Longitudinal Assessment of Serum 25-Hydroxyvitamin D Levels during Pregnancy and Postpartum—Are the Current Recommendations for Supplementation Sufficient?
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patient Characteristics and Pregnancy Outcomes
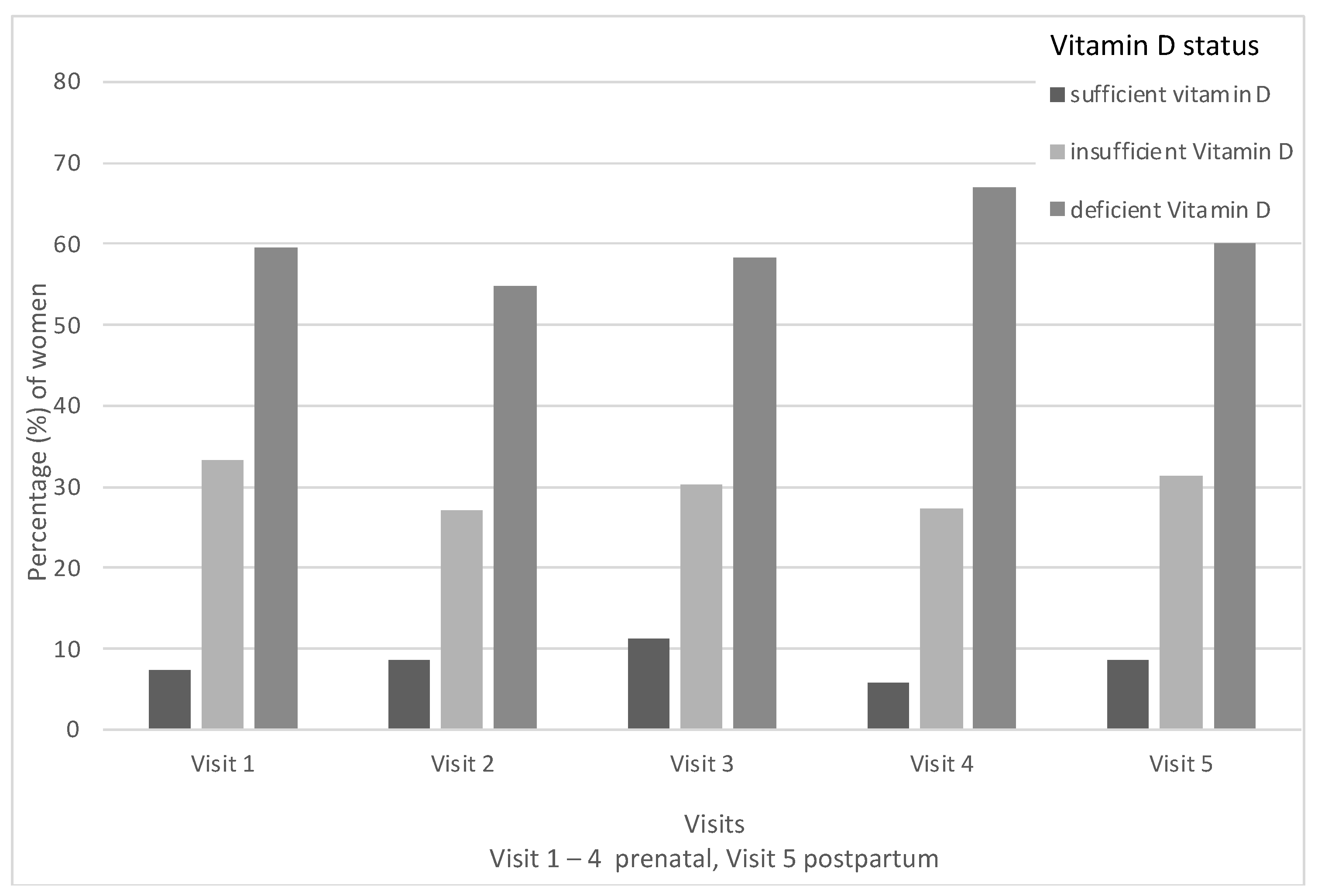
3.2. Vitamin D Deficiency during Pregnancy
3.3. Vitamin D Supplementation and Nutritional Vitamin D Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saraf, R.; Morton, S.M.B.; Camargo, C.A., Jr.; Grant, C.C. Global summary of maternal and newborn vitamin D status—A systematic review. Matern. Child Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef]
- Mulligan, M.L.; Felton, S.K.; Riek, A.E.; Bernal-Mizrachi, C. Implications of vitamin D deficiency in pregnancy and lactation. Am. J. Obstet. Gynecol. 2010, 202, 429.e1–429.e9. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.L.; Oliveri, B.; Giacoia, E.; Fusaro, D.; Costanzo, P.R. Vitamin D: Before, during and after Pregnancy: Effect on Neonates and Children. Nutrients 2022, 14, 1900. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Dawodu, A.; Wagner, C.L. Mother-child vitamin D deficiency: An international perspective. Arch. Dis. Child. 2007, 92, 737–740. [Google Scholar] [CrossRef]
- Shrestha, D.; Budhathoki, S.; Pokhrel, S.; Sah, A.K.; Shrestha, R.K.; Raya, G.B.; Shrestha, R.; Pasakhala, R.; Smith, C.; Dhoubhadel, B.G. Prevalence of vitamin D deficiency in pregnant women and their babies in Bhaktapur, Nepal. BMC Nutr. 2019, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.M.; de Klerk, N.H.; Davis, E.A.; Lucas, R.M.; Hart, P.H.; Haynes, A. Demographic and clinical predictors of vitamin D status in pregnant women tested for deficiency in Western Australia. Aust. N. Z. J. Public Health 2021, 45, 474–481. [Google Scholar] [CrossRef]
- Karras, S.N.; Wagner, C.L.; Castracane, V.D. Understanding vitamin D metabolism in pregnancy: From physiology to pathophysiology and clinical outcomes. Metabolism 2018, 86, 112–123. [Google Scholar] [CrossRef]
- Zerofsky, M.; Ryder, M.; Bhatia, S.; Stephensen, C.B.; King, J.; Fung, E.B. Effects of early vitamin D deficiency rickets on bone and dental health, growth and immunity. Matern. Child Nutr. 2016, 12, 898–907. [Google Scholar] [CrossRef]
- Cyprian, F.; Lefkou, E.; Varoudi, K.; Girardi, G. Immunomodulatory Effects of Vitamin D in Pregnancy and Beyond. Front. Immunol. 2019, 10, 2739. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W. The Implications of Vitamin D Status During Pregnancy on Mother and her Developing Child. Front. Endocrinol. 2018, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, R.P.; Thorp, J.M. Vitamin D in pregnancy: Current concepts. Curr. Opin. Obstet. Gynecol. 2012, 24, 57–64. [Google Scholar] [CrossRef]
- Harvey, N.C.; Holroyd, C.; Ntani, G.; Javaid, K.; Cooper, P.; Moon, R.; Cole, Z.; Tinati, T.; Godfrey, K.; Dennison, E.; et al. Vitamin D supplementation in pregnancy: A systematic review. Health Technol. Assess. 2014, 18, 1–190. [Google Scholar] [CrossRef]
