Maternal Intake of Vitamin D Supplements during Pregnancy and Pubertal Timing in Children: A Population-Based Follow-Up Study
Abstract
:1. Introduction
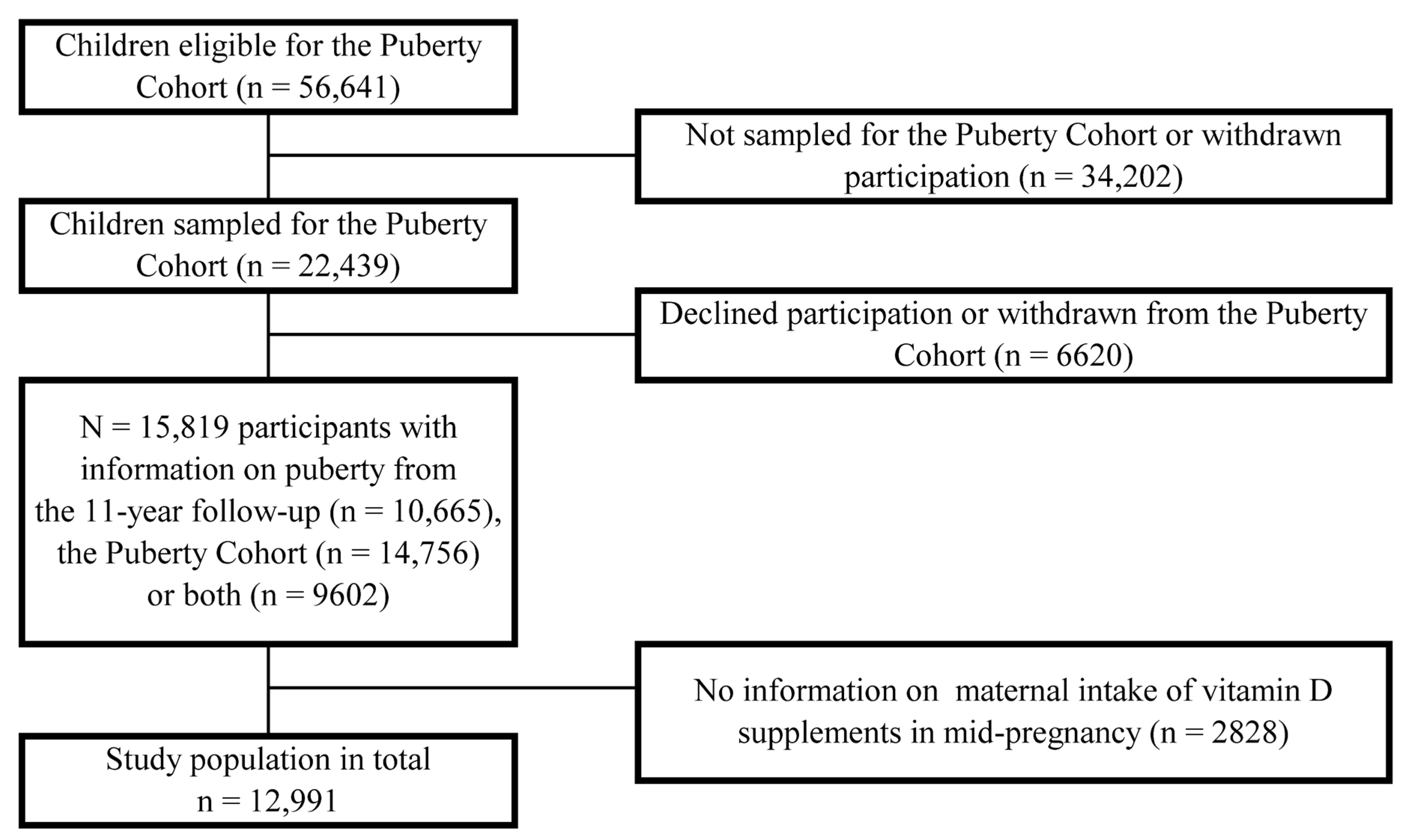
2. Materials and Methods
2.1. Vitamin D Supplements
2.2. Pubertal Timing
2.3. Covariates
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Strengths and Limitations
4.2. Interpretation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press (US); National Academy of Sciences: Washington, DC, USA, 2011. [Google Scholar]
- Snegarova, V.; Naydenova, D. Vitamin D: A Review of its Effects on Epigenetics and Gene Regulation. Folia Med. 2020, 62, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Zittermann, A.; Obeid, R.; Hahn, A.; Pludowski, P.; Trummer, C.; Lerchbaum, E.; Perez-Lopez, F.R.; Karras, S.N.; Marz, W. The Role of Vitamin D in Fertility and during Pregnancy and Lactation: A Review of Clinical Data. Int. J. Environ. Res. Public Health 2018, 15, 2241. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaum, E.; Obermayer-Pietsch, B. Vitamin D and fertility: A systematic review. Eur. J. Endocrinol. 2012, 166, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, M.; Boisen, I.M.; Mortensen, L.J.; Lanske, B.; Juul, A.; Blomberg Jensen, M. Reproductive endocrinology of vitamin D. Mol. Cell. Endocrinol. 2017, 453, 103–112. [Google Scholar] [CrossRef]
- Quaresima, P.; Angeletti, M.; Luziatelli, D.; Luziatelli, S.; Venturella, R.; Di Carlo, C.; Bernardo, S. Pregnancy associated transient osteoporosis of the hip (PR-TOH): A non-obstetric indication to caesarean section. A case report with literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 262, 28–35. [Google Scholar] [CrossRef]
- McGrath, J. Does ‘imprinting’ with low prenatal vitamin D contribute to the risk of various adult disorders? Med. Hypotheses 2001, 56, 367–371. [Google Scholar] [CrossRef]
- Larque, E.; Morales, E.; Leis, R.; Blanco-Carnero, J.E. Maternal and Foetal Health Implications of Vitamin D Status during Pregnancy. Ann. Nutr. Metab. 2018, 72, 179–192. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W. The Implications of Vitamin D Status During Pregnancy on Mother and her Developing Child. Front. Endocrinol. 2018, 9, 500. [Google Scholar] [CrossRef]
