Effects of Voluntary Sodium Consumption during the Perinatal Period on Renal Mechanisms, Blood Pressure, and Vasopressin Responses after an Osmotic Challenge in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sodium Overload (SO)
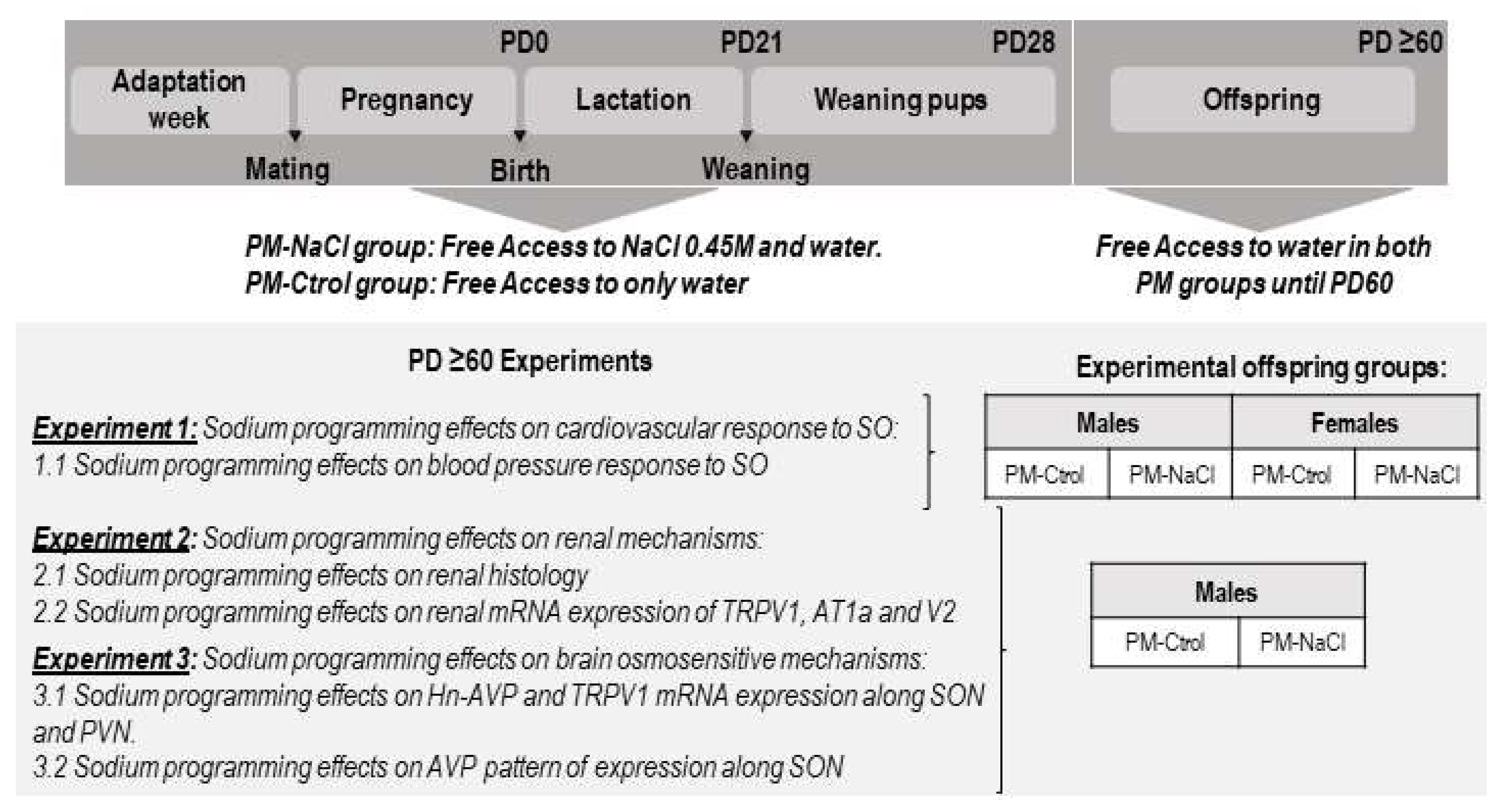
2.3. Experimental Protocols
2.4. Relative mRNA Expression of Agtr1a, Trpv1, Avpr2, and hn-Avp in the Brain and Kidney
Calculations of Relative Gene Expression
2.5. Renal Histological Staining and Analysis Using Hematoxylin Eosin
2.6. Immunofluorescence
2.7. Statistical Analysis
3. Results
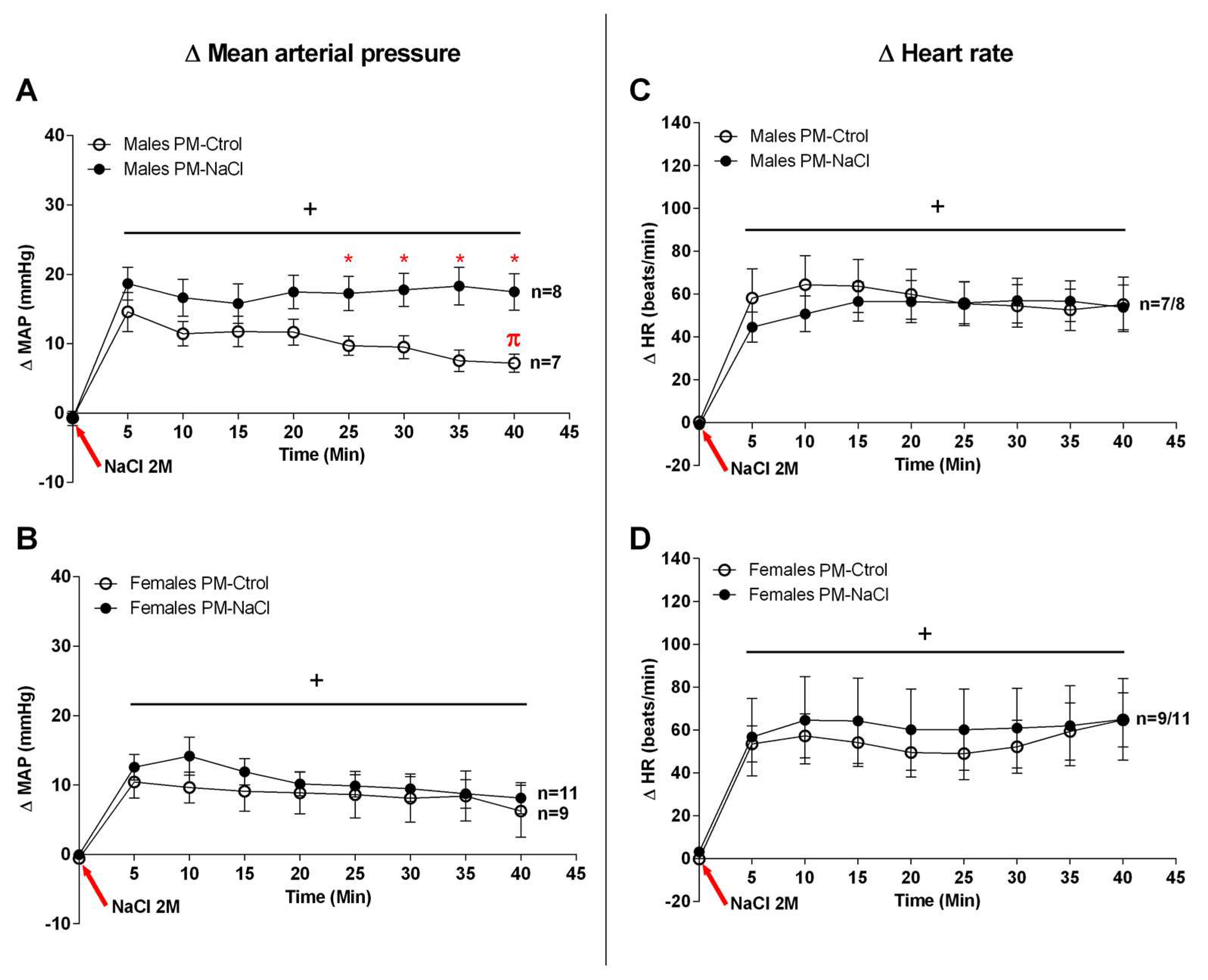
3.1. Sodium Programming Effects on Cardiovascular Response to SO
3.2. Sodium Programming Effects on Renal Mechanisms
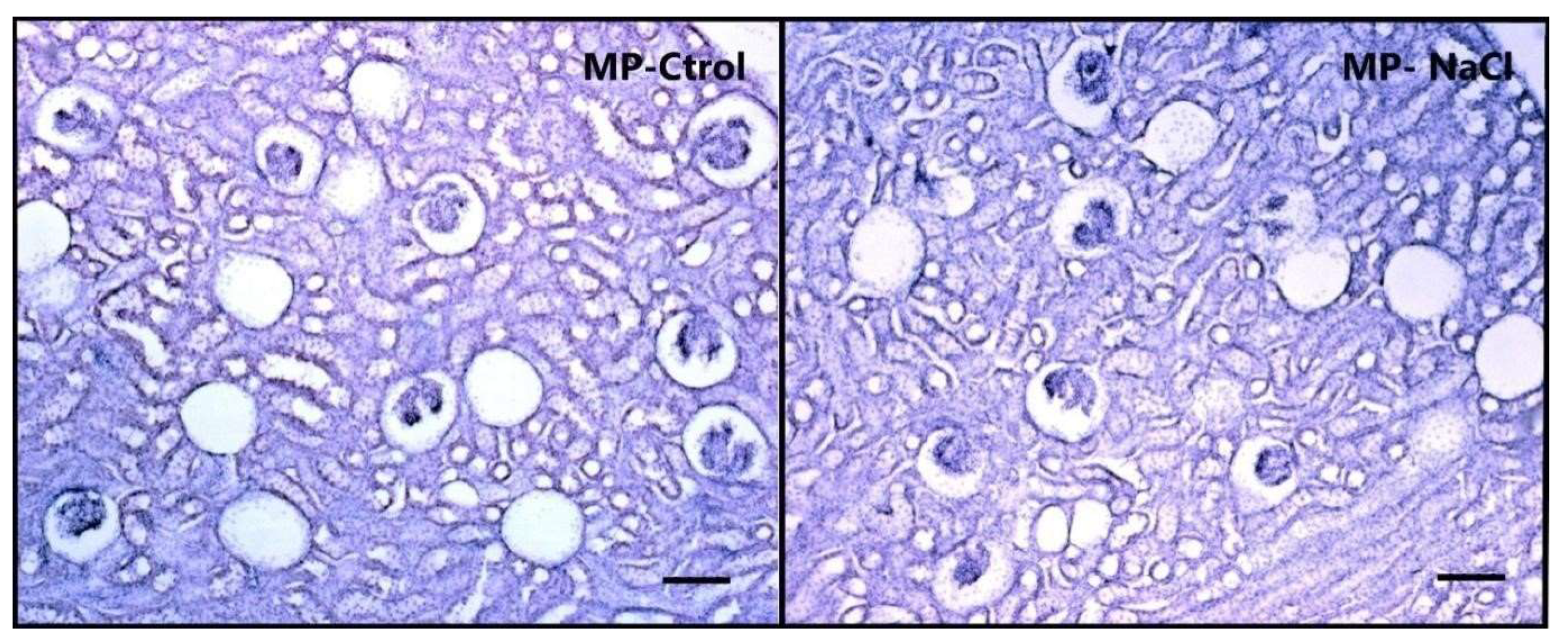
3.2.1. Sodium Programming Effects on Renal Histology
3.2.2. Sodium Programming Effects on Renal mRNA Expression of TRPV1, AT1a and V2 Receptor
3.3. Sodium Programming Effects on Brain Osmosensitive Mechanisms
3.3.1. Hn-Avp and Trpv1 mRNA Expression along SON and PVN
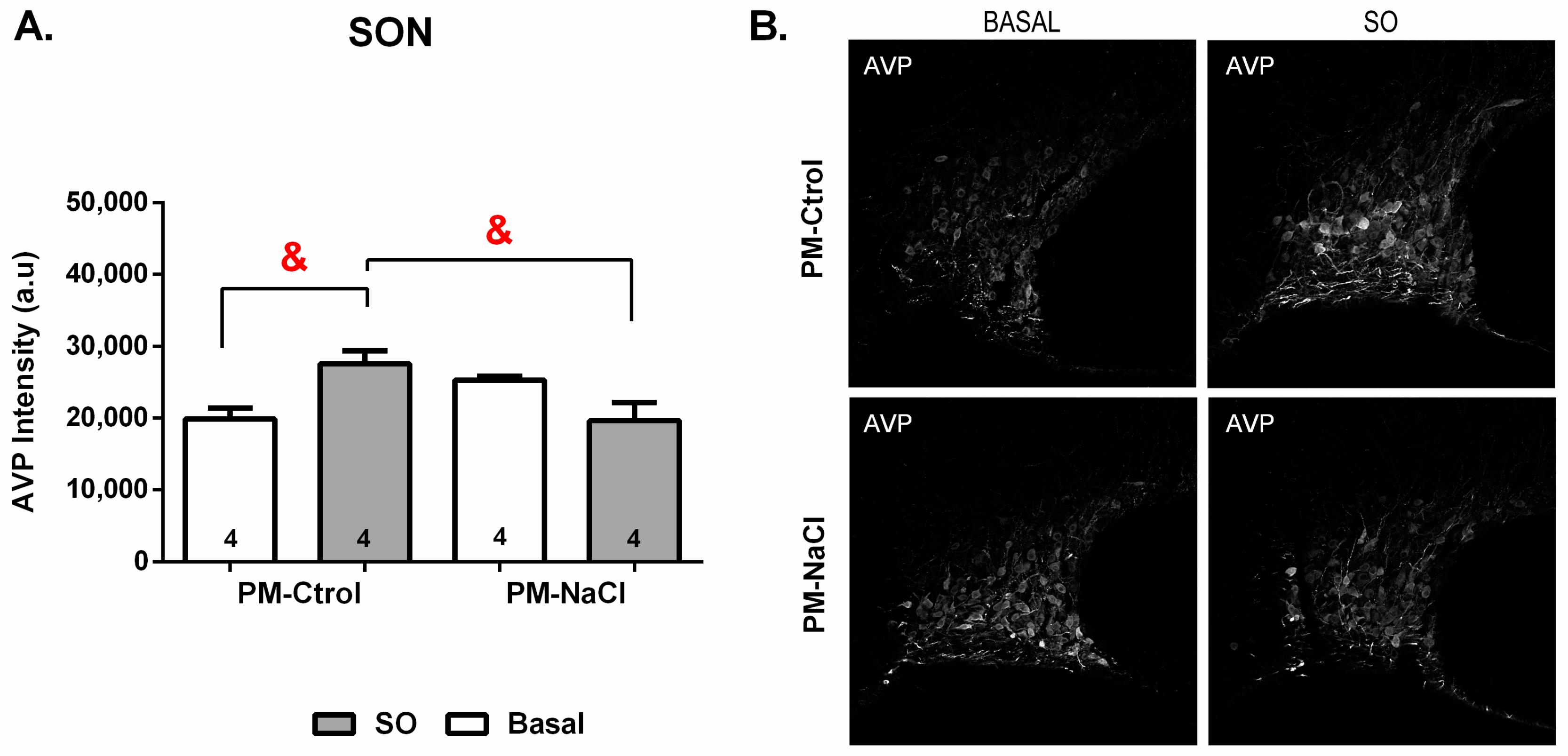
3.3.2. Sodium Programming Effects on AVP Pattern of Expression along SON
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leshem, M. Biobehavior of the human love of salt. Neurosci. Biobehav. Rev. 2009, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Galaverna, O.; Nicolaïdis, S.; Yao, S.Z.; Sakai, R.R.; Epstein, A.N. Endocrine consequences of prenatal sodium depletion prepare rats for high need-free NaCl intake in adulthood. Am. J. Physiol. Content 1995, 269, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.J.; Kosten, T. Prenatal and early postnatal sodium chloride intake modifies the solution preferences of adult rats. J. Nutr. 1983, 113, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Vijande, M.; Brime, J.I.; López-Sela, P.; Costales, M.; Arguelles, J. Increased salt preference in adult offspring raised by mother rats consuming excessive amounts of salt and water. Regul. Pept. 1996, 66, 105–108. [Google Scholar] [CrossRef]
