Acute Intake of Sucrose but Not of the Intense Sweetener Sucralose Is Associated with Post-Prandial Endotoxemia in Healthy Young Adults—A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Intervention Study
2.3. Anthropometry, Blood Pressure and Metabolic Parameters
2.4. Bacterial Endotoxin
2.5. Caco-2 Cells In Vitro Experiments
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Ex Vivo Everted Gut Sac Experiments and Xylose Permeation Measurement
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Nutritional Standardization
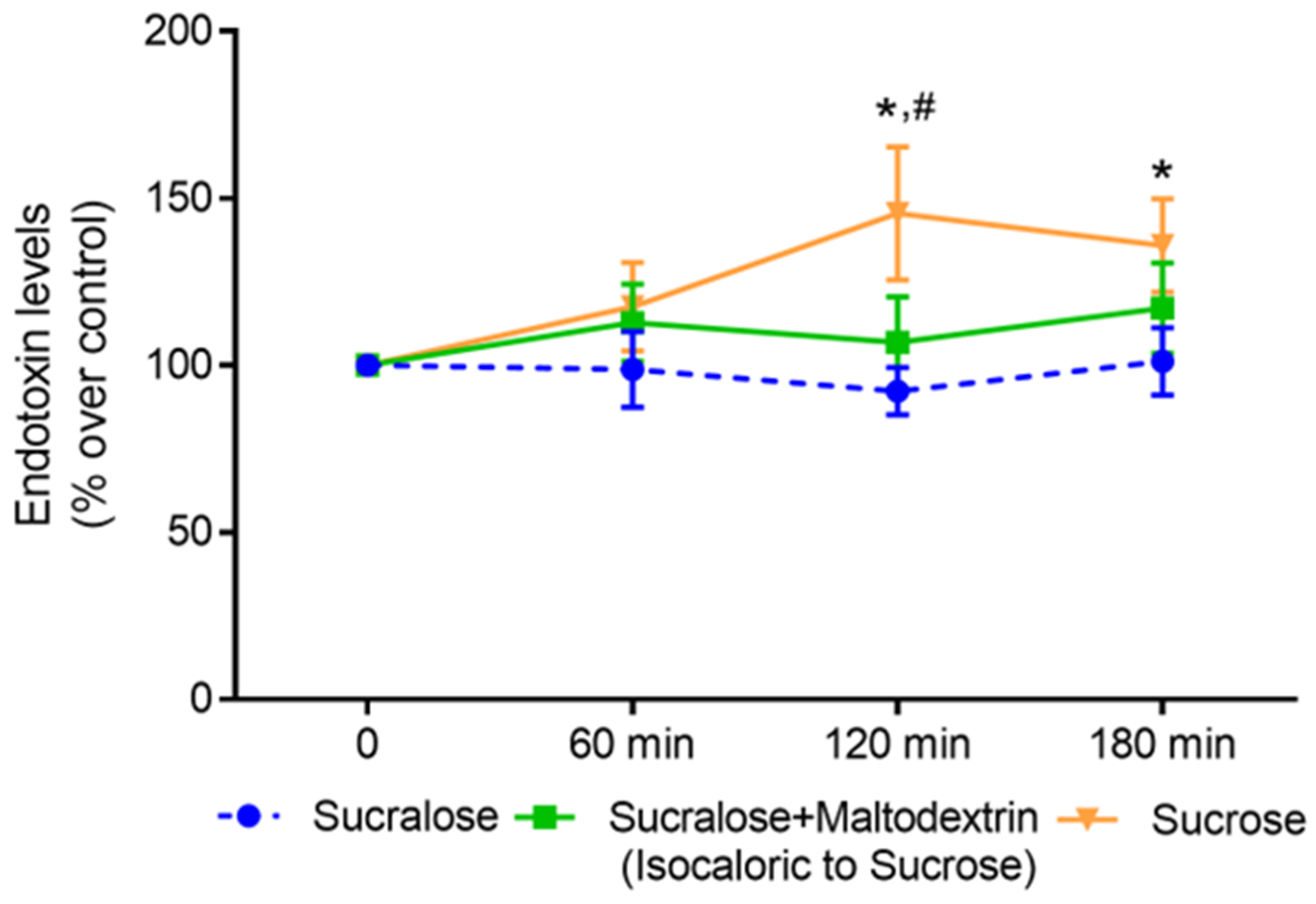
3.2. Effect of an Acute Intake of Sucralose, Sucrose and an Isocaloric Combination of Sucralose and Maltodextrin, Respectively, on Post-Prandial Bacterial Endotoxin Levels in Blood
3.3. Effect of Sucralose and Sucrose on Bacterial Endotoxin Permeation and Protein Levels of iFABP in Differentiated Caco-2 Cells
3.4. Effect of Sucralose and Sucrose on Markers of Intestinal Barrier Function in Small Intestinal Everted Tissue Sacs of C57BL/6J Mice
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 31 July 2023).