- Xue, J.; Schoenrock, S.A.; Valdar, W.; Tarantino, L.M.; Ideraabdullah, F.Y. Maternal vitamin D depletion alters DNA methylation at imprinted loci in multiple generations. Clin. Epigenetics 2016, 8, 107. [Google Scholar] [CrossRef]
- Abreu, A.P.; Kaiser, U.B. Pubertal development and regulation. Lancet. Diabetes Endocrinol. 2016, 4, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Chadio, S.; Kotsampasi, B. The role of early life nutrition in programming of reproductive function. J. Dev. Orig. Health Dis. 2014, 5, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Rhind, S.M.; Rae, M.T.; Brooks, A.N. Effects of nutrition and environmental factors on the fetal programming of the reproductive axis. Reproduction 2001, 122, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Juul, A. In utero programming of pubertal development? Arch. Dis. Child. 2011, 96, 703. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, L.; Cofini, M.; Leonardi, A.; Penta, L.; Esposito, S. Up-To-Date Review About Minipuberty and Overview on Hypothalamic-Pituitary-Gonadal Axis Activation in Fetal and Neonatal Life. Front. Endocrinol. 2018, 9, 410. [Google Scholar] [CrossRef]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Nicholas, C.; Davis, J.; Fisher, T.; Segal, T.; Petti, M.; Sun, Y.; Wolfe, A.; Neal-Perry, G. Maternal Vitamin D Deficiency Programs Reproductive Dysfunction in Female Mice Offspring Through Adverse Effects on the Neuroendocrine Axis. Endocrinology 2016, 157, 1535–1545. [Google Scholar] [CrossRef]
- Gaml-Sørensen, A.; Brix, N.; Hærvig, K.K.; Lindh, C.; Tøttenborg, S.S.; Hougaard, K.S.; Høyer, B.B.; Ernst, A.; Arendt, L.H.; Clemmensen, P.J.; et al. Maternal vitamin D levels and male reproductive health: A population-based follow-up study. Eur. J. Epidemiol. 2023, 38, 469–484. [Google Scholar] [CrossRef]
- Day, F.R.; Elks, C.E.; Murray, A.; Ong, K.K.; Perry, J.R.B. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: The UK Biobank study. Sci. Rep. 2015, 5, 11208. [Google Scholar] [CrossRef]
- Golub, M.S.; Collman, G.W.; Foster, P.M.; Kimmel, C.A.; Rajpert-De Meyts, E.; Reiter, E.O.; Sharpe, R.M.; Skakkebaek, N.E.; Toppari, J. Public health implications of altered puberty timing. Pediatrics 2008, 121 (Suppl. 3), S218–S230. [Google Scholar] [CrossRef]
