by pasteurization. Vass et al. [30] showed that pasteurization decreased leptin concentrations in a combined study cohort with human breast milk and term donor milk samples in a relatively small sample size (n = 55). In our study, different HM processing methods and their effect on leptin and adiponectin were investigated in the mothers’ own milk only. We demonstrated that pasteurization but not freezing alone had an impact on human breast milk in a large study cohort of 136 HM samples. To our knowledge, this is the largest study evaluating the effects of different milk processing methods on leptin and adiponectin levels in preterm human breast milk. Furthermore, we confirmed the hypothesis that pasteurization had a negative impact on leptin concentrations in human breast milk. Long-term follow-up studies, especially in preterm infants, are of major interest to evaluate whether this might translate into impaired neurodevelopment in these infants.
A plausible explanation for this finding could be that the functional activity of leptin is destroyed by temperature changes. The heating process, performed as pasteurization, decreased, even almost destroyed completely leptin levels. However, little is known about leptin and preterm infants and the optimal leptin intake. A study showed that adiponectin might affect short-term growth, but this was not seen with leptin [59]. Other results revealed that both hormones affected physical growth outcomes in preterm infants [60]. Furthermore, animal studies have demonstrated that leptin deficiency is associated with structural and functional brain alteration and lower brain weight [24,25,61]. Preterm infants are at high risk for leptin deficiency after birth [19,23], and the low leptin concentration in pasteurized human breast milk might have an additional effect on brain maturation and development. More research is needed, especially long-term studies on neurodevelopment. The risk of perinatal leptin deficiency is increased by breastfeeding, preterm birth, intrauterine growth restriction, and in male infants [62,63,64]. The leptin contents did not differ significantly between preterm and term milk, which is consistent with results in the literature [65]. This study highlights that unpasteurized HM is of major importance for preterm infants’ nutrients to avoid possible negative effects on brain maturation and development in this special group of vulnerable infants.
Although the effects of gestational age on HM composition are conflicting [8,66,67,68,69,70], our study indicates that GA has an impact on the nutritional content of HM. Mothers of preterm infants provided HM with significantly lower protein levels compared to lactating mothers of term infants. However, in interpreting these results, the following factors have to be mentioned in detail. Our group of term infants was relatively small compared to the preterm infant groups. Moreover, the group of term infants showed a longer lactation period, and it is well known that protein contents in HM decrease over the lactation period [8,69,71].
A strength of this study was the sample size compared to previous studies [46,51,52,72]. The collection, processing, and analysis of the HM were standardized in order to ensure comparability across all samples. This study was overall very well controlled, but the lower sample size in the term group has to be mentioned, and the measurements of the adipose tissue markers were not feasible in 15 HM samples.
In summary, we agree with Gidrewicz and Fenton, who reported that GA is an important predictor of HM composition [8]. Hence, GA should definitely be considered in the nutritional management of preterm infants to optimize their enteral daily protein intake and to prevent any protein malnutrition, which might result in inappropriate growth and development, especially in preterm infants.
5. Conclusions
In conclusion, our results show that HM processing had a significant impact on the leptin concentrations but had no effect on the protein levels. These data support the use of unpasteurized human milk for preterm infants’ nutrients to avoid possible negative effects on brain development. The milk of mothers from preterm compared to full-term infants differed, underlining the importance of individualized target fortification, especially focusing on protein.
Author Contributions
Conceptualization, A.T.; methodology, A.T.; formal analysis, A.T.; investigation, C.B. and A.T.; resources, A.B. and S.B.-P.; data curation, C.B., A.T., S.B.-P. and L.-I.G.; writing—original draft preparation, A.T.; writing—review and editing, C.B., S.B.-P., L.-I.G. and A.B.; visualization, A.T.; supervision, A.T.; project administration, C.B. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Medical University Vienna (protocol code: 2022/2015, date of approval: 5 February 2016).