- Curtis, K.S.; Krause, E.G.; Wong, D.L.; Contreras, R.J. Gestational and early postnatal dietary NaCl levels affect NaCl intake, but not stimulated water intake, by adult rats. Am. J. Physiol. Integr. Comp. Physiol. 2004, 286, R1043–R1050. [Google Scholar] [CrossRef]
- Málaga, I.; Arguelles, J.; Díaz, J.J.; Perillán, C.; Vijande, M.; Málaga, S.; Arguelles-Luis, J. Maternal pregnancy vomiting and offspring salt taste sensitivity and blood pressure. Pediatr. Nephrol. 2005, 20, 956–960. [Google Scholar] [CrossRef]
- Argüelles, J.; Brime, J.; López-Sela, P.; Perillán, C.; Vijande, M. Adult Offspring Long-Term Effects of High Salt and Water Intake during Pregnancy. Horm. Behav. 2000, 37, 156–162. [Google Scholar] [CrossRef]
- Perillán, C.; Núñez, P.; Costales, M.; Vijande, M.; Argüelles, J. Ingestive behavior in rat pups is modified by maternal sodium depletion. Psicothema 2012, 24, 422–426. [Google Scholar]
- Contreras, R.J. Differences in perinatal NaCl exposure alters blood pressure levels of adult rats. Am. J. Physiol. 1989, 256, R70–R77. [Google Scholar] [CrossRef]
- Contreras, R.J. High NaCl intake of rat dams alters maternal behavior and elevates blood pressure of adult offspring. Am. J. Physiol. Content 1993, 264, 296–304. [Google Scholar] [CrossRef]
- Argüelles, J.; López-Sela, P.; Brime, J.I.; Costales, M.; Vijande, M. Changes of blood pressure responsiveness in rats exposed in utero and perinatally to a high-salt environment. Regul. Pept. 1996, 66, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.J.; Ryan, K.W. Perinatal exposure to a high NaCl diet increases the NaCl intake of adult rats. Physiol. Behav. 1990, 47, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.J.; Wong, D.L.; Henderson, R.; Curtis, K.S.; Smith, J.C. High dietary NaCl early in development enhances mean arterial pressure of adult rats. Physiol. Behav. 