Genetically Predicted Levels of Serum Metabolites and Risk of Sarcopenia: A Mendelian Randomization Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Data on Genetic Instruments for Exposures
2.3. Data on Genetic Associations with Outcomes
2.4. Statistical Analysis
3. Results
3.1. Study Overview
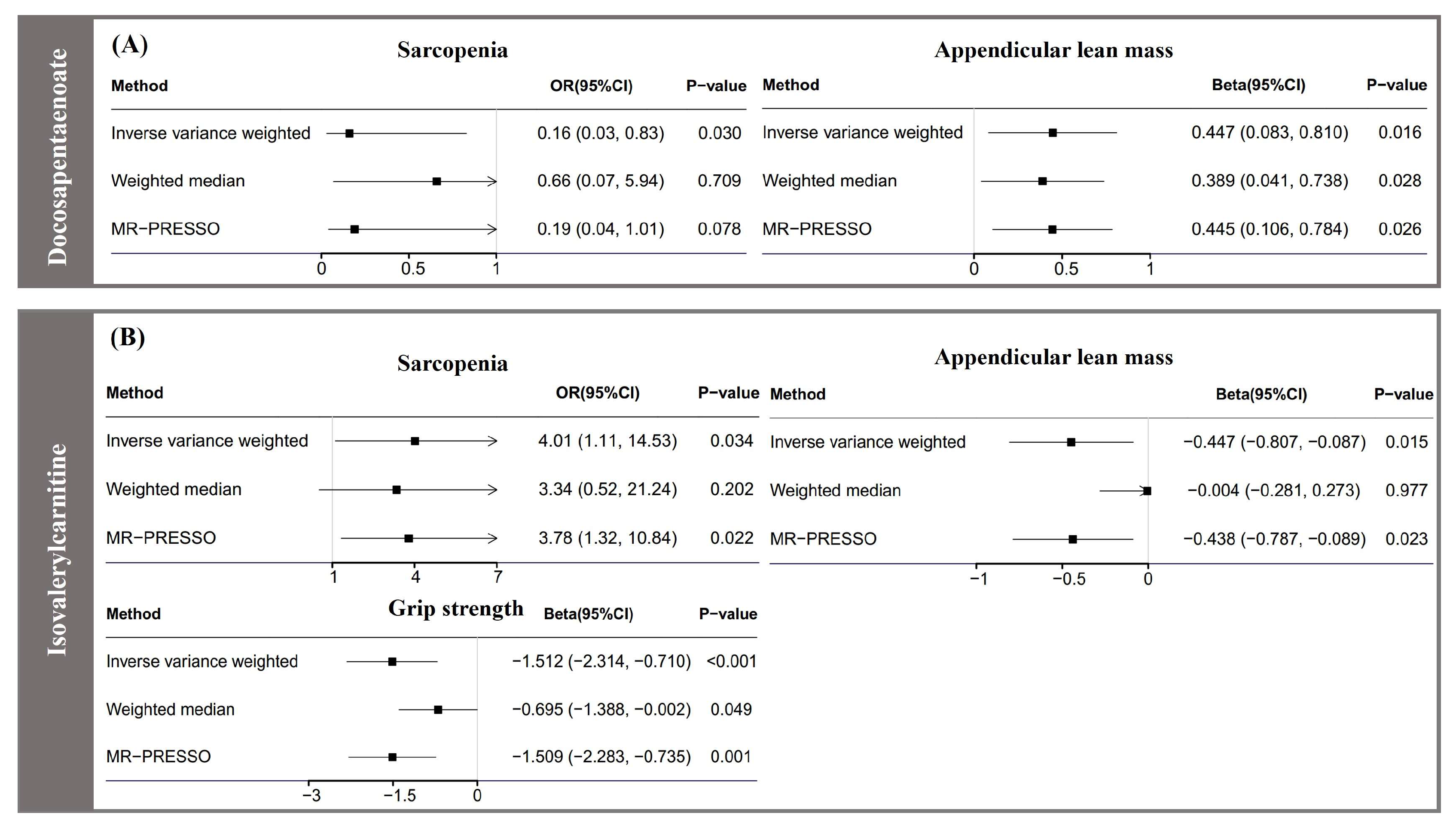
3.2. Association of Serum Metabolites with Sarcopenia
3.3. Association of Serum Metabolites with Appendicular Lean Mass
3.4. Association of Serum Metabolites with Grip Strength
3.5. Complementary Analyses
4. Discussion
4.1. Comparison with Previous Studies
4.2. Possible Explanations
4.3. Strengths and Limitations
4.4. Clinical and Research Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Coelho-Junior, H.J.; Calvani, R.; Marzetti, E.; Vetrano, D.L. Biomarkers shared by frailty and sarcopenia in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 73, 101530. [Google Scholar] [CrossRef]
- Robinson, S.M.; Reginster, J.Y.; Rizzoli, R.; Shaw, S.C.; Kanis, J.A.; Bautmans, I.; Bischoff-Ferrari, H.; Bruyère, O.; Cesari, M.; Dawson-Hughes, B.; et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin. Nutr. 2018, 37, 1121–1132. [Google Scholar] [CrossRef]
- Itoh, S.; Nagao, Y.; Morita, K.; Kurihara, T.; Tomino, T.; Kosai-Fujimoto, Y.; Harada, N.; Fujita, N.; Ushijima, Y.; Mori, M.; et al. Association between Sarcopenia and Omega-3 Polyunsaturated Fatty Acid in Patients with Hepatocellular Carcinoma. JMA J. 2022, 5, 169–176. [Google Scholar] [CrossRef]
- Kaur, G.; Cameron-Smith, D.; Garg, M.; Sinclair, A.J. Docosapentaenoic acid (22:5n-3): A review of its biological effects. Prog. Lipid Res. 2011, 50, 28–34. [Google Scholar] [CrossRef]
- Duan, J.; Song, Y.; Zhang, X.; Wang, C. Effect of omega-3 Polyunsaturated Fatty Acids-Derived Bioactive Lipids on Metabolic Disorders. Front. Physiol. 2021, 12, 646491. [Google Scholar] [CrossRef]
- Cespiati, A.; Meroni, M.; Lombardi, R.; Oberti, G.; Dongiovanni, P.; Fracanzani, A.L. Impact of Sarcopenia and Myosteatosis in Non-Cirrhotic Stages of Liver Diseases: Similarities and Differences across Aetiologies and Possible Therapeutic Strategies. Biomedicines 2022, 10, 182. [Google Scholar] [CrossRef]
- Indiveri, C.; Iacobazzi, V.; Tonazzi, A.; Giangregorio, N.; Infantino, V.; Convertini, P.; Console, L.; Palmieri, F. The mitochondrial carnitine/acylcarnitine carrier: Function, structure and physiopathology. Mol. Aspects Med. 2011, 32, 223–233. [Google Scholar] [CrossRef]