- Kiely, M.E.; Wagner, C.L.; Roth, D.E. Vitamin D in pregnancy: Where we are and where we should go. J. Steroid Biochem. Mol. Biol. 2020, 201, 105669. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; Hemmingway, A.; O’Callaghan, K.M. Vitamin D in pregnancy: Current perspectives and future directions. Ther. Adv. Musculoskelet. Dis. 2017, 9, 145–154. [Google Scholar] [CrossRef]
- Institute of Medicine (US); Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press: Washington, DC, USA, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK56070/ (accessed on 30 December 2022).
- Schaefer-Graf, U.; Ensenauer, R.; Gembruch, U.; Groten, T.; Flothkötter, M.; Hennicke, J.; Köhrle, J.; Möhler, J.; Kühnert, M.; Schmittendorf, A.; et al. Obesity and Pregnancy. Guideline of the German Society of Gynecology and Obstetrics (S3-Level, AWMF Registry No. 015-081, June 2019). Geburtshilfe Frauenheilkd. 2021, 81, 279–303. [Google Scholar] [CrossRef] [PubMed]
- German Nutrition Society. New Reference Values for Vitamin D. Ann. Nutr. Metab. 2012, 60, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Poon, L.C.Y.; Tan, M.Y.; Yerlikaya, G.; Syngelaki, A.; Nicolaides, K.H. Birth weight in live births and stillbirths. Ultrasound Obstet. Gynecol. 2016, 48, 602–606. [Google Scholar] [CrossRef]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guidelines Approved by the Guidelines Review Committee. In Guideline: Vitamin D Supplementation in Pregnant Women; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Krieger, J.-P.; Cabaset, S.; Canonica, C.; Christoffel, L.; Richard, A.; Schröder, T.; Von Wattenwyl, B.L.; Rohrmann, S.; Lötscher, K.Q. Prevalence and determinants of vitamin D deficiency in the third trimester of pregnancy: A multicentre study in Switzerland. Br. J. Nutr. 2018, 119, 299–309. [Google Scholar] [CrossRef]
- Cabaset, S.; Krieger, J.-P.; Richard, A.; Elgizouli, M.; Nieters, A.; Rohrmann, S.; Lötscher, K.C.Q. Vitamin D status and its determinants in healthy pregnant women living in Switzerland in the first trimester of pregnancy. BMC Pregnancy Childbirth 2019, 19, 10. [Google Scholar] [CrossRef]
- Wuertz, C.; Gilbert, P.; Baier, W.; Kunz, C. Cross-sectional study of factors that influence the 25-hydroxyvitamin D status in pregnant women and in cord blood in Germany. Br. J. Nutr. 2013, 110, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Cadario, F.; Savastio, S.; Magnani, C.; Cena, T.; Pagliardini, V.; Bellomo, G.; Bagnati, M.; Vidali, M.; Pozzi, E.; Pamparana, S.; et al. High Prevalence of Vitamin D Deficiency in Native versus Migrant Mothers and Newborns in the North of Italy: A Call to Act with a Stronger Prevention Program. PLoS ONE 2015, 10, e0129586. [Google Scholar] [CrossRef]
- Ceccaldi, P.-F.; Pejoan, H.; Breau, N.; Diallo, D.; Ducarme, G.; Poujade, O.; Davitian, C.; Luton, D. French prenatal Vitamin D recommended supplementation: Enough or not? J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 35–41. [Google Scholar] [CrossRef]
- Gallo, S.; McDermid, J.M.; Al-Nimr, R.I.; Hakeem, R.; Moreschi, J.M.; Pari-Keener, M.; Stahnke, B.; Papoutsakis, C.; Handu, D.; Cheng, F.W. Vitamin D Supplementation during Pregnancy: An Evidence Analysis Center Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2020, 120, 898–924.e4. [Google Scholar] [CrossRef]