2000, 71, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Koleganova, N.; Piecha, G.; Ritz, E.; Becker, L.E.; Müller, A.; Weckbach, M.; Nyengaard, J.R.; Schirmacher, P.; Gross-Weissmann, M.L. Both high and low maternal salt intake in pregnancy alter kidney development in the offspring. Am. J. Physiol. Renal. Physiol. 2011, 301, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Piecha, G.; Koleganova, N.; Ritz, E.; Müller, A.; Fedorova, O.V.; Bagrov, A.Y.; Lutz, D.; Schirmacher, P.; Gross-Weissmann, M.L. High salt intake causes adverse fetal programming—vascular effects beyond blood pressure. Nephrol. Dial. Transplant. 2012, 27, 3464–3476. [Google Scholar] [CrossRef] [PubMed]
- Macchione, A.; Beas, C.; Dadam, F.; Caeiro, X.; Godino, A.; Ponce, L.; Amigone, J.; Vivas, L. Early free access to hypertonic NaCl solution induces a long-term effect on drinking, brain cell activity and gene expression of adult rat offspring. Neuroscience 2015, 298, 120–136. [Google Scholar] [CrossRef]
- Macchione, A.; Caeiro, X.; Godino, A.; Amigone, J.; Antunes-Rodrigues, J.; Vivas, L. Availability of a rich source of sodium during the perinatal period programs the fluid balance restoration pattern in adult offspring. Physiol. Behav. 2012, 105, 1035–1044. [Google Scholar] [CrossRef]
- Johnson, A.K.; Gross, P.M. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993, 7, 678–686. [Google Scholar] [CrossRef]
- McKinley, M.J.; McAllen, R.; Davern, P.; Giles, M.E.; Penschow, J.; Sunn, N.; Uschakov, A.; Oldfield, B. The Sensory Circumventricular Organs of the Mammalian Brain. Adv. Anat. Embryol. Cell Biol. 2003, 172, 1–122. [Google Scholar]
- Murphy, D.; Konopacka, A.; Hindmarch, C.; Paton, J.F.R.; Sweedler, J.V.; Gillette, M.U.; Ueta, Y.; Grinevich, V.; Lozic, M.; Japundzic-Zigon, N. The Hypothalamic-Neurohypophyseal System: From Genome to Physiology. J. Neuroendocr. 2012, 24, 539–553. [Google Scholar] [CrossRef]