- Meng, L.; Yang, R.; Wang, D.; Wu, W.; Shi, J.; Shen, J.; Dang, Y.; Fan, G.; Shi, H.; Dong, J.; et al. Specific lysophosphatidylcholine and acylcarnitine related to sarcopenia and its components in older men. BMC Geriatr. 2022, 22, 249. [Google Scholar] [CrossRef] [PubMed]
- Sekula, P.; Del Greco, M.F.; Pattaro, C.; Kottgen, A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J. Am. Soc. Nephrol. 2016, 27, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Daniel, R.M.; Butterworth, A.S.; Thompson, S.G.; Consortium, E.P.-I. Network Mendelian randomization: Using genetic variants as instrumental variables to investigate mediation in causal pathways. Int. J. Epidemiol. 2015, 44, 484–495. [Google Scholar] [CrossRef]
- Hu, Q.; Hao, P.; Liu, Q.; Dong, M.; Gong, Y.; Zhang, C.; Zhang, Y. Mendelian randomization studies on atherosclerotic cardiovascular disease: Evidence and limitations. Sci. China Life Sci. 2019, 62, 758–770. [Google Scholar] [CrossRef]
- Lawlor, D.A. Commentary: Two-sample Mendelian randomization: Opportunities and challenges. Int. J. Epidemiol. 2016, 45, 908–915. [Google Scholar] [CrossRef]
- Shin, S.-Y.; The Multiple Tissue Human Expression Resource (MuTHER) Consortium; Fauman, E.B.; Petersen, A.-K.; Krumsiek, J.; Santos, R.; Huang, J.; Arnold, M.; Erte, I.; Forgetta, V.; et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014, 46, 543–550. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Vosa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- Sedgwick, P. Multiple hypothesis testing and Bonferroni’s correction. BMJ 2014, 349, g6284. [Google Scholar] [CrossRef] [PubMed]
- Medici, M.; Peeters, R.P.; Teumer, A.; Taylor, P. The importance of high-quality mendelian randomisation studies for clinical thyroidology. Lancet Diabetes Endocrinol. 2019, 7, 665–667. [Google Scholar] [CrossRef]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef]
- Ter Borg, S.; Luiking, Y.C.; van Helvoort, A.; Boirie, Y.; Schols, J.; de Groot, C. Low Levels of Branched Chain Amino Acids, Eicosapentaenoic Acid and Micronutrients Are Associated with Low Muscle Mass, Strength and Function in Community-Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Lopez-Maside, L.; Donapetry-Garcia, C.; Fernandez-Fernandez, C.; Sixto-Leal, C. Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids 2017, 49, 1005–1028. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Bertelli, A.; Falchi, M. Evaluation of carnitine, acetylcarnitine and isovalerylcarnitine on immune function and apoptosis. Drugs Exp. Clin. Res. 2005, 31, 109–114. [Google Scholar]