- Ernst, A.; Brix, N.; Lauridsen, L.L.B.; Strandberg-Larsen, K.; Bech, B.H.; Nohr, E.A.; Nybo Andersen, A.M.; Parner, E.T.; Meder, I.K.; Olsen, J.; et al. Cohort Profile: The Puberty Cohort in the Danish National Birth Cohort (DNBC). Int. J. Epidemiol. 2020, 49, 373–374g. [Google Scholar] [CrossRef]
- Olsen, J.; Melbye, M.; Olsen, S.F.; Sorensen, T.I.; Aaby, P.; Andersen, A.M.; Taxbol, D.; Hansen, K.D.; Juhl, M.; Schow, T.B.; et al. The Danish National Birth Cohort--its background, structure and aim. Scand. J. Public Health 2001, 29, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Brix, N.; Ernst, A.; Lauridsen, L.L.B.; Parner, E.T.; Olsen, J.; Henriksen, T.B.; Ramlau-Hansen, C.H. Maternal Smoking During Pregnancy and Timing of Puberty in Sons and Daughters: A Population-Based Cohort Study. Am. J. Epidemiol. 2019, 188, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.F.; Mikkelsen, T.B.; Knudsen, V.K.; Orozova-Bekkevold, I.; Halldorsson, T.I.; Strom, M.; Osterdal, M.L. Data collected on maternal dietary exposures in the Danish National Birth Cohort. Paediatr. Perinat. Epidemiol. 2007, 21, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef]
- Greenland, S.; Pearl, J.; Robins, J.M. Causal diagrams for epidemiologic research. Epidemiology 1999, 10, 37–48. [Google Scholar] [CrossRef]
- Bjerregaard, A.A.; Halldorsson, T.I.; Tetens, I.; Olsen, S.F. Mother’s dietary quality during pregnancy and offspring’s dietary quality in adolescence: Follow-up from a national birth cohort study of 19,582 mother-offspring pairs. PLoS Med. 2019, 16, e1002911. [Google Scholar] [CrossRef]
- Huber, P.J. (Ed.) The behavior of maximum likelihood estimates under nonstandard conditions. In Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Volume 1: Statistics; 1967; University of California Press: Berkeley, CA, USA, 1967. [Google Scholar]
- White, H. A Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica 1980, 48, 817–838. [Google Scholar] [CrossRef]
- National Food Institute. Food Data (frida.fooddata.dk): Technical University of Denmark; 2019. [Version 4]. Available online: https://frida.fooddata.dk/?lang=en (accessed on 14 September 2023).