- Oliet, S.H.; Bourque, C.W. Mechanosensitive channels transducer osmosensitivity in supraoptic neurons. Nature 1993, 364, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Sharif Naeini, R.; Witty, M.F.; Séguéla, P.; Bourque, C.W. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat. Neurosci. 2006, 9, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Sudbury, J.R.; Ciura, S.; Sharif-Naeini, R.; Bourque, C.W. Osmotic and thermal control of magnocellular neurosecretory neurons--role of an N-terminal variant of trpv1. Eur. J. Neurosci. 2010, 32, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Ciura, S.; Bourque, C.W. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J. Neurosci. 2006, 26, 9069–9075. [Google Scholar] [CrossRef] [PubMed]
- Prager-Khoutorsky, M.; Bourque, C.W. Mechanical basis of osmosensory transduction in magnocellular neurosecretory neu-rones of the rat supraoptic nucleus. J. Neuroendocrinol. 2015, 27, 507–515. [Google Scholar] [CrossRef]
- Prager-Khoutorsky, M.; Khoutorsky, A.; Bourque, C.W. Unique Interweaved Microtubule Scaffold Mediates Osmosensory Transduction via Physical Interaction with TRPV1. Neuron 2014, 83, 866–878. [Google Scholar] [CrossRef]
- Porcari, C.Y.; Cambiasso, M.J.; Mecawi, A.S.; Caeiro, X.E.; Antunes-Rodrigues, J.; Vivas, L.M.; Godino, A. Molecular neurobiological markers in the onset of sodium appetite. Sci. Rep. 2022, 12, 14224. [Google Scholar] [CrossRef]
- Porcari, C.; Debarba, L.; Amigone, J.; Caeiro, X.; Reis, L.; Cunha, T.; Mecawi, A.; Elias, L.; Antunes-Rodrigues, J.; Vivas, L.; et al. Brain osmo-sodium sensitive channels and the onset of sodium appetite. Horm. Behav. 2020, 118, 104658. [Google Scholar] [CrossRef]
- Wang, Y.; Babánková, D.; Huang, J.; Swain, G.M.; Wang, N.H. Deletion of Transient Receptor Potential Vanilloid Type 1 Receptors Exaggerates Renal Damage in Deoxycorticosterone Acetate-Salt Hypertension. Hypertension 2008, 52, 264–270. [Google Scholar] [CrossRef]
- Rayamajhi, S.; Contractor, T.; Wang, N.H. The potential of TRPV1 agonists for treating ischemia/reperfusion-induced renal injuries. Curr. Opin. Investig. Drugs 2009, 10, 963–970. [Google Scholar]
