Association of Serum Zinc and Inflammatory Markers with the Severity of COVID-19 Infection in Adult Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study Design
2.3. Patients and Data Collection
- Severe cases: COVID-19-positive patients who required admission to ICU (usually in respiratory distress or require>50% O2).
- Moderate cases: COVID-19-positive patients who required admission to the ward (usually requires <50% O2) and did not require ICU admission during their illness.
- Mild cases: COVID-19-positive patients who did not require hospital admission but home isolation (no O2 requirements).
- Control cases: Individuals who came to respiratory clinic with symptoms suggestive of COVID-19 infection and tested negative.
- Adult patients (18 years or older) who were symptomatic and tested positive for COVID-19 at King Abdulaziz Hospital whether they required admission to ICU/ward or just home isolation.
- Individuals who came to respiratory clinic with symptoms suggestive of COVID-19 infection and tested negative.
- Documented other infections apart from COVID-19.
- Any participant who was prescribed zinc supplement prior to blood sampling.
- Receiving immune-suppressant or immune-modulatory medications within 90 days prior to recruitment.
2.4. Serum Zinc, Biochemical and Hematological Assessment
2.5. Statistical Analysis
3. Results
3.1. Basic Characteristics and Comparisons between COVID-19 Patients and Controls
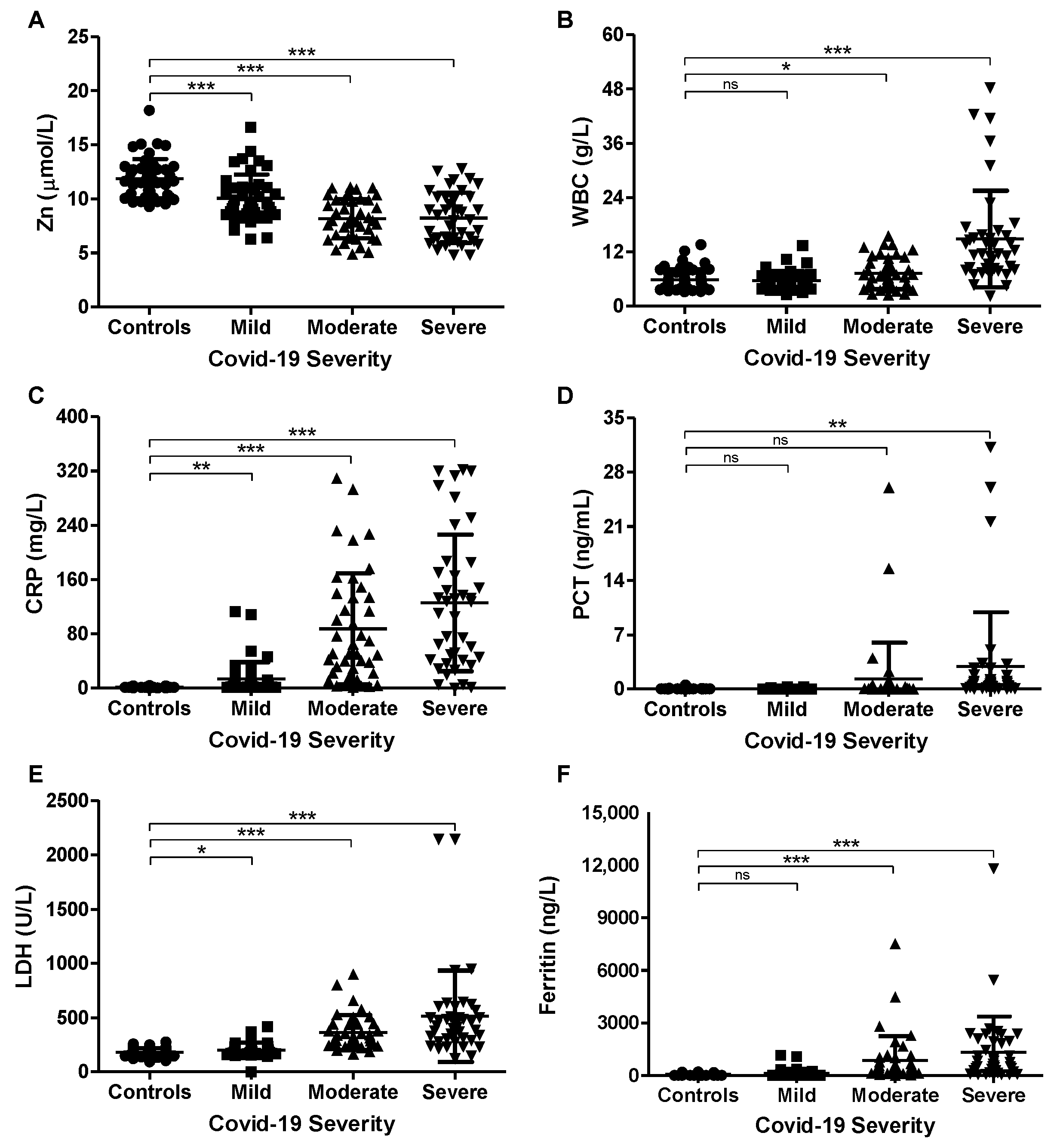
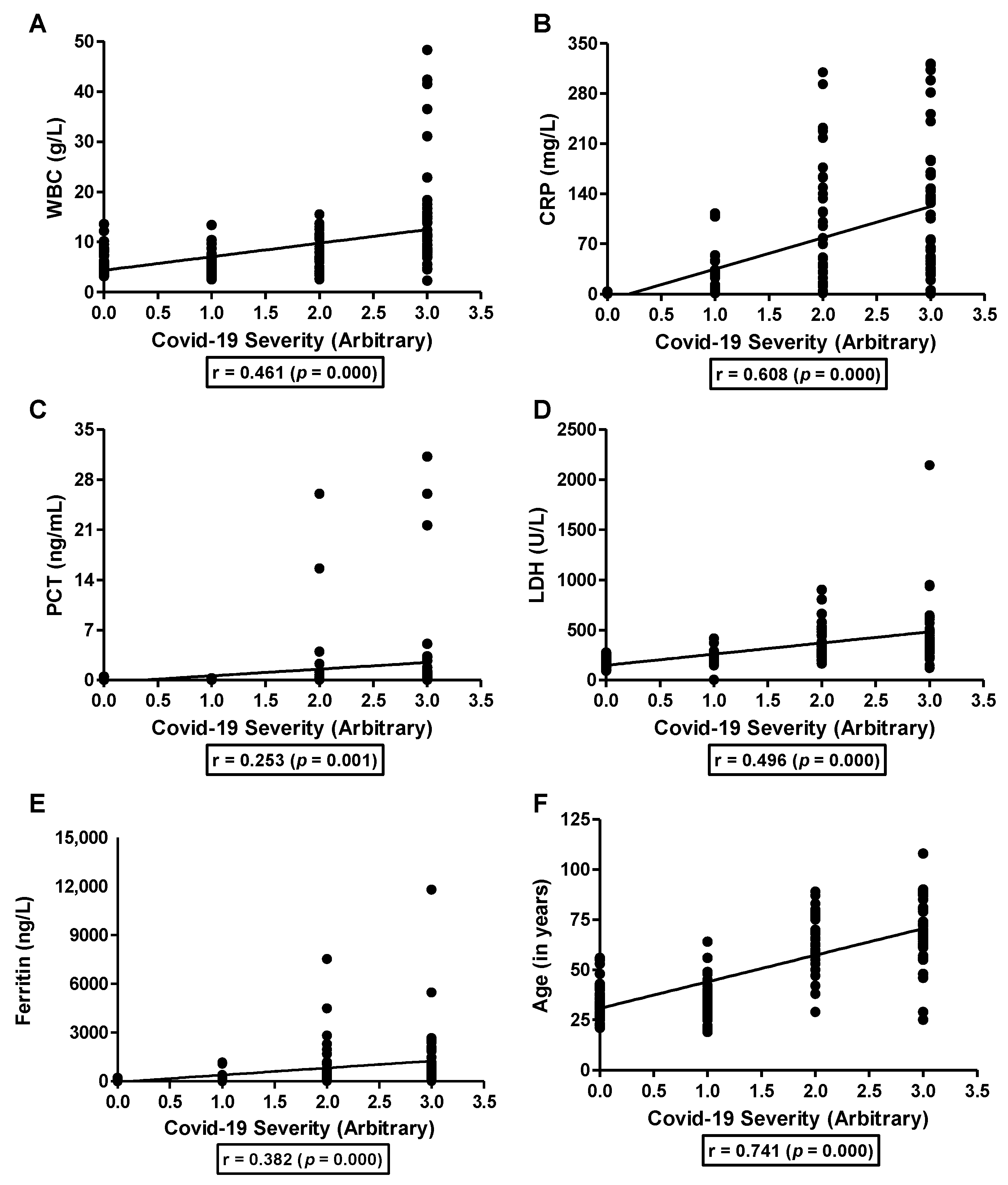
3.2. Association of Serum Zinc with COVID-19 Severity and Inflammatory Markers
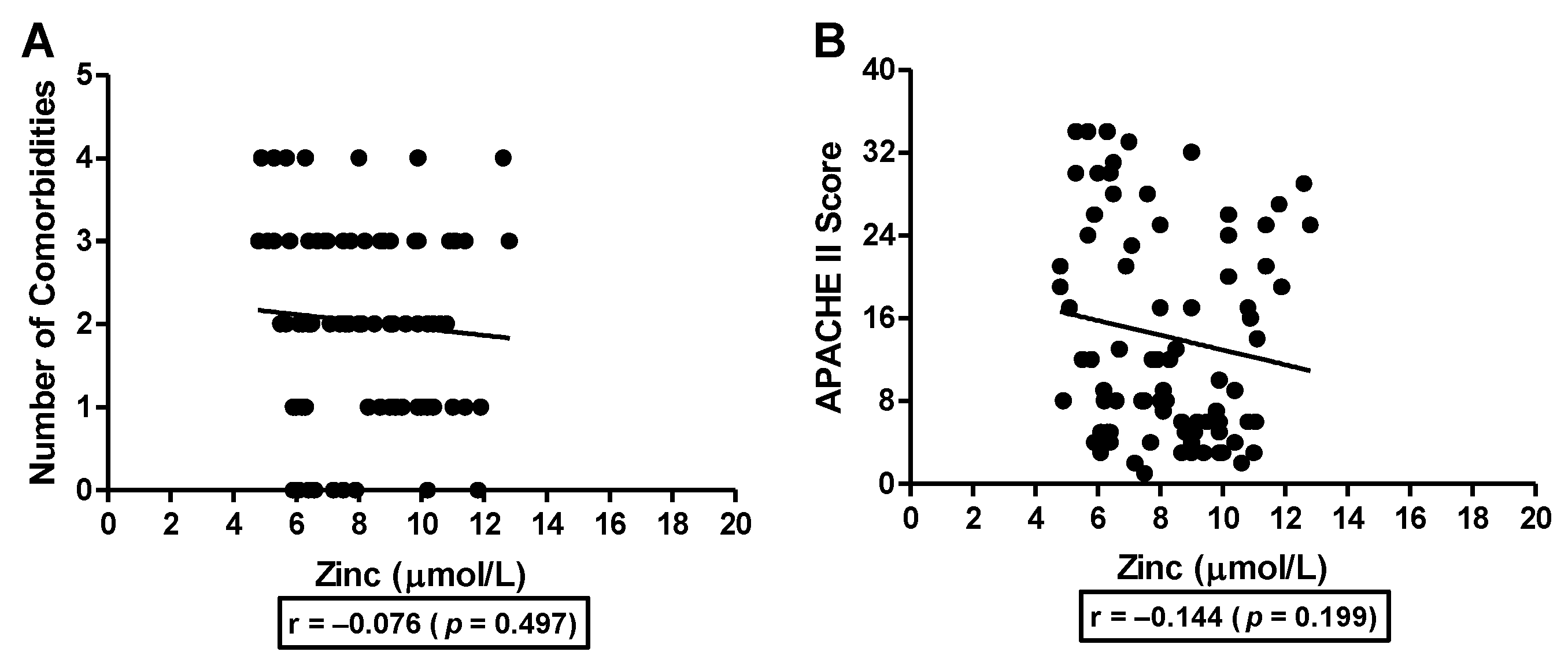