Informed Consent Statement
Informed consent was obtained from all mothers involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lucas, A.; Morley, R.; Cole, T.; Lister, G.; Leeson-Payne, C. Breast milk and subsequent intelligence quotient in children born preterm. Lancet 1992, 339, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Morley, R.; Cole, T.J.; Gore, S.M. A randomised multicentre study of human milk versus formula and later development in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 1994, 70, F141–F146. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Donovan, S.M. Role of human milk components in gastrointestinal development: Current knowledge and future Needs. J. Pediatr. 2006, 149, S49–S61. [Google Scholar] [CrossRef]
- Lönnerdal, B. Human Milk: Bioactive Proteins/Peptides and Functional Properties. In Protein in Neonatal and Infant Nutrition: Recent Updates; Karger Publishers: Basel, Switzerland, 2016; Volume 86, pp. 97–107. [Google Scholar] [CrossRef]
- Hamosh, M. Bioactive Factors in Human Milk. Pediatr. Clin. North Am. 2001, 48, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Perrella, S.; Gridneva, Z.; Lai, C.T.; Stinson, L.; George, A.; Bilston-John, S.; Geddes, D. Human milk composition promotes optimal infant growth, development and health. Semin. Perinatol. 2021, 45, 151380. [Google Scholar] [CrossRef] [PubMed]
- Gidrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, K.G.; Christensen, S.H.; Lind, M.V.; Michaelsen, K.F. Human milk composition and infant growth. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 200–206. [Google Scholar] [CrossRef]
- Andreas, N.J.; Kampmann, B.; Le-Doare, K.M. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Mitoulas, L.R.; Kent, J.C.; Cox, D.B.; Owens, R.A.; Sherriff, J.L.; Hartmann, P.E. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br. J. Nutr. 2002, 88, 29–37. [Google Scholar] [CrossRef]
- Paulaviciene, I.J.; Liubsys, A.; Molyte, A.; Eidukaite, A.; Usonis, V. Circadian changes in the composition of human milk macronutrients depending on pregnancy duration: A cross-sectional study. Int. Breastfeed. J. 2020, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Rochow, N.; Fusch, G.; Choi, A.; Chessell, L.; Elliott, L.; McDonald, K.; Kuiper, E.; Purcha, M.; Turner, S.; Chan, E.; et al. Target Fortification of Breast Milk with Fat, Protein, and Carbohydrates for Preterm Infants. J. Pediatr. 2013, 163, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Rochow, N.; Fusch, G.; Ali, A.; Bhatia, A.; So, H.Y.; Iskander, R.; Chessell, L.; el Helou, S.; Fusch, C. Individualized target fortification of breast milk with protein, carbohydrates, and fat for preterm infants: A double-blind randomized controlled trial. Clin. Nutr. 2020, 40, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Embleton, N.D.; Akker, C.H.V.D. Protein intakes to optimize outcomes for preterm infants. Semin. Perinatol. 2019, 43, 151154. [Google Scholar] [CrossRef]
- Kunz, C.; Lönnerdal, B. Re-evaluation of the whey protein/casein ratio of human milk. Acta Paediatr. 1992, 81, 107–112. [Google Scholar] [CrossRef]
- Hay, W.W.; Thureen, P. Protein for Preterm Infants: How Much is Needed? How Much is Enough? How Much is Too Much? Pediatr. Neonatol. 2010, 51, 198–207. [Google Scholar] [CrossRef]
- Isaacs, E.B.; Morley, R.; Lucas, A. Early Diet and General Cognitive Outcome at Adolescence in Children Born at or Below 30 Weeks Gestation. J. Pediatr. 2009, 155, 229–234. [Google Scholar] [CrossRef]
- Chatmethakul, T.; Schmelzel, M.L.; Johnson, K.J.; Walker, J.R.; Santillan, D.A.; Colaizy, T.T.; Roghair, R.D. Postnatal Leptin Levels Correlate with Breast Milk Leptin Content in Infants Born before 32 Weeks Gestation. Nutrients 2022, 14, 5224. [Google Scholar] [CrossRef]
