1. Introduction
Traditional medicines, also known as herbal medicines, are raw, fresh, and dried extracts and whole dried plants, including roots, seeds, leaves, fruits, flowers, etc.; they have contributed to the development of the economy, health care, and pharmaceuticals [
1]. Herbal medicines have been reported to have various beneficial effects, and they are economical, cheap, available, safe, and low in side effects and toxicity; however, safety assessments are necessary to confirm the safe dose for the consumption of herbal products [
2]. The toxicity of herbs is related to the chemical constituents present in these plants; this toxicity may also contribute to acute or chronic, mutagenic, or carcinogenic effects [
3]. Toxic plants may affect different or multiple organ systems. For example, daily doses of aqueous extracts of
Aphania senegalensis leaves (1000 to 2000 mg/kg) used to treat humans may cause liver toxicity;
Herniaria cinerea is toxic and may cause digestive and alveolar destruction, bloody diarrhea, and respiratory problems [
4,
5]. Given the general desire to use herbs, further studies on their safety and toxicity are needed.
C. cajan (L.) Millsp. is known as the pigeon pea and belongs to the legume family, whose distribution is found in Asia, Egypt, and Africa [
6]. This plant is supplied as a protein-rich food and medicine; it is also used as a traditional medicine by Taiwanese aborigines. Some of the young stems of
C. cajan are used as toothbrushes; the leaves treat mouth ulcers, inflammation, and various skin problems; the seeds are high in protein and are known as poor man’s meat, contributing protein to vegetarian diets; and the seeds are used to treat various chronic diseases [
7,
8,
9,
10]. Basically, the antioxidant, anti-inflammatory, antibacterial, antidiabetic, and anti-oral-cancer effects of
C. cajan have been documented [
8,
9,
10,
11,
12,
13,
14]. Nowadays, the research interest in medicinal plants is manifested in potential compounds (flavonoids and other phenolic compounds), indicating that more potential biological activities and safe doses of these phytochemicals need to be further investigated.
Recently, Yang et al. 2020 demonstrated that the seeds of
C. cajan are an excellent source of protein in the legume family; its roots are a good source of dietary fiber, which plays an important role in cholesterol-lowering effects; and its seeds and roots can be beneficial for health in hypocalcemia and magnesium deficiency [
12]. This plant also has many potential health benefits in terms of traditional medicine and commercial exploitation. The roots and leaves contain approximately 5 and 2.5 times the phenolic content of
Astragalus L.; these compounds, such as genistein, daidzein, and cajanol, are present in its roots, which contribute to antioxidant, anti-inflammatory, antibacterial, and anti-oral-cancer effects [
6,
11,
12,
13]. The root helps in reducing the risk of obesity, diabetes, and decreased serum cholesterol levels in the diet due to its low glycemic index and high fiber content. Similarly, Yang et al. reported that EECR95-protected male Wistar rats from methylglyoxal (MGO)-induced insulin resistance (IR) and hyperlipidemia by inhibiting the formation of advanced glycation end products (AGEs) through the inhibition of carbohydrate hydrolases (α-glucosidase and α-amylase) and enhanced MGO trapping [
14]. However, there are no toxicological data on
C. cajan root to know the range of safe doses for consumption. Therefore, genetic, cellular, and animal safety assessments are necessary to confirm the safe dose for consumption.
Flavonoids are a large group of plant polyphenols that exert their possible beneficial effects on human health and contribute to growth and healthy plants [
15]; flavonoids are present in most plants and mainly in legumes, such as beans, white and red clover, and alfalfa [
16]. Isoflavones are known as phytoestrogens or soy isoflavones, given that they are structurally similar to the estrogen-like compound 17β-estradiol; thus, they have beneficial health properties for bone health, cardiovascular risk, cancer, and menopausal symptoms [
16]. Food isoflavones were found in chickpeas, nuts, fruits, and vegetables in which soy products are the most interesting [
17]. Zaheer et al. reported that the main isoflavones, like daidzein, genistein, glycitein, biochanin A, and formononetin, were present in most soy products [
17]; were called soy isoflavones [
18]. Nix et al. indicated that pigeon pea has presented with 27 flavonoids, including six flavones, eight isoflavones, four flavanols, two anthocyanins, three flavanones, three isoflavones, and a single chalcone [
19]. Our previous studies have also found that EECR95 is rich in genistein, cajanol, and daidzein [
11,
13]. However, we do not know if there are other isoflavones or isoflavone glycosides (e.g., genistein or daidzein) that deserve further investigation.