- Yu, S.-Q.; Ma, S.; Wang, D.H. Activation of TRPV1 Prevents Salt-Induced Kidney Damage and Hypertension After Renal Ischemia-Reperfusion Injury in Rats. Kidney Blood Press. Res. 2018, 43, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Caeiro, X.; Vivas, L. Beta-Endorphin in the median preoptic nucleus modulates the pressor response induced by subcutaneous hypertonic sodium chloride. Exp. Neurol. 2008, 210, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Porcari, C.Y.; Araujo, I.G.; Urzedo-Rodrigues, L.; De Luca, L.A., Jr.; Menani, J.V.; Caeiro, X.E.; Imboden, H.; Antunes-Rodrigues, J.; Reis, L.C.; Vivas, L.; et al. Whole body sodium depletion modifies AT1 mRNA expression 581 and serotonin content in the dorsal raphe nucleus. J. Neuroendocrinol. 2019, 31, 12703. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: New York, NY, USA, 1997. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Ben-Barak, Y.; Russell, J.T.; Whitnall, M.H.; Ozato, K.; Gainer, H. Neurophysin in the hypothalamo-neurohypophysial system. I. Production and characterization of monoclonal antibodies. J. Neurosci. 1985, 5, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef]
- Mcdonald, J.H.; Dunn, K.W. Statistical tests for measures of colocalization in biological microscopy. J. Microsc. 2013, 252, 295–302. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- da Silva, E.F.; Bassi, M.; Menani, J.V.; Colombari, D.S.A.; Zoccal, D.B.; Pedrino, G.R.; Colombari, E. Carotid bodies contribute to sympathoexcitation induced by acute salt overload. Exp. Physiol. 2019, 104, 15–27. [Google Scholar] [CrossRef]
- Pamidimukkala, J.; Xue, B.; Newton, L.G.; Lubahn, D.B.; Hay, M. Estrogen receptor-α mediates estrogen facilitation of baroreflex heart rate responses in conscious mice. Am. J. Physiol. Circ. Physiol. 2005, 288, H1063–H1070. [Google Scholar] [CrossRef]
- Pourshanazari, A.; Ciriello, J.; Tajadini, H. Role of 17-beta estradiol in baroreflex sensitivity in the nucleus tractus solitarii via the autonomic system in ovariectomized rats. Neurosciences 2013, 18, 126–132. [Google Scholar] [PubMed]