3.3. Association of Serum Zinc with Number of Comorbidities and APACHE II Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVID-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses 2020, 143, 110102. [Google Scholar] [CrossRef] [PubMed]
- Overbeck, S.; Rink, L.; Haase, H. Modulating the immune response by oral zinc supplementation: A single approach for multiple diseases. Arch. Immunol. Ther. Exp. 2008, 56, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Rink, L.; Haase, H. Chelation of Free Zn2+ Impairs Chemotaxis, Phagocytosis, Oxidative Burst, Degranulation, and Cytokine Production by Neutrophil Granulocytes. Biol. Trace Elem. Res. 2016, 171, 79–88. [Google Scholar] [CrossRef]
- Ibs, K.H.; Rink, L. Zinc-altered immune function. J. Nutr. 2003, 133, 1452S–1456S. [Google Scholar] [CrossRef]
- Jiang, P.; Mizushima, N. Autophagy and human diseases. Cell Res. 2014, 24, 69–79. [Google Scholar] [CrossRef]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef]
- Meijer, A.J.; Codogno, P. Autophagy: Regulation and role in disease. Crit. Rev. Clin. Lab. Sci. 2009, 46, 210–240. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Yoo, C. Role of zinc in the regulation of autophagy during ethanol exposure in human hepatoma cells. Biol. Trace Elem. Res. 2013, 156, 350–356. [Google Scholar] [CrossRef]
- Korant, B.D.; Kauer, J.C.; Butterworth, B.E. Zinc ions inhibit replication of rhinoviruses. Nature 1974, 248, 588–590. [Google Scholar] [CrossRef]
- Lanke, K.; Krenn, B.M.; Melchers, W.J.G.; Seipelt, J.; van Kuppeveld, F.J.M. PDTC inhibits picornavirus polyprotein processing and RNA replication by transporting zinc ions into cells. J. Gen. Virol. 2007, 88, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Krenn, B.M.; Gaudernak, E.; Holzer, B.; Lanke, K.; Van Kuppeveld, F.J.; Seipelt, J. Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. J. Virol. 2009, 83, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Te Velthuis, A.J.; van den Worm, S.H.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Cakman, I.; Kirchner, H.; Rink, L. Zinc supplementation reconstitutes the production of interferon-alpha by leukocytes from elderly persons. J. Interferon Cytokine Res. 1997, 17, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Bolt, G.; Andersen, H.; Owen, T.C. Zinc potentiates the antiviral action of human IFN-alpha tenfold. J. Interferon Cytokine Res. 2001, 21, 471–474. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef]
- Razeghi Jahromi, S.; Moradi Tabriz, H.; Togha, M.; Ariyanfar, S.; Ghorbani, Z.; Naeeni, S.; Haghighi, S.; Jazayeri, A.; Montazeri, M.; Talebpour, M.; et al. The correlation between serum selenium, zinc, and COVID-19 severity: An observational study. BMC Infect. Dis. 2021, 21, 899. [Google Scholar] [CrossRef]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68, 447S–463S. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Laur, N.; Kinscherf, R.; Pomytkin, K.; Kaiser, L.; Knes, O.; Deigner, H.P. ICP-MS trace element analysis in serum and whole blood. PLoS ONE 2020, 15, e0233357. [Google Scholar] [CrossRef]
- Xu, J.; Gong, Y.; Sun, Y.; Cai, J.; Liu, Q.; Bao, J.; Yang, J.; Zhang, Z. Impact of Selenium Deficiency on Inflammation, Oxidative Stress, and Phagocytosis in Mouse Macrophages. Biol. Trace Elem. Res. 2020, 194, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.S.; Haider Kazmi, S.J.; Khan, N.A.; Akram, M.; Hassan, M.; Rasheed, U.; Ahmed Khan, S. Poor Prognostic Biochemical Markers Predicting Fatalities Caused by COVID-19: A Retrospective Observational Study From a Developing Country. Cureus 2020, 12, e9575. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, K.; Kaur, H.; Sarma, P.; Bhattacharyya, A.; Sharma, D.J.; Prajapat, M.; Pathak, M.; Kothari, A.; Kumar, S.; Rana, S.; et al. Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J. Crit. Care 2022, 67, 172–181. [Google Scholar] [CrossRef]
- Jothimani, D.; Kailasam, E.; Danielraj, S.; Nallathambi, B.; Ramachandran, H.; Sekar, P.; Manoharan, S.; Ramani, V.; Narasimhan, G.; Kaliamoorthy, I.; et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020, 100, 343–349. [Google Scholar] [CrossRef]
- Wessels, I.; Rolles, B.; Rink, L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front. Immunol. 2020, 11, 1712. [Google Scholar] [CrossRef]
- Broman, L.M.; Bernardson, A.; Bursell, K.; Wernerman, J.; Flaring, U.; Tjader, I. Serum selenium in critically ill patients: Profile and supplementation in a depleted region. Acta Anaesthesiol. Scand. 2020, 64, 803–809. [Google Scholar] [CrossRef]
- Pal, A.; Squitti, R.; Picozza, M.; Pawar, A.; Rongioletti, M.; Dutta, A.K.; Sahoo, S.; Goswami, K.; Sharma, P.; Prasad, R. Zinc and COVID-19: Basis of Current Clinical Trials. Biol. Trace Elem. Res. 2021, 199, 2882–2892. [Google Scholar] [CrossRef]
- Besecker, B.; Bao, S.; Bohacova, B.; Papp, A.; Sadee, W.; Knoell, D.L. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cytoprotection in lung epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L1127–L1136. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Liles, W.C.; Steinberg, K.P.; Kiener, P.A.; Mongovin, S.; Chi, E.Y.; Jonas, M.; Martin, T.R. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS). J. Immunol. 1999, 163, 2217–2225. [Google Scholar] [PubMed]