- Portincasa, P.; Bonfrate, L.; Khalil, M.; Angelis, M.; Calabrese, F.M.; D’Amato, M.; Wang, D.Q.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2021, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2020, 11, 594150. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Staltner, R.; Burger, K.; Baumann, A.; Bergheim, I. Fructose: A modulator of intestinal barrier function and hepatic health? Eur. J. Nutr. 2023. [Google Scholar] [CrossRef] [PubMed]
- Guideline: Sugars Intake for Adults and Children. Available online: https://www.who.int/publications/i/item/9789241549028 (accessed on 31 July 2023).
- Ernst, J.B.; Arens-Azevêdo, U.; Bitzer, B.; Bosy-Westphal, A.; de Zwaan, M.; Egert, S.; Fritsche, A.; Gerlach, S.; Hauner, H.; Heseker, H. Quantitative Empfehlung zur Zuckerzufuhr in Deutschland; Ernährungsumschau: Bonn, Germany, 2018. [Google Scholar]
- Sluik, D.; van Lee, L.; Engelen, A.I.; Feskens, E.J. Total, Free, and Added Sugar Consumption and Adherence to Guidelines: The Dutch National Food Consumption Survey 2007–2010. Nutrients 2016, 8, 70. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, H.; Zheng, F.; Song, J.; Lu, Y.; Yu, X.; Zhao, C. Sugar Is the Key Cause of Overweight/Obesity in Sugar-Sweetened Beverages (SSB). Front. Nutr. 2022, 9, 885704. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, N.D.; Khan, T.A.; Wang, L.; Zhang, R.; Chiavaroli, L.; Au-Yeung, F.; Lee, J.J.; Noronha, J.C.; Comelli, E.M.; Blanco Mejia, S.; et al. Association of Low- and No-Calorie Sweetened Beverages as a Replacement for Sugar-Sweetened Beverages With Body Weight and Cardiometabolic Risk: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e222092. [Google Scholar] [CrossRef]
- Lee, J.J.; Khan, T.A.; McGlynn, N.; Malik, V.S.; Hill, J.O.; Leiter, L.A.; Jeppesen, P.B.; Rahelic, D.; Kahleova, H.; Salas-Salvado, J.; et al. Relation of Change or Substitution of Low- and No-Calorie Sweetened Beverages With Cardiometabolic Outcomes: A Systematic Review and Meta-analysis of Prospective Cohort Studies. Diabetes Care 2022, 45, 1917–1930. [Google Scholar] [CrossRef] [PubMed]
- Mbambo, N.P.; Dlamini, S.N.; Chukwuma, C.I.; Islam, M.S. Comparative effects of commonly used commercially available non-nutritive sweeteners on diabetes-related parameters in non-diabetic rats. J. Food Biochem. 2020, 44, e13453. [Google Scholar] [CrossRef]
- Yu, Z.; Henderson, I.R.; Guo, J. Non-caloric artificial sweeteners modulate conjugative transfer of multi-drug resistance plasmid in the gut microbiota. Gut Microbes 2023, 15, 2157698. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.; Lee, K.M.; Sylvetsky, A.C.; Kirkpatrick, S.I. Low-calorie sweeteners and human health: A rapid review of systematic reviews. Nutr. Rev. 2021, 79, 1145–1164. [Google Scholar] [CrossRef]
- Lohner, S.; Toews, I.; Meerpohl, J.J. Health outcomes of non-nutritive sweeteners: Analysis of the research landscape. Nutr. J. 2017, 16, 55. [Google Scholar] [CrossRef]
- Zhang, R.; Noronha, J.C.; Khan, T.A.; McGlynn, N.; Back, S.; Grant, S.M.; Kendall, C.W.C.; Sievenpiper, J.L. The Effect of Non-Nutritive Sweetened Beverages on Postprandial Glycemic and Endocrine Responses: A Systematic Review and Network Meta-Analysis. Nutrients 2023, 15, 1050. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). Safety of the proposed extension of use of sucralose (E 955) in foods for special medical purposes in young children. EFSA J. 2016, 14, 4361. [Google Scholar] [CrossRef]
- Food Additives Permitted for Direct Addition to Food for Human Consumption; Sucralose. Available online: https://www.govinfo.gov/content/pkg/FR-1998-04-03/pdf/98-8750.pdf (accessed on 1 August 2023).