- Caeiro, X.E.; Mir, F.R.; Vivas, L.M.; Carrer, H.F.; Cambiasso, M.J. Sex chromosome complement contributes to sex differences in bradycardic baroreflex response. Hypertension 2011, 58, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.; Blache, D.; Gregg, K.; Revell, D. Consumption of a high-salt diet by ewes during pregnancy alters nephrogenesis in 5-month-old offspring. Animal 2012, 6, 1803–1810. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marin, E.C.; Balbi, A.P.; Francescato, H.D.; Alves da Silva, C.G.; Costa, R.S.; Coimbra, T.M. Renal structure and function evaluation of rats from dams that received increased sodium intake during pregnancy and lactation submitted or not to 5/6 nephrectomy. Ren. Fail. 2008, 30, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Garcia, D.L.; Anderson, S. Glomeruli and blood pressure. Less of one, more the other? Am. J. Hypertens. 1988, 1, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.L.; Rasch, R. Perinatal ANG II programs adult blood pressure, glomerular number, and renal function in rats. Am. J. Physiol. Integr. Comp. Physiol. 1998, 275, R1593–R1599. [Google Scholar] [CrossRef]
- Miyata, N.; Park, F.; Li, X.F.; Cowley, A.W., Jr. Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am. J. Physiol. 1999, 277, F437–F446. [Google Scholar]
- Wang, Y.; Wang, D.H. A Novel Mechanism Contributing to Development of Dahl Salt–Sensitive Hypertension. Hypertension 2006, 47, 609–614. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.H. Increased GFR and renal excretory function by activation of TRPV1 in the isolated perfused kidney. Pharmacol. Res. 2008, 57, 239–246. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.H. Aggravated renal inflammatory responses in TRPV1 gene knockout mice subjected to DOCA-salt hypertension. Am. J. Physiol. Physiol. 2009, 297, F1550–F1559. [Google Scholar] [CrossRef]