- Valleau, J.C.; Sullivan, E.L. The impact of leptin on perinatal development and psychopathology. J. Chem. Neuroanat. 2014, 61–62, 221–232. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Brunner, S.; Schmid, D.; Zang, K.; Much, D.; Knoeferl, B.; Kratzsch, J.; Amann-Gassner, U.; Bader, B.L.; Hauner, H. Breast milk leptin and adiponectin in relation to infant body composition up to 2 years. Pediatr. Obes. 2014, 10, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Valūnienė, M.; Verkauskienė, R.; Boguszewski, M.; Dahlgren, J.; Lašienė, D.; Lašas, L.; Wikland, K.A. Leptin levels at birth and in early postnatal life in small- and appropriate-for-gestational-age infants. Medicina 2007, 43, 784. [Google Scholar] [CrossRef] [PubMed]
- Calió, M.L.; Mosini, A.C.; Marinho, D.S.; Salles, G.N.; Massinhani, F.H.; Ko, G.M.; Porcionatto, M.A. Leptin enhances adult neurogenesis and reduces pathological features in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2020, 148, 105219. [Google Scholar] [CrossRef] [PubMed]
- Erkonen, G.E.; Hermann, G.M.; Miller, R.L.; Thedens, D.L.; Nopoulos, P.C.; Wemmie, J.A.; Roghair, R.D. Neonatal Leptin Administration Alters Regional Brain Volumes and Blocks Neonatal Growth Restriction-Induced Behavioral and Cardiovascular Dysfunction in Male Mice. Pediatr. Res. 2011, 69, 406–412. [Google Scholar] [CrossRef]
- Bouret, S.G. Neurodevelopmental actions of leptin. Brain Res. 2010, 1350, 2–9. [Google Scholar] [CrossRef]
- Steppan, C.M.; Swick, A.G. A Role for Leptin in Brain Development. Biochem. Biophys. Res. Commun. 1999, 256, 600–602. [Google Scholar] [CrossRef]
- Devaskar, S.U.; Ollesch, C.; Rajakumar, R.A.; Rajakumar, P.A. Developmental Changes inobGene Expression and Circulating Leptin Peptide Concentrations. Biochem. Biophys. Res. Commun. 1997, 238, 44–47. [Google Scholar] [CrossRef]
- Ilcol, Y.O.; Hizli, Z.B.; Ozkan, T. Leptin concentration in breast milk and its relationship to duration of lactation and hormonal status. Int. Breastfeed. J. 2006, 1, 21. [Google Scholar] [CrossRef]
- Vass, R.A.; Bell, E.F.; Colaizy, T.T.; Schmelzel, M.L.; Johnson, K.J.; Walker, J.R.; Ertl, T.; Roghair, R.D. Hormone levels in preterm and donor human milk before and after Holder pasteurization. Pediatr. Res. 2020, 88, 612–617. [Google Scholar] [CrossRef]
- Wesolowska, A.; Sinkiewicz-Darol, E.; Barbarska, O.; Bernatowicz-Lojko, U.; Borszewska-Kornacka, M.K.; van Goudoever, J.B. Innovative Techniques of Processing Human Milk to Preserve Key Components. Nutrients 2019, 11, 1169. [Google Scholar] [CrossRef]
- Pitino, M.A.; O’Connor, D.L.; McGeer, A.J.; Unger, S. The impact of thermal pasteurization on viral load and detectable live viruses in human milk and other matrices: A rapid review. Appl. Physiol. Nutr. Metab. 2021, 46, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Miris, A. Miris HMA User Manual. 2022, 2, 1–39. Available online: https://www.mirissolutions.com/media/19fad927-9b29-447b-adf1-877494a7c61d (accessed on 6 December 2022).
- Miris, A. Miris Heater. 2022, 1–52. Available online: https://www.mirissolutions.com/media/408cc59c-3be8-430f-b37f-a59fd6a303a5 (accessed on 6 December 2022).
- Miris, A. Miris Ultrasonic Processor. 2018, 1–9. Available online: https://www.mirissolutions.com/media/a918b1d8-8283-4fd7-b495-78a23e168c69 (accessed on 6 December 2022).
- Bronský, J.; Karpisek, M.; Bronská, E.; Pechová, M.; Jančíková, B.; Kotolová, H.; Stejskal, D.; Pruša, R.; Nevoral, J. Adiponectin, Adipocyte Fatty Acid Binding Protein, and Epidermal Fatty Acid Binding Protein: Proteins Newly Identified in Human Breast Milk. Clin. Chem. 2006, 52, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Bronsky, J.; Mitrova, K.; Karpisek, M.; Mazoch, J.; Durilova, M.; Fisarkova, B.; Stechova, K.; Prusa, R.; Nevoral, J. Adiponectin, AFABP, and Leptin in Human Breast Milk During 12 Months of Lactation. J. Craniofacial Surg. 2011, 52, 474–477. [Google Scholar] [CrossRef]
- BioVendor. Application Protocol: Determination of Leptin in Breast Milk with Human Leptin ELISA; BioVendor: Brno, Czech Republic, 2011; pp. 1–3. [Google Scholar]
- Peila, C.; Moro, G.E.; Bertino, E.; Cavallarin, L.; Giribaldi, M.; Giuliani, F.; Cresi, F.; Coscia, A. The Effect of Holder Pasteurization on Nutrients and Biologically-Active Components in Donor Human Milk: A Review. Nutrients 2016, 8, 477. [Google Scholar] [CrossRef]