- González-Blanco, L.; Bermúdez, M.; Bermejo-Millo, J.C.; Gutiérrez-Rodríguez, J.; Solano, J.J.; Antuña, E.; Menéndez-Valle, I.; Caballero, B.; Vega-Naredo, I.; Potes, Y.; et al. Cell interactome in sarcopenia during aging. J. Cachexia Sarcopenia Muscle 2022, 13, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.S.; Gao, F.; Liu, J.; Fridianto, K.T.; Ching, J.; Tan, R.S.; Wong, J.-I.; Chua, S.J.; Leng, S.; Zhong, L.; et al. Metabolomic profile of arterial stiffness in aged adults. Diab. Vasc. Dis. Res. 2018, 15, 74–80. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Kovalik, J.P.; Ching, J.; Chan, A.W.; Matchar, D.B. Novel metabolomics markers are associated with pre-clinical decline in hand grip strength in community-dwelling older adults. Mech. Ageing Dev. 2021, 193, 111405. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H.; Derbyshire, E.; Toribio-Mateas, M. Role of fatty acids and micronutrients in healthy ageing: A systematic review of randomised controlled trials set in the context of European dietary surveys of older adults. J. Hum. Nutr. Diet. 2016, 29, 308–324. [Google Scholar] [CrossRef]
- Li, K.; Sinclair, A.J.; Zhao, F.; Li, D. Uncommon Fatty Acids and Cardiometabolic Health. Nutrients 2018, 10, 1559. [Google Scholar] [CrossRef]
- Guo, X.F.; Sinclair, A.J.; Kaur, G.; Li, D. Differential effects of EPA, DPA and DHA on cardio-metabolic risk factors in high-fat diet fed mice. Prostaglandins Leukot Essent Fat. Acids 2018, 136, 47–55. [Google Scholar] [CrossRef]
- Pistorius, K.; Souza, P.R.; De Matteis, R.; Austin-Williams, S.; Primdahl, K.G.; Vik, A.; Mazzacuva, F.; Colas, R.A.; Marques, R.M.; Hansen, T.V.; et al. PDn-3 DPA Pathway Regulates Human Monocyte Differentiation and Macrophage Function. Cell Chem. Biol. 2018, 25, 749–760.e9. [Google Scholar] [CrossRef]
- Colas, R.A.; Souza, P.R.; Walker, M.E.; Burton, M.; Zasłona, Z.; Curtis, A.M.; Marques, R.M.; Dalli, J. Impaired Production and Diurnal Regulation of Vascular RvDn-3 DPA Increase Systemic Inflammation and Cardiovascular Disease. Circ. Res. 2018, 122, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.A.; Holmes, M.V. Mendelian randomisation in cardiovascular research: An introduction for clinicians. Heart 2017, 103, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | |
|---|---|
| All subjects at recruitment, n | 324,976 |
| Age, mean (SD), years | 56.4 (8.1) |
| Body mass index, mean (SD), kg/m2 | 27.4 (4.8) |
| Female sex, n (%) | 174,139 (53.6) |
| Sarcopenia, n (%) | 561 (0.2) |
| Grip strength, mean (SD), kg | 22.4 (5.3) |
| Appendicular lean mass, mean (SD), kg | 32.9 (11.3) |
| Metabolites | N (SNPs) | Variance, % | OR (95% CI) | p |
|---|---|---|---|---|
| 4-acetamidobutanoate * | 35 | 16.3 | 0.10 (0.01, 0.84) | 0.034 |
| Biliverdin * | 19 | 26.1 | 2.49 (1.06, 5.82) | 0.036 |
| Taurocholate | 16 | 21.4 | 1.52 (1.03, 2.24) | 0.037 |
| Myo-inositol * | 40 | 18.6 | 0.16 (0.03, 0.93) | 0.042 |
| Phenyllactate * | 19 | 11.3 | 0.13 (0.02, 0.81) | 0.029 |
| Levulinate (4-oxovalerate) * | 61 | 22.7 | 5.50 (1.12, 26.89) | 0.035 |
| Acetylcarnitine * | 21 | 10.3 | 10.83 (1.55, 76.81) | 0.016 |
| Docosapentaenoate (n3) | 11 | 7.3 | 0.16 (0.03, 0.83) | 0.029 |
| 5α-pregnan-3β,20α-disulfate ** | 15 | 15.3 | 0.42 (0.20, 0.87) | 0.019 |
| Gamma-glutamylmethionine | 9 | 10.8 | 7.61 (1.07, 53.99) | 0.042 |
| Valerate ** | 10 | 6.0 | 0.01 (0.00, 0.89) | <0.001 |
| Isovalerylcarnitine * | 21 | 13.5 | 4.00 (1.11, 14.52) | 0.034 |
| 1-arachidonoylglycerophosphoethanolamine * | 26 | 11.7 | 0.19 (0.04, 0.95) | 0.042 |
| Hydroxyisovaleroyl carnitine * | 8 | 6.4 | 10.96 (1.65, 72.86) | 0.013 |
| Metabolites | N (SNPs) | Variance, % | β (95% CI) | p |
|---|---|---|---|---|
| Mannose ** | 21 | 13.9 | 0.91 (0.34, 1.48) | 0.002 |
| Citrate * | 46 | 15.8 | 0.46 (0.10, 0.83) | 0.013 |
| Guanosine * | 13 | 13.0 | −0.12 (−0.24, −0.00) | 0.050 |
| Betaine * | 25 | 13.4 | −0.90 (−1.50, −0.28) | 0.004 |
| Beta-hydroxyisovalerate * | 23 | 9.9 | −0.49 (−0.89, −0.09) | 0.014 |
| 3-methylhistidine * | 11 | 6.5 | −0.14 (−0.25, −0.03) | 0.016 |
| Serine * | 35 | 13.1 | 0.91 (0.08, 1.74) | 0.031 |
| Hexanoylcarnitine ** | 19 | 13.0 | −0.40 (−0.72, −0.07) | 0.016 |
| Glycine ** | 26 | 18.7 | 0.51 (0.19, 0.84) | 0.002 |
| O-methylascorbate ** | 49 | 28.2 | −0.44 (−0.74, −0.14) | 0.003 |
| Docosapentaenoate (n3) ** | 11 | 7.3 | 0.45 (0.08, 0.81) | 0.016 |
| 1-arachidonoylglycerophosphocholine ** | 21 | 13.4 | 0.33 (0.08, 0.57) | 0.008 |
| Decanoylcarnitine ** | 15 | 9.9 | −0.34 (−0.55, −0.12) | 0.002 |
| 1-palmitoylglycerophosphocholine * | 34 | 11.2 | −0.48 (−0.92, −0.03) | 0.037 |