- Antunes, V.R.; Yao, S.T.; Pickering, A.E.; Murphy, D.; Paton, J.F. A spinal vasopressinergic mechanism mediates hyperosmolality-induced sympathoexcitation. J. Physiol. 2006, 576, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Holbein, W.W.; Toney, G.M. Activation of the hypothalamic paraventricular nucleus by forebrain hypertonicity selectively increases tonic vasomotor sympathetic nerve activity. Am. J. Physiol. Integr. Comp. Physiol. 2015, 308, R351–R359. [Google Scholar] [CrossRef] [PubMed]
- McKinley, M.J.; Yao, S.T.; Uschakov, A.; McAllen, R.M.; Rundgren, M.; Martelli, D. The median preoptic nucleus: Front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol. 2015, 214, 8–32. [Google Scholar] [CrossRef] [PubMed]
- Pedrino, G.R.; Monaco, L.R.; Cravo, S.L. Renal vasodilation induced by hypernatraemia: Role of α-adrenoceptors in the median preoptic nucleus. Clin. Exp. Pharmacol. Physiol. 2009, 36, e83–e89. [Google Scholar] [CrossRef] [PubMed]
- Brooks, V.L.; Freeman, K.L.; O’Donaughy, T.L. Acute and chronic increases in osmolality increase excitatory amino acid drive of the rostral ventrolateral medulla in rats. Am. J. Physiol. Integr. Comp. Physiol. 2004, 287, R1359–R1368. [Google Scholar] [CrossRef] [PubMed]
- Hochstenbach, S.L.; Ciriello, J. Effects of plasma hypernatremia on nucleus tractus solitarius neurons. Am. J. Physiol. Content 1994, 266, 1916–1921. [Google Scholar] [CrossRef]
- Blanch, G.T.; Freiria-Oliveira, A.H.; Murphy, D.; Paulin, R.F.; Antunes-Rodrigues, J.; Colombari, E.; Colombari, D.S. Inhibitory mechanism of the nucleus of the solitary tract involved in the control of cardiovascular, dipsogenic, hormonal, and renal responses to hyperosmolality. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, 531–542. [Google Scholar] [CrossRef]


| Gene | Forward Primer 5′-3′ | Reverse Primer 5′-3′ | Product Size (bp) |
|---|---|---|---|
| Gapdh | TGTGAACGGATTTGGCCGTA | ATGAAGGGGTCGTTGATGGC | 20 |
| Agtr1a | AACCCTCTGTTCTACGGC | ACCTGTCACTCCACCTCA | 18 |
| Trpv1 | TTCACCGAATGGGCCTATGG | TGAC- GGTTAGGGGTCTCACT | 20 |
| Avp hnRNA | GACGCAA- GAGGGCCACATC | CTCTCCTAGCCCATGA CCCTT | 20/19 |