In this study, the safe dose of EECR95 in vitro and in vivo toxicity tests were conducted on Wistar rats. In vitro, we examined the effects of EECR95 on toxic and genotoxic bacteria on Salmonella typhimurium TA98 and TA100, as well as cytotoxicity in three kinds of cells: RAW 264.7, L-929, and HGF-1. In vivo, Wistar rats were treated with low or high doses of EECR95 for 90 days, and we measured body and organ weights, hematological tests, biochemical analysis, and histopathology. We also determined its safety and provided recommendations for the safe use of this plant in medicine and commerce. Additionally, we designed a method for the determination of soy isoflavones (daidzin, daidzein, genistein, cajanol, and biochanin A) components using an HPLC-DAD-UV/Vis system.
4. Discussion
Previous studies have reported that genistein and daidzein show no mutagenicity in the bacterial gene mutation test on
S. typhimurium TA98 and TA100 strains (Ames tests) [
24,
25,
26]. The genotoxicity assay results indicated that EECR95 at a very high concentration (1.0 mg/plate) did not increase the number of histidine revertant colonies over the negative control in the TA100 and TA98 tester strains, either with or without S9 metabolic activation. The standard mutagens used in this study (4-NQNO and 2-AF) induced a clear positive response. The above results indicate that EECR95 was not mutagenic in this assay. The absence of mutagenicity for EECR95 in the tested
S. typhimurium strains indicates that EECR95 does not affect the structural integrity of DNA. In addition, we also investigated the cytotoxic effects evaluated by MTT assay of EECR95 at concentrations from 10 to 1000 µg/mL. As the results showed, the highest doses of EECR95 (1000 µg/mL) had noncytotoxic effects on RAW264.7, L-929, and HGF-1 cells; their percentage of viable cells was more than 90%. Therefore, in vitro bacterial mutagenicity and cytotoxicity assays have confirmed that EECR95 is not mutagenic or cytotoxic at high doses.
Many recent studies have shown that changes in body weight are a simple and sensitive predictor of the effects of extracts; abnormal increases or decreases in body weight can indicate the degree of toxicity of drugs and chemicals [
27,
28]. We found no statistical differences in the body weight or food and water intake of female and male rats fed low or high doses of EECR95 compared to the control group (
p > 0.05). Therefore, we obtained preliminary evidence for the use of the highest safe dose for consumption (1.0 g/kg bw) of EECR95.
Clinicopathologic (urine biochemical and hematological analyses) results showed no statistically significant differences (p > 0.05) between female and male rats fed EECR95 compared to the controls. As with other organs and systems in the human body, urine biochemical analysis is the most basic test for the routine examination of urinary system function. The results of the urine analysis showed that no RBC, WBC, glucose, proteins, or hematuria were detected in the urine of female or male rats fed low or high doses of EECR95. The presence of hematuria is associated with infection, inflammation, trauma, hemorrhage, urolithiasis, toxemia, etc. Therefore, low and high doses of EECR95 did not cause infection or inflammation in either the female or male rats.
Hematological analysis is considered an important element in toxicity studies and has been elucidated as a pathological reflection of pharmacological reactions, pathogenic processes, or normal biological processes [
29]. Consumption of toxic plants or agents can cause alterations in hematological characteristics [
30,
31]. Delclos et al. conducted a study in which rats were impregnated with soy and alfalfa-free diets at doses of 0, 5, 25, 100, 250, 625, or 1250 ppm (genistein and daidzein) and showed that in any clinical chemistry or hematological parameter, there were no significant treatment-related differences in measurements [
32]. Yangzom et al. also reported no significant differences in hematological parameters between two isoflavones (e.g., kaempferol and biochanin A) administered orally to mice for 28 days [
33]. The results of this study showed that there were no statistically significant differences in various hematological parameters (including red blood cells (RBC), white blood cells (WBC), hemoglobin (Hb), hematocrit (HCT), mean red blood cell volume (MCV), platelet count (PLT), mean hemoglobin (MCH)m and mean hemoglobin concentration (MCHC)) between female and male rats administered with either low doses or high doses of EECR95, as compared to the control group (
p > 0.05).