- Devaux, C.A.; Rolain, J.M.; Raoult, D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020, 53, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Li, L.; Zhang, Y.; Wang, X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Hardigan, P.C. A Case-Control Study for the Effectiveness of Oral Zinc in the Prevention and Mitigation of COVID-19. Front. Med. 2021, 8, 756707. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Controls (48) | COVID-19 Positive (123) | p |
|---|---|---|---|
| Age (years) | 33.5 ± 8.3 | 56.2 ± 20.3 | 0.000 |
| Zinc (µmol/L) | 11.9 ± 1.8 | 8.8 ± 2.3 | 0.000 |
| WBC (g/L) | 5.83 ± 2.36 | 9.19 ± 7.60 | 0.003 |
| CRP (mg/L) | 1.6 ± 0.9 | 74.6 ± 88.7 | 0.000 |
| PCT (ng/mL) | 0.03 ± 0.07 | 1.37 ± 4.90 | 0.060 |
| LDH (U/L) | 180.4 ± 37.6 | 356.5 ± 286.5 | 0.000 |
| Ferritin (ng/L) | 61.0 ± 61.2 | 758.7 ± 1483.0 | 0.001 |
| Parameters | Controls | Mild | Moderate | Severe | p |
|---|---|---|---|---|---|
| Age (years) (n) | 33.5 ± 8.3 (48) | 35.3 ± 9.9 (42) | 65.6 ± 13.5 (41) | 68.6 ± 16.7 (40) | 0.000 |
| Sex (M/F) | 23/25 | 17/25 | 19/22 | 20/20 | 0.839 |
| Death (%) | 0 (0%) | 0 (0%) | 1 (2.4%) | 25 (62.5%) | |
| Zinc (µmol/L) | 11.9 ± 1.8 | 10.1 ± 2.2 | 8.2 ± 1.8 | 8.2 ± 2.3 | 0.000 |
| WBC (g/L) | 5.83 ± 2.36 | 5.68 ± 2.04 | 7.27 ± 3.50 | 14.86 ± 10.67 | 0.000 |
| CRP (mg/L) | 1.6 ± 0.9 | 13.6 ± 25.0 | 87.3 ± 82.2 | 125.5 ± 100.8 | 0.000 |
| PCT (ng/mL) | 0.03 ± 0.07 | 0.04 ± 0.05 | 1.30 ± 4.67 | 2.88 ± 7.01 | 0.004 |
| LDH (U/L) | 180.4 ± 37.6 | 203.1 ± 66.2 | 361.8 ± 159.4 | 512.2 ± 419.3 | 0.000 |
| Ferritin (ng/L) | 61.0 ± 61.2 | 127.0 ± 240.2 | 854.4 ± 1373.5 | 1323.8 ± 2029.9 | 0.000 |
| Parameters | Hospital Discharge | Death | p |