| Avpr2 | AAGCTCCTCTGGAAAGACCC | CAAAGCAGGCTACGCAACTC | 20 |
| Factors Analyzed | PM-Ctrol Mean ± SE | PM-NaCl Mean ± SE | p-Value |
|---|---|---|---|
| Kidney size (g/100 gpc) | 0.43 ± 0.01 (n = 23) | 0.42 ± 0.01 (n = 26) | 0.204 |
| Renal cortex size (mm) | 14.73 ± 0.79 (n = 6) | 15.02 ± 0.42 (n = 7) | 0.745 |
| N° Total of glomeruli (5× microscope magnifications) | 14.54 ± 0.42 (n = 6) | 12.39 ± 0.56 (n = 6) | 0.012 * |
| N° of glomeruli/mm2 | 3.0 × 10−2 ± 9.1 × 10−4 (n = 6) | 2.0 × 10−2 ± 9.8 × 10−4 (n = 6) | 0.007 ** |
| Glomerular capillary/Bowman’s capsule (%) | 83 ± 4 (n = 7) | 80 ± 2 (n = 7) | 0.559 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcari, C.Y.; Macagno, A.; Mecawi, A.S.; Anastasía, A.; Caeiro, X.E.; Godino, A. Effects of Voluntary Sodium Consumption during the Perinatal Period on Renal Mechanisms, Blood Pressure, and Vasopressin Responses after an Osmotic Challenge in Rats. Nutrients 2023, 15, 254. https://doi.org/10.3390/nu15020254
Porcari CY, Macagno A, Mecawi AS, Anastasía A, Caeiro XE, Godino A. Effects of Voluntary Sodium Consumption during the Perinatal Period on Renal Mechanisms, Blood Pressure, and Vasopressin Responses after an Osmotic Challenge in Rats. Nutrients. 2023; 15(2):254. https://doi.org/10.3390/nu15020254
Chicago/Turabian StylePorcari, Cintia Y., Agustina Macagno, André S. Mecawi, Agustín Anastasía, Ximena E. Caeiro, and Andrea Godino. 2023. "Effects of Voluntary Sodium Consumption during the Perinatal Period on Renal Mechanisms, Blood Pressure, and Vasopressin Responses after an Osmotic Challenge in Rats" Nutrients 15, no. 2: 254. https://doi.org/10.3390/nu15020254
APA StylePorcari, C. Y., Macagno, A., Mecawi, A. S., Anastasía, A., Caeiro, X. E., & Godino, A. (2023). Effects of Voluntary Sodium Consumption during the Perinatal Period on Renal Mechanisms, Blood Pressure, and Vasopressin Responses after an Osmotic Challenge in Rats. Nutrients, 15(2), 254. https://doi.org/10.3390/nu15020254