- Barebring, L.; Amberntsson, A.; Winkvist, A.; Augustin, H. Validation of Dietary Vitamin D Intake from Two Food Frequency Questionnaires, Using Food Records and the Biomarker 25-Hydroxyvitamin D among Pregnant Women. Nutrients 2018, 10, 745. [Google Scholar] [CrossRef]
- Marsal, K.; Persson, P.H.; Larsen, T.; Lilja, H.; Selbing, A.; Sultan, B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996, 85, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wei, S.Q.; Bi, W.G.; Weiler, H.A.; Wen, S.W. Effect of Vitamin D Supplementation in Early Life on Children’s Growth and Body Composition: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Hernan, M.A.; Hernandez-Diaz, S.; Robins, J.M. A structural approach to selection bias. Epidemiology 2004, 15, 615–625. [Google Scholar] [CrossRef]
- Wellner, J.A.; Zhan, Y. A Hybrid Algorithm for Computation of the Nonparametric Maximum Likelihood Estimator From Censored Data. J. Am. Stat. Assoc. 1997, 92, 945–959. [Google Scholar] [CrossRef]
- Turnbull, B.W. The Empirical Distribution Function with Arbitrarily Grouped, Censored and Truncated Data. J. R. Stat. Society. Ser. B 1976, 38, 290–295. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Brix, N.; Ernst, A.; Lauridsen, L.L.B.; Parner, E.T.; Arah, O.A.; Olsen, J.; Henriksen, T.B.; Ramlau-Hansen, C.H. Risk of selection bias due to non-participation in a cohort study on pubertal timing. Paediatr. Perinat. Epidemiol. 2020, 34, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A. Self-assessment of pubertal development in a puberty cohort. J. Pediatr. Endocrinol. Metab. JPEM 2018, 31, 763–772. [Google Scholar] [CrossRef]
- Bailey, R.L.; Dodd, K.W.; Gahche, J.J.; Dwyer, J.T.; Cowan, A.E.; Jun, S.; Eicher-Miller, H.A.; Guenther, P.M.; Bhadra, A.; Thomas, P.R.; et al. Best Practices for Dietary Supplement Assessment and Estimation of Total Usual Nutrient Intakes in Population-Level Research and Monitoring. J. Nutr. 2019, 149, 181–197. [Google Scholar] [CrossRef]
- Perez-Lopez, F.R.; Pasupuleti, V.; Mezones-Holguin, E.; Benites-Zapata, V.A.; Thota, P.; Deshpande, A.; Hernandez, A.V. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: A systematic review and meta-analysis of randomized controlled trials. Fertil. Steril. 2015, 103, 1278–1288.e4. [Google Scholar] [CrossRef]
- Keglberg Hærvig, K.; Bonde, J.P.; Ramlau-Hansen, C.H.; Toft, G.; Hougaard, K.S.; Specht, I.O.; Giwercman, A.; Nybo Andersen, A.M.; Olsen, J.; Lindh, C.; et al. Fetal Programming of Semen Quality (FEPOS) Cohort—A DNBC Male-Offspring Cohort. Clin Epidemiol 2020, 12, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Gaml-Sørensen, A.; Brix, N.; Ernst, A.; Lunddorf, L.L.H.; Lindh, C.; Toft, G.; Henriksen, T.B.; Arah, O.A.; Ramlau-Hansen, C.H. The estimated effect of season and vitamin D in the first trimester on pubertal timing in girls and boys: A cohort study and an instrumental variable analysis. Int. J. Epidemiol. 2023; online ahead of print. [Google Scholar] [CrossRef]