| 1-oleoylglycerophosphocholine * | 17 | 4.2 | −0.37 (−0.65, −0.09) | 0.010 |
| 10-heptadecenoate (17:1n7) * | 6 | 2.5 | 0.40 (0.03, 0.78) | 0.034 |
| Isovalerylcarnitine * | 21 | 13.5 | −0.45 (−0.81, −0.09) | 0.015 |
| nonanoylcarnitine | 16 | 15.7 | 0.13 (0.00, 0.26) | 0.048 |
| Tetradecanedioate * | 20 | 19.4 | 0.13 (0.02, 0.25) | 0.026 |
| Octadecanedioate | 10 | 6.3 | 0.32 (0.01, 0.63) | 0.043 |
| 5α-Adiol disulfate ** | 18 | 12.2 | 0.14 (0.02, 0.27) | 0.028 |
| 4α-Adiol disulfate 2 * | 19 | 6.9 | 0.33 (0.06, 0.61) | 0.018 |
| Metabolites | N (SNPs) | Variance, % | β (95% CI) | p |
|---|---|---|---|---|
| Laurate (12:0) * | 41 | 16.0 | −1.04 (−1.86, −0.21) | 0.014 |
| Gamma-glutamyltyrosine | 47 | 17.3 | 0.76 (0.01, 1.51) | 0.047 |
| Glucose * | 38 | 15.4 | −0.80 (−1.59, −0.01) | 0.046 |
| 1-oleoylglycerol (1-monoolein) | 16 | 7.5 | −0.54 (−1.05, −0.02) | 0.040 |
| Hyodeoxycholate ** | 17 | 12.5 | 0.29 (0.05, 0.52) | 0.017 |
| Androsterone sulfate ** | 22 | 20.7 | −0.17 (−0.30, −0.03) | 0.014 |
| Glycine ** | 26 | 18.7 | 0.66 (0.19, 1.13) | 0.006 |
| 3-dehydrocarnitine ** | 27 | 11.6 | −1.51 (−2.27, −0.75) | <0.001 |
| 1-heptadecanoyl glycerophosphocholine * | 10 | 7.3 | 1.10 (0.26, 1.93) | 0.010 |
| Epiandrosterone sulfate ** | 13 | 12.3 | −0.33 (−0.53, −0.13) | 0.001 |
| Isovalerylcarnitine * | 21 | 13.5 | −1.51 (−2.31, −0.71) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, T.; Wang, N.; Wei, J.; He, H.; Wang, Y.; Zeng, C.; Lei, G. Genetically Predicted Levels of Serum Metabolites and Risk of Sarcopenia: A Mendelian Randomization Study. Nutrients 2023, 15, 3964. https://doi.org/10.3390/nu15183964
Sha T, Wang N, Wei J, He H, Wang Y, Zeng C, Lei G. Genetically Predicted Levels of Serum Metabolites and Risk of Sarcopenia: A Mendelian Randomization Study. Nutrients. 2023; 15(18):3964. https://doi.org/10.3390/nu15183964
Chicago/Turabian StyleSha, Tingting, Ning Wang, Jie Wei, Hongyi He, Yilun Wang, Chao Zeng, and Guanghua Lei. 2023. "Genetically Predicted Levels of Serum Metabolites and Risk of Sarcopenia: A Mendelian Randomization Study" Nutrients 15, no. 18: 3964. https://doi.org/10.3390/nu15183964
APA StyleSha, T., Wang, N., Wei, J., He, H., Wang, Y., Zeng, C., & Lei, G. (2023). Genetically Predicted Levels of Serum Metabolites and Risk of Sarcopenia: A Mendelian Randomization Study. Nutrients, 15(18), 3964. https://doi.org/10.3390/nu15183964