- Suez, J.; Cohen, Y.; Valdes-Mas, R.; Mor, U.; Dori-Bachash, M.; Federici, S.; Zmora, N.; Leshem, A.; Heinemann, M.; Linevsky, R.; et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell 2022, 185, 3307–3328.e19. [Google Scholar] [CrossRef]
- Zani, F.; Blagih, J.; Gruber, T.; Buck, M.D.; Jones, N.; Hennequart, M.; Newell, C.L.; Pilley, S.E.; Soro-Barrio, P.; Kelly, G.; et al. The dietary sweetener sucralose is a negative modulator of T cell-mediated responses. Nature 2023, 615, 705–711. [Google Scholar] [CrossRef]
- Nier, A.; Brandt, A.; Rajcic, D.; Bruns, T.; Bergheim, I. Short-Term Isocaloric Intake of a Fructose- but not Glucose-Rich Diet Affects Bacterial Endotoxin Concentrations and Markers of Metabolic Health in Normal Weight Healthy Subjects. Mol. Nutr. Food Res. 2019, 63, e1800868. [Google Scholar] [CrossRef] [PubMed]
- Sellmann, C.; Baumann, A.; Brandt, A.; Jin, C.J.; Nier, A.; Bergheim, I. Oral Supplementation of Glutamine Attenuates the Progression of Nonalcoholic Steatohepatitis in C57BL/6J Mice. J. Nutr. 2017, 147, 2041–2049. [Google Scholar] [CrossRef]
- Jung, F.; Burger, K.; Staltner, R.; Brandt, A.; Mueller, S.; Bergheim, I. Markers of Intestinal Permeability Are Rapidly Improved by Alcohol Withdrawal in Patients with Alcohol-Related Liver Disease. Nutrients 2021, 13, 1659. [Google Scholar] [CrossRef]
- Hamilton, K.L.; Butt, A.G. Glucose transport into everted sacs of the small intestine of mice. Adv. Physiol. Educ. 2013, 37, 415–426. [Google Scholar] [CrossRef]
- Eberts, T.J.; Sample, R.H.; Glick, M.R.; Ellis, G.H. A simplified, colorimetric micromethod for xylose in serum or urine, with phloroglucinol. Clin. Chem. 1979, 25, 1440–1443. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Ernährung e.V.; Schweizerische Gesellschaft für Ernährungsforschung; Schweizerische Vereinigung für Ernährung (Hrsg.). Referenzwerte für die Nährstoffzufuhr; Deutsche Gesellschaft für Ernährung e.V.: Bonn, Germany, 2021. [Google Scholar]
- Sarikaya, M.; Ergul, B.; Dogan, Z.; Filik, L.; Can, M.; Arslan, L. Intestinal fatty acid binding protein (I-FABP) as a promising test for Crohn’s disease: A preliminary study. Clin. Lab. 2015, 61, 87–91. [Google Scholar] [CrossRef]
- Laviada-Molina, H.; Molina-Segui, F.; Perez-Gaxiola, G.; Cuello-Garcia, C.; Arjona-Villicana, R.; Espinosa-Marron, A.; Martinez-Portilla, R.J. Effects of nonnutritive sweeteners on body weight and BMI in diverse clinical contexts: Systematic review and meta-analysis. Obes. Rev. 2020, 21, e13020. [Google Scholar] [CrossRef]
- Poelman, M.P.; Eyles, H.; Dunford, E.; Schermel, A.; L’Abbe, M.R.; Neal, B.; Seidell, J.C.; Steenhuis, I.H.; Ni Mhurchu, C. Package size and manufacturer-recommended serving size of sweet beverages: A cross-sectional study across four high-income countries. Public Health Nutr. 2016, 19, 1008–1016. [Google Scholar] [CrossRef]
- Yan, T.; Shi, L.; Xu, K.; Bai, J.; Wen, R.; Liao, X.; Dai, X.; Wu, Q.; Zeng, L.; Peng, W.; et al. Habitual intakes of sugar-sweetened beverages associated with gut microbiota-related metabolites and metabolic health outcomes in young Chinese adults. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 359–368. [Google Scholar] [CrossRef]