- Guillaume, M.; Riquet, S.; Zakarian, C.; Comte, F. Iron, folic acid, and vitamin D supplementation during pregnancy: Patient compliance. Sante Publique 2020, 32, 161–170. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Substantial Vitamin D Supplementation Is Required during the Prenatal Period to Improve Birth Outcomes. Nutrients 2022, 14, 899. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Morris, S.K.; Zlotkin, S.; Gernand, A.D.; Ahmed, T.; Shanta, S.S.; Papp, E.; Korsiak, J.; Shi, J.; Islam, M.M.; et al. Vitamin D Supplementation in Pregnancy and Lactation and Infant Growth. N. Engl. J. Med. 2018, 379, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Trak-Fellermeier, M.A.; Martinez, R.X.; Lopez-Perez, L.; Lips, P.; Salisi, J.A.; John, J.C.; Peña-Rosas, J.P. Regimens of vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 10, CD013446. [Google Scholar] [CrossRef]
- Lewis, S.; Lucas, R.M.; Halliday, J.; Ponsonby, A.-L. Vitamin D deficiency and pregnancy: From preconception to birth. Mol. Nutr. Food Res. 2010, 54, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Kinshella, M.-L.W.; Pickerill, K.; Bone, J.N.; Prasad, S.; Campbell, O.; Vidler, M.; Craik, R.; Volvert, M.-L.; Mistry, H.D.; Tsigas, E.; et al. An evidence review and nutritional conceptual framework for pre-eclampsia prevention. Br. J. Nutr. 2022, 1–35. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7, CD008873. [Google Scholar] [CrossRef]
- Vivanti, A.J.; Monier, I.; Salakos, E.; Elie, C.; Tsatsaris, V.; Senat, M.-V.; Jani, J.; Jouannic, J.-M.; Winer, N.; Zeitlin, J.; et al. Vitamin D and pregnancy outcomes: Overall results of the FEPED study. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101883. [Google Scholar] [CrossRef]
- Rostami, M.; Tehrani, F.R.; Simbar, M.; Bidhendi Yarandi, R.; Minooee, S.; Hollis, B.W.; Hosseinpanah, F. Effectiveness of Prenatal Vitamin D Deficiency Screening and Treatment Program: A Stratified Randomized Field Trial. J. Clin. Endocrinol. Metab. 2018, 103, 2936–2948. [Google Scholar] [CrossRef] [PubMed]
- Nassar, N.; Halligan, G.H.; Roberts, C.L.; Morris, J.M.; Ashton, A.W. Systematic review of first-trimester vitamin D normative levels and outcomes of pregnancy. Am. J. Obstet. Gynecol. 2011, 205, 208.e1–208.e7. [Google Scholar] [CrossRef]
- Lee, S.S.; Ling, K.H.; Tusimin, M.; Subramaniam, R.; Rahim, K.F.; Loh, S.P. Interplay between Maternal and Neonatal Vitamin D Deficiency and Vitamin-D-Related Gene Polymorphism with Neonatal Birth Anthropometry. Nutrients 2022, 14, 564. [Google Scholar] [CrossRef]
- Roth, D.E.; Leung, M.; Mesfin, E.; Qamar, H.; Watterworth, J.; Papp, E. Vitamin D supplementation during pregnancy: State of the evidence from a systematic review of randomised trials. BMJ 2017, 359, j5237. [Google Scholar] [CrossRef] [PubMed]
| Study Participants n = 249 | |
|---|---|
| GA at study inclusion in weeks, median (IQR) | 12.43 (12.0–13.0) |
| Maternal age in years, median (IQR) | 31 (26–34) |