- World Health Organization. Vitamin D Supplementation during Pregnancy: World Health Organization; 2016. Available online: https://www.who.int/elena/titles/guidance_summaries/vitamind_supp_pregnancy/en/ (accessed on 20 January 2023).
- Poulsen, A.; Brot, C. Anbefalinger for Svangreomsorgen; Sundhedsstyrelsen: København, Denmark, 2009. [Google Scholar]
- Vestergaard, A.L.; Justesen, S.; Volqvartz, T.; Aagaard, S.K.; Andreasen, M.F.; Lesnikova, I.; Uldbjerg, N.; Larsen, A.; Bor, P. Vitamin D insufficiency among Danish pregnant women-Prevalence and association with adverse obstetric outcomes and placental vitamin D metabolism. Acta Obstet. Et Gynecol. Scand. 2021, 100, 480–488. [Google Scholar] [CrossRef] [PubMed]

| Average Daily Intake of Vitamin D from Supplements (µg/day) | |||||
|---|---|---|---|---|---|
| ≥10 | 0.01–9.99 | 0 | Missings | ||
| n = 6191 (48%) | n = 4823 (37%) | n = 1977 (15%) | |||
| Alcohol 1st trimester (drinks/week) b | >20 a (0) | ||||
| 0 | 3312 (54) | 2326 (48) | <976 a (49) | ||
| 0.1–1.0 | 1894 (31) | 1617 (34) | 612 (31) | ||
| 1.1–3.0 | 712 (12) | 602 (12) | 264 (13) | ||
| > 3.0 | 263 (4) | 268 (6) | 125 (6) | ||
| Maternal age at delivery (years (SD)) | 30.5 (4.3) | 31.0 (4.2) | 30.5 (4.7) | 5 (0) | |
| Couple fecundity c | 32 (0) | ||||
| Unplanned pregnancy | 843 (14) | 712 (15) | 395 (20) | ||
| 0–5 months TTP | 3338 (54) | 2670 (55) | 1032 (52) | ||
| 6–12 months TTP | 806 (13) | 621 (13) | 248 (13) | ||
| >12 months TTP + MAR | 1193 (19) | 806 (17) | 295 (15) | ||
| Maternal age at menarche | 90 (1) | ||||
| Earlier than peers | 1538 (25) | 1265 (26) | 470 (24) | ||
| Same as peers | 3554 (57) | 2724 (56) | 1135 (57) | ||
| Later than peers | 1059 (17) | 796 (17) | 360 (18) | ||
| Maternal BMI (kg/m2) | 175 (1) | ||||
| <18.5 | 399 (6) | 326 (7) | 131 (7) | ||
| 18.5–≤24.9 | 3812 (62) | 2991 (62) | 1176 (59) | ||
| 25–≤29.9 | 1259 (20) | 1009 (21) | 410 (21) | ||
| ≥30 | 638 (10) | 434 (9) | 231 (12) | ||
| Parental cohabitation | 5 (0) | ||||
| Yes | <6077 a (98) | <4740 a (98) | 1930 (98) | ||
| No | 114 (2) | 83 (2) | 47 (2) | ||
| Parity | 0 (0) | ||||
| Primipara | 3575 (58) | 2305 (48) | 806 (41) | ||
| Multipara | 2616 (42) | 2518 (52) | 1171 (59) | ||
| Highest parental socioeconomic status | 25 (0) | ||||
| High-grade professional | 1426 (23) | 1280 (27) | 416 (21) | ||
| Low-grade professional | 2040 (33) | 1662 (34) | 595 (30) | ||
| Skilled worker | 1753 (28) | 1201 (25) | 578 (29) | ||
| Unskilled worker | 816 (13) | 559 (12) | 319 (16) | ||