The kidneys play an important role in the excretion of wastes and toxins, such as urea, creatinine, and uric acid; the regulation of extracellular fluid volume, serum osmolality, and electrolyte concentrations; and the production of hormones, such as erythropoietin, 1, 25 dihydroxyvitamin D, and renin. Markers of renal function help to diagnose clinical disease and determine the progression of renal disease. Uric acid, BUN, CRE, and ALB are commonly used to measure renal function. As shown in
Table 7, the serum BUN, CRE, and ALB values of the control rats were mostly within the normal range, but the serum uric acid and GLU values of the control rats were significantly higher than the normal range. However, the serum uric acid and GLU values of both the male and female control rats were significantly decreased after feeding L- and H-EECR95.
According to the National Institutes of Health, the overall prevalence of chronic kidney disease (CKD) is about 14%, and the most common causes of CKD are high blood pressure and diabetes [
34]. Uric acid has a role in the development of abnormal glucose metabolism by causing insulin resistance, impaired insulin secretion, and beta-cell dysfunction. Thus, hyperuricemia conditions are implicated in the pathogenesis of diabetes [
35]. In the past, we demonstrated the hypoglycemic potential of EECR95 by inhibiting the activities of key carbohydrate digestive enzymes (α-amylase and α-glucosidase) and anti-glycation (AGEs formation) [
12]. In addition, Yang et al. (2022) also found that EECR95 had a protective effect against methylglyoxal (MGO)-induced insulin resistance (IR) and hyperlipidemia in male Wistar rats [
14]. The present study also supports these findings and confirms the ability of EECR95 to protect the kidneys and prevent diabetes by lowering uric acid and GLU in the renal serum of male and female rats.
In the present study, serum liver function indices (e.g., CHOL, TG, and GPT) were not within the normal range in the control rats. However, the levels of CHOL, TG, and GPT were significantly decreased (
p < 0.05) (that is, the liver function markers were significantly improved) after the administration of low-dose or high-dose EECR95 (
Table 8). In the past, there have been considerable studies confirming the hepatoprotective effects of flavonoids. Soy isoflavones (e.g., genistein, soy isoflavones, bioflavonoid A, and formononetin) have been shown to have a protective effect against liver and kidney injury [
36,
37,
38,
39]. Elmarakby et al. remarked that genistein (10 mg/kg, i.p. three times a week for 10 weeks) exerted renal-protective properties related to reduced renal inflammation, oxidative stress, and apoptosis in diabetic mice [