- McNaughton, S.A.; Pendergast, F.J.; Worsley, A.; Leech, R.M. Eating occasion situational factors and sugar-sweetened beverage consumption in young adults. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 71. [Google Scholar] [CrossRef]
- Vors, C.; Drai, J.; Pineau, G.; Laville, M.; Vidal, H.; Laugerette, F.; Michalski, M.C. Emulsifying dietary fat modulates postprandial endotoxemia associated with chylomicronemia in obese men: A pilot randomized crossover study. Lipids Health Dis. 2017, 16, 97. [Google Scholar] [CrossRef]
- Erridge, C.; Attina, T.; Spickett, C.M.; Webb, D.J. A high-fat meal induces low-grade endotoxemia: Evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 2007, 86, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.; Wylie, A.T.; Tucker, K.L.; Hamp, T.J.; Gharaibeh, R.Z.; Fodor, A.A.; Cullen, J.M. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. Am. J. Clin. Nutr. 2013, 98, 349–357. [Google Scholar] [CrossRef]
- Jin, R.; Willment, A.; Patel, S.S.; Sun, X.; Song, M.; Mannery, Y.O.; Kosters, A.; McClain, C.J.; Vos, M.B. Fructose induced endotoxemia in pediatric nonalcoholic Fatty liver disease. Int. J. Hepatol. 2014, 2014, 560620. [Google Scholar] [CrossRef]
- Bergheim, I.; Weber, S.; Vos, M.; Kramer, S.; Volynets, V.; Kaserouni, S.; McClain, C.J.; Bischoff, S.C. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: Role of endotoxin. J. Hepatol. 2008, 48, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Tapia, M.; Miller, A.W.; Granados-Portillo, O.; Tovar, A.R.; Torres, N. The development of metabolic endotoxemia is dependent on the type of sweetener and the presence of saturated fat in the diet. Gut Microbes 2020, 12, 1801301. [Google Scholar] [CrossRef]
- McRae, M.P. Dietary Fiber Is Beneficial for the Prevention of Cardiovascular Disease: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2017, 16, 289–299. [Google Scholar] [CrossRef]
- Borgi, L.; Muraki, I.; Satija, A.; Willett, W.C.; Rimm, E.B.; Forman, J.P. Fruit and Vegetable Consumption and the Incidence of Hypertension in Three Prospective Cohort Studies. Hypertension 2016, 67, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Madsen, H.; Sen, A.; Aune, D. Fruit and vegetable consumption and the risk of hypertension: A systematic review and meta-analysis of prospective studies. Eur. J. Nutr. 2023, 62, 1941–1955. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef]


| Sucralose | Sucralose + Maltodextrin | Sucrose | |
|---|---|---|---|
| Water (L) containing | 1 | 1 | 1 |
| Sucrose (g) | - | - | 110 |
| Sucralose (mg) | 180 | 180 | - |
| Maltodextrin (g) | - | 110 | - |
| Butter (g) | 10 | 10 | 10 |
| Roll (g) | 65 | 65 | 65 |
| Energy (kcal) | 235.4 | 681.5 | 681.5 |
| Carbohydrates (E%) | 57.0 | 85.0 | 85.0 |
| Fat (E%) | 35.0 | 12.0 | 12.0 |
| Protein (E%) | 8.0 | 3.0 | 3.0 |
| Parameter | Baseline |
|---|---|
| Gender (m/f) | 5/6 |
| Age (years) | 26.2 ± 0.8 |
| BMI (kg/m2) | 21.9 ± 0.5 |
| WHR | 0.85 ± 0.01 |
| Blood glucose (mg/dL) | 81.4 ± 2.6 |
| Total cholesterol (mg/dL) | 172.1 ± 6.8 |
| Triglycerides (mg/dL) | 71.9 ± 7.1 |
| HDL (mg/dL) | 60.2 ± 2.5 |
| LDL (mg/dL) | 98.8 ± 6.9 |
| Blood pressure | |