| Student | 117 (2) | 89 (2) | 46 (2) | ||
| Economically inactive | 29 (0) | 22 (0) | 18 (1) | ||
| Smoking 1st trimester (cigarettes/day) | 47 (0) | ||||
| 0 | 4514 (73) | 3690 (77) | 1357 (69) | ||
| 1–10 | 1355 (22) | 897 (19) | 457 (23) | ||
| >10 | 299 (5) | 217 (5) | 158 (8) | ||
| Season at birth | 0 | ||||
| Winter | 1597 (26) | 1190 (25) | 528 (27) | ||
| Spring | 1605 (26) | 1356 (28) | 495 (25) | ||
| Summer | 1546 (25) | 1217 (25) | 489 (25) | ||
| Fall | 1443 (23) | 1060 (22) | 465 (24) | ||
| Healthy eating index d (score (SD)) | 23.0 (7) | 23.0 (7) | 22.7 (7) | 5429 (42) | |
| Birth weight (grams (SD)) | 3524 (604) | 3556 (588) | 3514 (585) | 41 (0) | |
| Gestational age at delivery (days (SD)) | 279 (13) | 279 (13) | 279 (13) | 42 (0) | |
| Child BMI (kg/m2) | 15.6 (1.7) | 15.6 (1.7) | 15.8 (1.8) | 3418 (26) | |
| Average Daily Intake of Vitamin D from Supplements (µg/day) | ||||||||
|---|---|---|---|---|---|---|---|---|
| ≥10 (Reference) | 0.01–9.99 | 0 | Pr. 5 µg/day Decrease | |||||
| Pubertal Milestones | Mean Age in Years | Crude Difference | Adjusted Difference | Crude Difference | Adjusted Difference | Crude Difference | Adjusted Difference | |
| Boys n = 6031 b | ||||||||
| Tanner Genital stage 2 | 10.8 | 0.5 | 0.9 (−0.3; 2.1) | 0.7 | 1.4 (−0.3; 3.0) | 0.5 | 0.7 (0.1; 1.2) | |
| Tanner Genital stage 3 | 12.5 | 0.2 | 0.1 (−1.1; 1.2) | 0.1 | 0.5 (−1.1; 2.1) | 0.1 | 0.2 (−0.3; 0.8) | |
| Tanner Genital stage 4 | 13.7 | 0.2 | 0.0 (−1.2; 1.2) | 0.5 | 1.1 (−0.4; 2.7) | 0.2 | 0.4 (−0.2; 1.0) | |
| Tanner Genital stage 5 | 15.8 | 0.1 | 0.1 (−1.9; 2.0) | 0.0 | 0.3 (−2.2; 2.8) | 0.3 | 0.7 (−0.3; 1.6) | |
| Tanner Pubic Hair stage 2 | 11.3 | 0.5 | 0.7 (−0.5; 1.8) | 0.7 | 1.0 (−0.5; 2.6) | 0.4 | 0.5 (0.0; 1.1) | |
| Tanner Pubic Hair stage 3 | 12.7 | 0.4 | 0.5 (−0.6; 1.5) | 0.1 | 0.6 (−0.7; 1.9) | 0.1 | 0.3 (−0.2; 0.8) | |
| Tanner Pubic Hair stage 4 | 13.5 | 0.6 | 0.6 (−0.3; 1.6) | −0.1 | 0.7 (−0.6; 1.9) | 0.1 | 0.4 (−0.1; 0.9) | |
| Tanner Pubic Hair stage 5 | 14.8 | 0.2 | 0.1 (−1.2; 1.4) | 0.1 | 0.9 (−0.9; 2.6) | 0.2 | 0.5 (−0.1; 1.1) | |
| Axillary hair | 13.2 | 1.4 | 1.6 (0.4; 2.9) | −0.1 | −0.2 (−1.9; 1.4) | 0.2 | 0.5 (0.0; 1.1) | |
| Acne | 12.2 | 1.2 | 1.2 (0.1; 2.4) | 1.3 | 2.1 (0.5; 3.7) | 0.7 | 0.9 (0.4; 1.5) | |
| Voice break | 13.0 | 0.8 | 0.6 (−0.6; 1.7) | −0.5 | −0.5 (−2.2; 1.1) | 0.2 | 0.2 (−0.4; 0.7) | |
| First ejaculation | 13.3 | 0.8 | 1.4 (0.3; 2.6) | 0.8 | 1.2 (−0.4; 2.7) | 0.5 | 0.8 (0.3; 1.4) | |
| Combined estimate | 0.6 | 0.6 (−0.2; 1.5) | 0.3 | 0.7 (−0.4; 1.8) | 0.4 | 0.5 (0.1; 0.9) | ||
| Girls n = 6445 b | ||||||||
| Tanner Breast stage 2 | 9.8 | −0.3 | 0.4 (−1.2; 2.0) | −0.7 | −0.5 (−2.6; 1.6) | −0.4 | 0.2 (−0.5; 0.9) | |