|---|---|---|---|
| Age (years) (n) | 63.7 ± 14.3 (55) | 74.2 ± 14.5 (26) | 0.003 |
| Sex (M/F) | 23/32 | 16/10 | 0.253 |
| ICU required (%) | 15 (37.5%) | 25 (62.5%) | |
| Zinc (µmol/L) | 8.4 ± 1.8 | 7.9 ± 2.6 | 0.317 |
| WBC (g/L) | 7.77 ± 3.59 | 17.88 ± 11.98 | 0.000 |
| CRP (mg/L) | 82.6 ± 76.9 | 155.4 ± 107.7 | 0.004 |
| PCT (ng/mL) | 1.09 ± 4.12 | 4.10 ± 8.33 | 0.032 |
| LDH (U/L) | 347.3 ± 136.9 | 623.8 ± 488.7 | 0.000 |
| Ferritin (ng/L) | 966.2 ± 1939.2 | 1340.1 ± 1184.6 | 0.368 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almasaud, A.S.; Chalabi, J.; Arfaj, A.A.; Qarni, A.A.; Alkroud, A.; Nagoor, Z.; Akhtar, S.; Iqbal, J. Association of Serum Zinc and Inflammatory Markers with the Severity of COVID-19 Infection in Adult Patients. Nutrients 2023, 15, 340. https://doi.org/10.3390/nu15020340
Almasaud AS, Chalabi J, Arfaj AA, Qarni AA, Alkroud A, Nagoor Z, Akhtar S, Iqbal J. Association of Serum Zinc and Inflammatory Markers with the Severity of COVID-19 Infection in Adult Patients. Nutrients. 2023; 15(2):340. https://doi.org/10.3390/nu15020340
Chicago/Turabian StyleAlmasaud, Abdulaziz Saad, Jamal Chalabi, Abdulmajid Al Arfaj, Ali Al Qarni, Ammar Alkroud, Zuheb Nagoor, Sana Akhtar, and Jahangir Iqbal. 2023. "Association of Serum Zinc and Inflammatory Markers with the Severity of COVID-19 Infection in Adult Patients" Nutrients 15, no. 2: 340. https://doi.org/10.3390/nu15020340
APA StyleAlmasaud, A. S., Chalabi, J., Arfaj, A. A., Qarni, A. A., Alkroud, A., Nagoor, Z., Akhtar, S., & Iqbal, J. (2023). Association of Serum Zinc and Inflammatory Markers with the Severity of COVID-19 Infection in Adult Patients. Nutrients, 15(2), 340. https://doi.org/10.3390/nu15020340