| BMI in kg/m2, median (IQR), booking | 23.31 (20.55–27.05) |
| Ethnicity | |
| Caucasian, n (%) | 221 (94) |
| African, n (%) | 3 (1.3) |
| Asian, n (%) | 8 (3.4) |
| Hispanic, n (%) | 2 (0.9) |
| Mixed, n (%) | 1 (0.4) |
| Conception | |
| Spontaneous, n (%) | 208 (87.8) |
| Assisted, n (%) | 29 (12.2) |
| Smoking, n (%) | 40 (16.1) |
| Nulliparous, n (%) | 113 (47.7) |
| Systolic blood pressure, mmHg, median (IQR) | 120 (112–127) |
| Diastolic blood pressure, mmHg, median (IQR) | 78 (72–84) |
| Twin pregnancy, n (%) | 51 (20.5) |
| Monochorionic diamniotic, n (%) | 17 (33.3) |
| Monochorionic monoamniotic, n (%) | 2 (3.9) |
| Dichorionic diamniotic, n (%) | 32 (62.8) |
| Vitamin D < 50 nmol/L n = 114 | Vitamin D > 50 nmol/L n = 67 | p-Value | |
|---|---|---|---|
| Preterm birth, n (%) | 12 (10.5) | 5 (7.5) | 0.49 |
| HDP, n (%) | 6 (5.3) | 2 (3.0) | 0.47 |
| GDM, n (%) | 13 (11.4) | 4 (6.0) | 0.23 |
| IGDM, n (%) | 12 (10.5) | 3 (4.5) | 0.15 |
| PROM, n (%) | 10 (8.8) | 8 (11.9) | 0.49 |
| Preterm labor, n (%) | 5 (4.4) | 4 (6.0) | 0.64 |
| Cervical insufficiency, n (%) | 2 (1.8) | 1 (1.5) | 0.89 |
| NICU admission, n (%) | 6 (5.3) | 2 (3.0) | 0.46 |
| RDS, n (%) SGA, n (%) | 6 (5.3) 17 (15.2) | 3 (4.5) 6 (9.2) | 0.80 0.26 |
| Mean Change nmol/L | 95% CI | p-Value | |
|---|---|---|---|
| Visit 2 | 0.69 | −1.56–2.93 | 0.55 |
| Visit 3 | 0.31 | −2.47–3.08 | 0.83 |
| Visit 4 | −2.89 | −6.26–0.48 | 0.09 |
| Vitamin D supplementation | 11.72 | 7.92–15.51 | <0.001 |
| Summer | 9.91 | 6.0–13.81 | <0.001 |
| Winter | −4.4 | −7.73–1.07 | 0.009 |
| Autumn | 3.64 | −0.25–7.52 | 0.07 |
| Suntan in the past 3 months | 9.16 | 4.62–13.70 | <0.001 |
| BMI at booking | −0.68 | −1.07–0.30 | <0.001 |
| Covering | −10.19 | −17.28–3.11 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmrich, P.; Thajer, A.; Schirwani, N.; Haberl, C.; Zeisler, H.; Ristl, R.; Binder, J. Longitudinal Assessment of Serum 25-Hydroxyvitamin D Levels during Pregnancy and Postpartum—Are the Current Recommendations for Supplementation Sufficient? Nutrients 2023, 15, 339. https://doi.org/10.3390/nu15020339
Palmrich P, Thajer A, Schirwani N, Haberl C, Zeisler H, Ristl R, Binder J. Longitudinal Assessment of Serum 25-Hydroxyvitamin D Levels during Pregnancy and Postpartum—Are the Current Recommendations for Supplementation Sufficient? Nutrients. 2023; 15(2):339. https://doi.org/10.3390/nu15020339
Chicago/Turabian StylePalmrich, Pilar, Alexandra Thajer, Nawa Schirwani, Christina Haberl, Harald Zeisler, Robin Ristl, and Julia Binder. 2023. "Longitudinal Assessment of Serum 25-Hydroxyvitamin D Levels during Pregnancy and Postpartum—Are the Current Recommendations for Supplementation Sufficient?" Nutrients 15, no. 2: 339. https://doi.org/10.3390/nu15020339
APA StylePalmrich, P., Thajer, A., Schirwani, N., Haberl, C., Zeisler, H., Ristl, R., & Binder, J. (2023). Longitudinal Assessment of Serum 25-Hydroxyvitamin D Levels during Pregnancy and Postpartum—Are the Current Recommendations for Supplementation Sufficient? Nutrients, 15(2), 339. https://doi.org/10.3390/nu15020339