36]. Daidzein also possesses effects on oxidative stress and inflammation and the mediation of the angiotensin AT1 and Mas receptors in a fibrotic model of kidney disease of ovariectomized (OVX) rats, suggesting that daidzein can be able to replace estrogen for therapy in postmenopausal or older women against postmenopausal kidney damage [
37]. In addition, biochanin A (10 mg/kg and 20 mg/kg) was found to be protective against acetaminophen-induced hepatotoxicity in mice by inhibiting oxidative stress pathways and attenuating hepatic inflammation [
38].
Barańska et al. indicated that the influence of soy isoflavones on CHOL and GLU levels as well as the modulation of lipid profiles, suggests benefits in preventing cardiovascular disease and type 2 diabetes [
40]. Soy isoflavones have been found in the stems and roots of pigeon pea, consisting of biochanin A, formononetin, genistein, cajanol, 2′-hydroxygenistein, and cajanin [
19]. However, the presence of genistein, daidzein, and cajanol has already been reported previously for
C. cajan roots [
11,
13]; genistin is a glycoside form of genistein and is mainly found in soy-derived foods. Kwon et al. indicated that the oral bioavailability of genistin is greater than that of genistein [
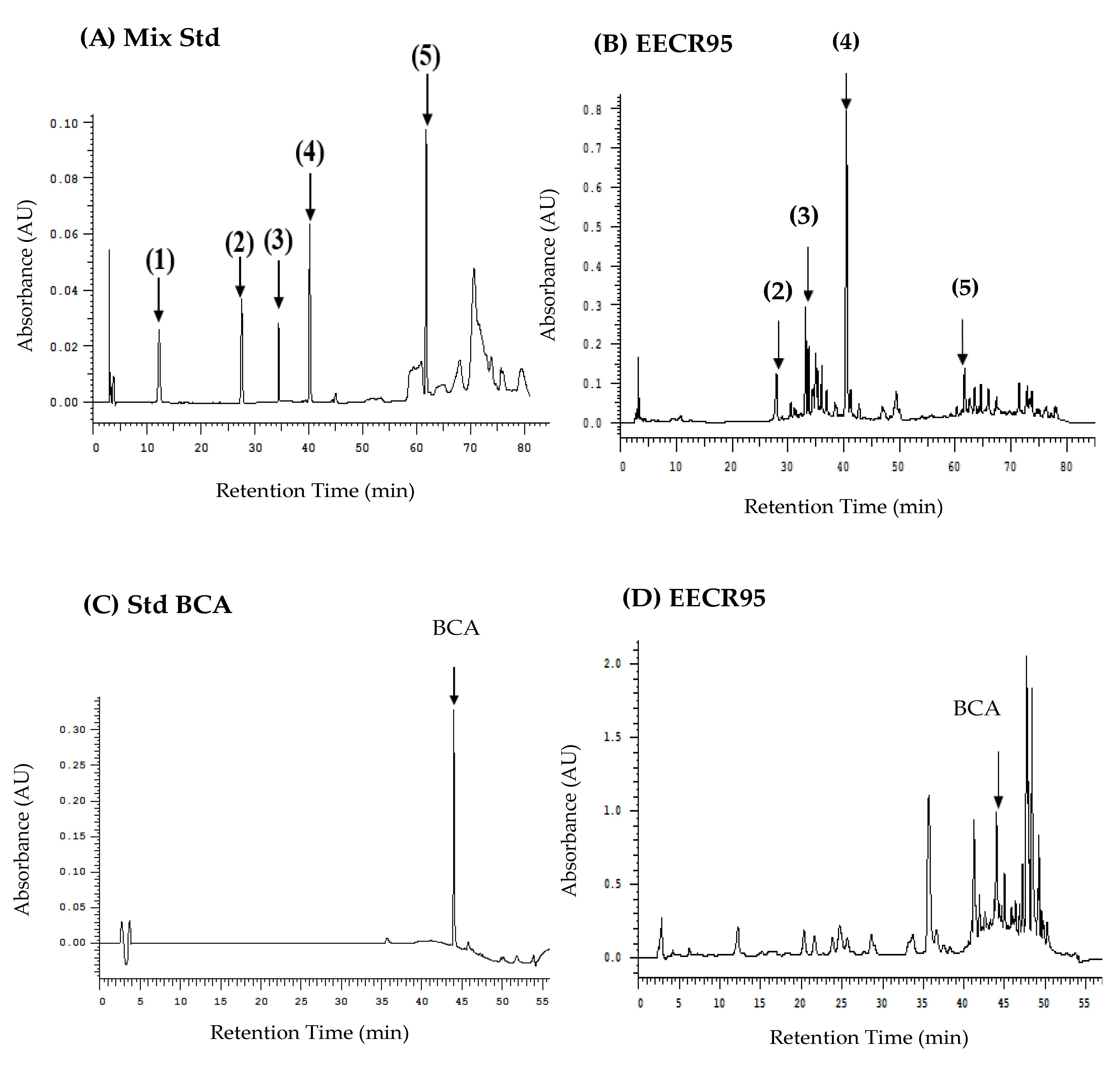
41]. Therefore, we further analyzed the content of biochanin A, cajanol, genistein, daidzein, and its glycosides (genistin and daidzein) in EECR95 using an HPLC-DAD-UV/Vis system.
The results of the HPLC chromatogram indicated that five soy isoflavones (daidzein, genistin, genistein, cajanol, and biochanin A) were found in EECR95. Thus, this study demonstrated that C. cajan (L.) Millsp. roots contain soy isoflavones, which exhibit several pharmacological properties. Therefore, EECR95 was effective in lowering lipids, cholesterol, and GPT, suggesting its hepatoprotective effects, and its main potent components were hypothesized to be related to flavonoids.
The immunohistochemical (IHC) staining results provided conclusive evidence of organ toxicity and correlated with changes in biochemical tests. The analysis of pathological examinations by IHC staining showed no histopathological changes in organs or tissues (brain, heart, liver, spleen, lungs, kidneys, thymus, and adrenal glands (ovaries, testes)) of male and female rats fed EECR95 continuously for 90 days at low or high doses (0.2 or 1.0 g/kg/day). Therefore, based on calculations from the literature [
42], we estimated a no-observed adverse effect level (NOAEL) value for EECR95 of approximately 1.0 g/kg bw extrapolated from rats to humans, which corresponds to approximately 972 mg/60 kg man/day.