- García-Lara, N.R.; Escuder-Vieco, D.; García-Algar, O.; De La Cruz, J.; Lora, D.; Pallás-Alonso, C. Effect of Freezing Time on Macronutrients and Energy Content of Breastmilk. Breastfeed. Med. 2012, 7, 295–301. [Google Scholar] [CrossRef]
- Păduraru, L.; Zonda, G.I.; Avasiloaiei, A.-L.; Moscalu, M.; Dimitriu, D.C.; Stamatin, M. Influence of refrigeration or freezing on human milk macronutrients and energy content in early lactation: Results from a tertiary centre survey. Paediatr. Child Health 2018, 24, 250–257. [Google Scholar] [CrossRef]
- Orbach, R.; Mandel, D.; Mangel, L.; Marom, R.; Lubetzky, R. The Effect of Deep Freezing on Human Milk Macronutrients Content. Breastfeed. Med. 2019, 14, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Fenaille, F.; Parisod, V.; Tabet, J.-C.; Guy, P.A. Carbonylation of milk powder proteins as a consequence of processing conditions. Proteomics 2005, 5, 3097–3104. [Google Scholar] [CrossRef]
- Van Boekel, M. Effect of heating on Maillard reactions in milk. Food Chem. 1998, 62, 403–414. [Google Scholar] [CrossRef]
- Vieira, A.A.; Soares, F.V.M.; Pimenta, H.P.; Abranches, A.D.; Moreira, M.E.L. Analysis of the influence of pasteurization, freezing/thawing, and offer processes on human milk’s macronutrient concentrations. Early Hum. Dev. 2011, 87, 577–580. [Google Scholar] [CrossRef]
- Thajer, A.; Fusch, G.; Binder, C.; Berger, A.; Fusch, C. Human milk analyser underestimated protein content of unfortified and fortified samples compared to elemental analysis. Acta Paediatr. 2019, 108, 2298–2300. [Google Scholar] [CrossRef]
- Kotrri, G.; Fusch, G.; Kwan, C.; Choi, D.; Choi, A.; Al Kafi, N.; Rochow, N.; Fusch, C. Validation of Correction Algorithms for Near-IR Analysis of Human Milk in an Independent Sample Set—Effect of Pasteurization. Nutrients 2016, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Peila, C.; Coscia, A.; Bertino, E.; Cavaletto, M.; Spertino, S.; Icardi, S.; Tortone, C.; Visser, G.H.A.; Gazzolo, D. Effects of Holder pasteurization on the protein profile of human milk. Ital. J. Pediatr. 2016, 42, 36. [Google Scholar] [CrossRef]
- Valentine, C.J.; Morrow, G.; Fernandez, S.; Gulati, P.; Bartholomew, D.; Long, D.; Welty, S.E.; Morrow, A.L.; Rogers, L.K. Docosahexaenoic Acid and Amino Acid Contents in Pasteurized Donor Milk are Low for Preterm Infants. J. Pediatr. 2010, 157, 906–910. [Google Scholar] [CrossRef]
- Espinosa-Martos, I.; Montilla, A.; de Segura, A.G.; Escuder, D.; Bustos, G.; Pallás, C.; Rodríguez, J.; Corzo, N.; Fernández, L. Bacteriological, Biochemical, and Immunological Modifications in Human Colostrum After Holder Pasteurisation. J. Craniofacial Surg. 2013, 56, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Kreissl, A.; Zwiauer, V.; Repa, A.; Binder, C.; Haninger, N.; Jilma, B.; Berger, A.; Haiden, N. Effect of Fortifiers and Additional Protein on the Osmolarity of Human Milk. J. Craniofacial Surg. 2013, 57, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Paulaviciene, I.J.; Liubsys, A.; Eidukaite, A.; Molyte, A.; Tamuliene, L.; Usonis, V. The Effect of Prolonged Freezing and Holder Pasteurization on the Macronutrient and Bioactive Protein Compositions of Human Milk. Breastfeed. Med. 2020, 15, 583–588. [Google Scholar] [CrossRef]
- Andersson, Y.; Sävman, K.; Bläckberg, L.; Hernell, O. Pasteurization of mother’s own milk reduces fat absorption and growth in preterm infants. Acta Paediatr. 2007, 96, 1445–1449. [Google Scholar] [CrossRef]
- Takahashi, K.; Mizuno, K.; Itabashi, K. The Freeze-Thaw Process and Long Intervals after Fortification Denature Human Milk Fat Globules. Am. J. Perinatol. 2011, 29, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.R.; Fay, T.N.; Hamosh, M. Effect of pasteurization on long chain polyunsaturated fatty acid levels and enzyme activities of human milk. J. Pediatr. 1998, 132, 876–878. [Google Scholar] [CrossRef]