| Tanner Breast stage 3 | 11.6 | 0.1 | 0.3 (−0.7; 1.3) | −0.7 | −1.0 (−2.3; 0.4) | −0.2 | 0.0 (−0.5; 0.4) | |
| Tanner Breast stage 4 | 13.1 | −0.1 | 0.3 (−0.8; 1.3) | −0.7 | −0.5 (−2.0; 0.9) | −0.2 | 0.0 (−0.5; 0.5) | |
| Tanner Breast stage 5 | 16.0 | −0.5 | −0.2 (−2.2; 1.8) | −3.3 | −2.2 (−4.9; 0.5) | −1.0 | −0.3 (−1.3; 0.8) | |
| Tanner Pubic Hair stage 2 | 11.2 | 0.2 | 0.5 (−0.3; 1.4) | 0.4 | 0.4 (−0.7; 1.5) | 0.2 | 0.4 (0.0; 0.9) | |
| Tanner Pubic Hair stage 3 | 12.4 | 0.3 | 0.5 (−0.3; 1.4) | 0.1 | 0.3 (−0.7; 1.5) | 0.1 | 0.3 (−0.1; 0.7) | |
| Tanner Pubic Hair stage 4 | 13.5 | 0.0 | 0.4 (−0.7; 1.6) | 0.6 | 0.6 (−0.9; 2.2) | 0.3 | 0.4 (−0.1; 0.9) | |
| Tanner Pubic Hair stage 5 | 15.6 | −0.3 | 0.4 (−1.3; 2.1) | 0.0 | −0.5 (−2.9; 1.9) | −0.1 | −0.1 (−1.0; 0.7) | |
| Axillary hair | 11.9 | −0.3 | 0.1 (−1.1; 1.2) | −0.2 | 0.2 (−1.3; 1.8) | −0.1 | 0.3 (−0.3; 0.8) | |
| Acne | 11.4 | −0.1 | −0.1 (−1.5; 1.2) | −1.5 | −2.2 (−3.9; −0.4) | −0.5 | −0.7 (−1.3; −0.1) | |
| Menarche | 13.0 | 0.8 | 0.8 (−0.1; 1.7) | −0.8 | −0.7 (−1.9; 0.4) | −0.2 | 0.1 (−0.4; 0.5) | |
| Combined estimate | 0.1 | 0.4 (−0.4; 1.2) | −0.5 | −0.5 (−1.6; 0.6) | −0.1 | 0.1 (−0.3; 0.5) | ||
| Boys | Girls | ||||||
|---|---|---|---|---|---|---|---|
| Crude Difference | Adjusted Difference | Crude Difference | Adjusted Difference | ||||
| (1) Total intake of vitamin D | |||||||
| Highest quartile (13.48−45.65) | Ref. | Ref. | Ref. | Ref. | |||
| Third quartile (11−38−13.48) | −0.2 | 0.1 (−1.0; 1.1) | −0.3 | 0.6 (−0.5; 1.6) | |||
| Second quartile (7.17−11−38) | 0.1 | 0.5 (−0.5; 1.6) | 0.1 | 1.0 (0.0; 2.0) | |||
| Lowest quartile (0−7.17) | 0.4 | 0.8 (−0.2; 1.9) | −0.5 | 0.2 (−0.9; 1.2) | |||
| Pr. 5 µg/day decrease in vitamin D | 0.3 | 0.5 (0.1; 0.8) | −0.2 | 0.1 (−0.3; 0.4) | |||
| (2a) Main model further adjusted for healthy eating index | |||||||
| ≥10 µg | Ref. | Ref. | Ref. | Ref. | |||
| 0.01–9.99 µg | 0.7 | 0.6 (−0.5; 1.7) | −0.3 | 0.1 (−1.0; 1.1) | |||
| 0 µg | 0.5 | 0.7 (−0.8; 2.2) | −1.0 | −0.9 (−2.3; 0.4) | |||
| Pr. 5 µg/day decrease in vitamin D | 0.3 | 0.4 (−0.1; 0.9) | −0.4 | −0.2 (−0.7; 0.3) | |||
| (2b) Main model with restriction to participants with information on healthy eating index | |||||||
| ≥10 µg | Ref. | Ref. | Ref. | Ref. | |||
| 0.01–9.99 µg | 0.7 | 0.6 (−0.5; 1.7) | −0.3 | 0.1 (−1.0; 1.1) | |||
| 0 µg | 0.5 | 0.7 (−0.8; 2.2) | −1.0 | −0.9 (−2.3; 0.4) | |||
| Pr. 5 µg/day decrease in vitamin D | 0.3 | 0.4 (−0.1; 0.9) | −0.4 | −0.2 (−0.7; 0.3) | |||
| (3) Main model further adjusted for birth weight z−scores | |||||||
| ≥10 µg | Ref. | Ref. | Ref. | Ref. | |||
| 0.01–9.99 µg | 0.6 | 0.6 (−0.2; 1.4) | 0.1 | 0.3 (−0.5; 1.2) | |||
| 0 µg | 0.3 | 0.7 (−0.4; 1.8) | −0.5 | −0.4 (−1.5; 0.7) | |||
| Pr. 5 µg/day decrease in vitamin D | 0.4 | 0.5 (0.1;0.9) | −0.1 | 0.1 (−0.3;0.5) | |||