| Systolic blood pressure (mm Hg) | 118.9 ± 1.8 |
| Diastolic blood pressure (mm Hg) | 75.2 ± 2.7 |
| Uric acid (mg/dL) | 4.3 ± 0.3 |
| Bilirubin (mg/dL) | 2.2 ± 1.7 |
| ALT (U/L) | 24.0 ± 1.9 |
| AST (U/L) | 24.4 ± 3.4 |
| Alkaline phosphatase (U/L) | 59.5 ± 6.3 |
| Gamma-GT (U/L) | 14.2 ± 1.6 |
| C-reactive protein (mg/dL) | 0.1 ± 0.0 |
| Parameters and Recommendations a | 24 h Recall | Standardization |
|---|---|---|
| Energy (kcal/day) | 2194 ± 112.4 | 2237 ± 95.4 |
| Carbohydrates (E%) (>50 E%) a | 41.7 ± 3.2 | 59.0 ± 0.3 * |
| Total polysaccharides (g) | 141.8 ± 21.9 | 202.0 ± 11.5 * |
| Total vegetable consumption (g) (≥400 g/day) a | 253.5 ± 41.7 | 551.1 ± 18.0 * |
| Total fruit consumption (g) (≥250 g/day) a | 229.8 ± 60.2 | 373.5 ± 16.0 * |
| Total fructose b (g) | 30.8 ± 3.9 | 53.8 ± 1.8 * |
| Added fructose (g) | 14.5 ± 2.5 | 15.7 ± 1.5 |
| Total glucose c (g) | 30.4 ± 5.1 | 50.4 ± 1.7 * |
| Added glucose (g) | 17.0 ± 4.2 | 15.4 ± 1.8 |
| Total sucrose (g) | 26.4 ± 3.8 | 49.7 ± 2.3 * |
| Added sucrose (g) | 15.4 ± 3.4 | 14.9 ± 1.2 |
| Total added sugar (E%) (<10 E%) a | 8.3 ± 1.8 | 8.3 ± 0.8 |
| Protein (g/kg body weight/day) (0.8 g/kg body weight/day) a | 1.4 ± 0.1 | 0.8 ± 0.0 * |
| Fat (E%) (30 E% d) a | 45.5 ± 4.5 | 31.3 ± 0.2 * |
| SFA (E%) (<10 E%) a | 14.6 ± 1.4 | 8.6 ± 0.3 * |
| Fiber (g) (≥30 g/day) a | 27.2 ± 3.0 | 34.8 ± 1.0 * |
| Parameters | Before Standardization | After Standardization |
|---|---|---|
| Body weight (kg) | 67.5 ± 2.5 | 67.6 ± 2.5 |
| BMI (kg/m2) | 21.9 ± 0.5 | 22.0 ± 0.5 |
| Blood glucose (mg/dL) | 96.5 ± 0.9 | 97.6 ± 1.4 |
| Triglycerides (mg/dL) | 84.2 ± 7.2 | 72.8 ± 4.6 a |
| Total Cholesterol (mg/dL) | 161.3 ± 12.4 | 150.6 ± 10.7 * |
| HDL (mg/dL) | 47.9 ± 4.3 | 45.6 ± 4.1 * |
| LDL (mg/dL) | 96.3 ± 10.8 | 89.6 ± 9.8 * |
| Systolic blood pressure (mm Hg) | 122.5 ± 3.3 | 120.5 ± 3.0 |
| Diastolic blood pressure (mm Hg) | 79.4 ± 1.8 | 76.7 ± 1.5 * |
| Endotoxin (EU/mL) | 1.8 ± 0.2 | 1.5 ± 0.1 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staltner, R.; Sánchez, V.; Bergheim, I.; Baumann, A. Acute Intake of Sucrose but Not of the Intense Sweetener Sucralose Is Associated with Post-Prandial Endotoxemia in Healthy Young Adults—A Randomized Controlled Trial. Nutrients 2023, 15, 4038. https://doi.org/10.3390/nu15184038
Staltner R, Sánchez V, Bergheim I, Baumann A. Acute Intake of Sucrose but Not of the Intense Sweetener Sucralose Is Associated with Post-Prandial Endotoxemia in Healthy Young Adults—A Randomized Controlled Trial. Nutrients. 2023; 15(18):4038. https://doi.org/10.3390/nu15184038
Chicago/Turabian StyleStaltner, Raphaela, Victor Sánchez, Ina Bergheim, and Anja Baumann. 2023. "Acute Intake of Sucrose but Not of the Intense Sweetener Sucralose Is Associated with Post-Prandial Endotoxemia in Healthy Young Adults—A Randomized Controlled Trial" Nutrients 15, no. 18: 4038. https://doi.org/10.3390/nu15184038
APA StyleStaltner, R., Sánchez, V., Bergheim, I., & Baumann, A. (2023). Acute Intake of Sucrose but Not of the Intense Sweetener Sucralose Is Associated with Post-Prandial Endotoxemia in Healthy Young Adults—A Randomized Controlled Trial. Nutrients, 15(18), 4038. https://doi.org/10.3390/nu15184038