- Adhisivam, B.; Bhat, B.V.; Rao, K.; Kingsley, S.M.; Plakkal, N.; Palanivel, C. Effect of Holder pasteurization on macronutrients and immunoglobulin profile of pooled donor human milk. J. Matern. Neonatal Med. 2018, 32, 3016–3019. [Google Scholar] [CrossRef] [PubMed]
- Fidler, N.; Sauerwald, T.U.; Koletzko, B.; Demmelmair, H. Effects of Human Milk Pasteurization and Sterilization on Available Fat Content and Fatty Acid Composition. J. Craniofacial Surg. 1998, 27, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Ewaschuk, J.B.; Unger, S.; Harvey, S.; O’Connor, D.; Field, C. Effect of pasteurization on immune components of milk: Implications for feeding preterm infants. Appl. Physiol. Nutr. Metab. 2011, 36, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Koksal, E.; Ozcan, K.E.; Turkyilmaz, C. Do the adiponectin and leptin levels in preterm and term breast milk samples relate to infants’ short-term growth? J. Dev. Orig. Health Dis. 2019, 10, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Joung, K.E.; Martin, C.R.; Cherkerzian, S.; Kellogg, M.; Belfort, M.B. Human Milk Hormone Intake in the First Month of Life and Physical Growth Outcomes in Preterm Infants. J. Clin. Endocrinol. Metab. 2021, 106, 1793–1803. [Google Scholar] [CrossRef]
- Schepers, J.; Gebhardt, C.; Bracke, A.; Eiffler, I.; Halbach, O.V.B.U. Structural and functional consequences in the amygdala of leptin-deficient mice. Cell Tissue Res. 2020, 382, 421–426. [Google Scholar] [CrossRef]
- Steinbrekera, B.; Roghair, R. Modeling the impact of growth and leptin deficits on the neuronal regulation of blood pressure. J. Endocrinol. 2016, 231, R47–R60. [Google Scholar] [CrossRef]
- Hellgren, G.; Engström, E.; Smith, L.E.; Löfqvist, C.; Hellström, A. Effect of Preterm Birth on Postnatal Apolipoprotein and Adipocytokine Profiles. Neonatology 2015, 108, 16–22. [Google Scholar] [CrossRef]
- Ertl, T.; Funke, S.; Sárkány, I.; Szabó, I.; Rascher, W.; Blum, W.; Sulyok, E. Postnatal Changes of Leptin Levels in Full-Term and Preterm Neonates: Their Relation to Intrauterine Growth, Gender and Testosterone. Neonatology 1999, 75, 167–176. [Google Scholar] [CrossRef]
- Eilers, E.; Ziska, T.; Harder, T.; Plagemann, A.; Obladen, M.; Loui, A. Leptin determination in colostrum and early human milk from mothers of preterm and term infants. Early Hum. Dev. 2011, 87, 415–419. [Google Scholar] [CrossRef]
- Fumeaux, C.J.F.; Garcia-Rodenas, C.L.; De Castro, C.A.; Courtet-Compondu, M.-C.; Thakkar, S.K.; Beauport, L.; Tolsa, J.-F.; Affolter, M. Longitudinal Analysis of Macronutrient Composition in Preterm and Term Human Milk: A Prospective Cohort Study. Nutrients 2019, 11, 1525. [Google Scholar] [CrossRef] [PubMed]
- Maly, J.; Burianova, I.; Vitkova, V.; Ticha, E.; Navratilova, M.; Cermakova, E. Preterm human milk macronutrient concentration is independent of gestational age at birth. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 104, F50–F56. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Williams, F.H.; Merkatz, R.B.; Schulman, P.K.; Kerr, D.S.; Pittard, W.B. Length of gestation and nutritional composition of human milk. Am. J. Clin. Nutr. 1983, 37, 810–814. [Google Scholar] [CrossRef]
- Butte, N.F.; Garza, C.; Johnson, C.A.; Smith, E.; Nichols, B.L. Longitudinal changes in milk composition of mothers delivering preterm and term infants. Early Hum. Dev. 1984, 9, 153–162. [Google Scholar] [CrossRef]
- A Lemons, J.; Moye, L.; Hall, D.; Simmons, M. Differences in the Composition of Preterm and Term Human Milk during Early Lactation. Pediatr. Res. 1982, 16, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Kreissl, A.; Zwiauer, V.; Repa, A.; Binder, C.; Thanhaeuser, M.; Jilma, B.; Berger, A.; Haiden, N. Human Milk Analyser shows that the lactation period affects protein levels in preterm breastmilk. Acta Paediatr. 2016, 105, 635–640. [Google Scholar] [CrossRef]
- De Oliveira, M.N.S.; Rodrigues, A.M.; De Faria, A.M.C.; Pereira, S.C.L.; Maioli, T.U. Effects of Holder Pasteurization on Immune Composition of Human Milk. Breastfeed. Med. 2020, 15, 803–808. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).