| (4a) Main model further adjusted for childhood BMI at age 7 years | |||||||
| ≥10 µg | Ref. | Ref. | Ref. | Ref. | |||
| 0.01–9.99 µg | 0.6 | 0.4 (−0.5; 1.4) | −0.1 | 0.2 (−0.7; 1.1) | |||
| 0 µg | 0.3 | 0.5 (−0.7; 1.7) | −0.6 | −0.6 (−1.8; 0.7) | |||
| Pr. 5 µg/day decrease in vitamin D | 0.3 | 0.3 (−0.1;0.7) | −0.2 | 0.0 (−0.5; 0.4) | |||
| (4b) Main model with restriction to participants with information on childhood BMI | |||||||
| ≥10 µg | Ref. | Ref. | Ref. | Ref. | |||
| 0.01–9.99 µg | 0.6 | 0.6 (−0.3; 1.5) | −0.1 | 0.2 (−0.7; 1.1) | |||
| 0 µg | 0.3 | 0.5 (−0.8; 1.7) | −0.6 | −0.7 (−2.0; 0.5) | |||
| Pr. 5 µg/day decrease in vitamin D | 0.3 | 0.3 (−0.1;0.7) | −0.2 | −0.1 (−0.5; 0.4) | |||
| (5) Main model with 10 µg and >10 µg as separate exposure categories | |||||||
| >10 µg | −0.3 | −0.7 (−2.1; 0.7) | 0.6 | −0.3 (−1.8; 1.1) | |||
| 10 µg | Ref. | Ref. | Ref. | Ref. | |||
| 0.01–9.99 µg | 0.6 | 0.5 (−0.3; 1.4) | 0.2 | 0.4 (−0.5; 1.2) | |||
| 0 µg | 0.3 | 0.6 (−0.5; 1.7) | −0.5 | −0.5 (−1.6; 0.6) | |||
| (6) Early pregnancy supplement intake | |||||||
| Vitamin D with/without calcium | Ref. | Ref. | Ref. | Ref. | |||
| Multivitamin | 0.0 | 0.2 (−1.0; 1.3) | −0.1 | 0.3 (−0.9; 1.4) | |||
| Other vitamin | 0.3 | 0.1 (−1.5; 1.7) | 0.0 | 0.1 (−1.5; 1.7) | |||
| No vitamin | 0.5 | 1.3 (−0.1; 2.7) | −1.5 | −1.0 (−2.3; 0.3) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaml-Sørensen, A.; Brix, N.; Lunddorf, L.L.H.; Ernst, A.; Høyer, B.B.; Toft, G.; Henriksen, T.B.; Ramlau-Hansen, C.H. Maternal Intake of Vitamin D Supplements during Pregnancy and Pubertal Timing in Children: A Population-Based Follow-Up Study. Nutrients 2023, 15, 4039. https://doi.org/10.3390/nu15184039
Gaml-Sørensen A, Brix N, Lunddorf LLH, Ernst A, Høyer BB, Toft G, Henriksen TB, Ramlau-Hansen CH. Maternal Intake of Vitamin D Supplements during Pregnancy and Pubertal Timing in Children: A Population-Based Follow-Up Study. Nutrients. 2023; 15(18):4039. https://doi.org/10.3390/nu15184039
Chicago/Turabian StyleGaml-Sørensen, Anne, Nis Brix, Lea Lykke Harrits Lunddorf, Andreas Ernst, Birgit Bjerre Høyer, Gunnar Toft, Tine Brink Henriksen, and Cecilia Høst Ramlau-Hansen. 2023. "Maternal Intake of Vitamin D Supplements during Pregnancy and Pubertal Timing in Children: A Population-Based Follow-Up Study" Nutrients 15, no. 18: 4039. https://doi.org/10.3390/nu15184039
APA StyleGaml-Sørensen, A., Brix, N., Lunddorf, L. L. H., Ernst, A., Høyer, B. B., Toft, G., Henriksen, T. B., & Ramlau-Hansen, C. H. (2023). Maternal Intake of Vitamin D Supplements during Pregnancy and Pubertal Timing in Children: A Population-Based Follow-Up Study. Nutrients, 15(18), 4039. https://doi.org/10.3390/nu15184039






