The Effects of Dietary Chromium Supplementation along with Discontinuing a High-Fat Diet on the Microbial Enzymatic Activity and the Production of SCFAs in the Faeces of Rats
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
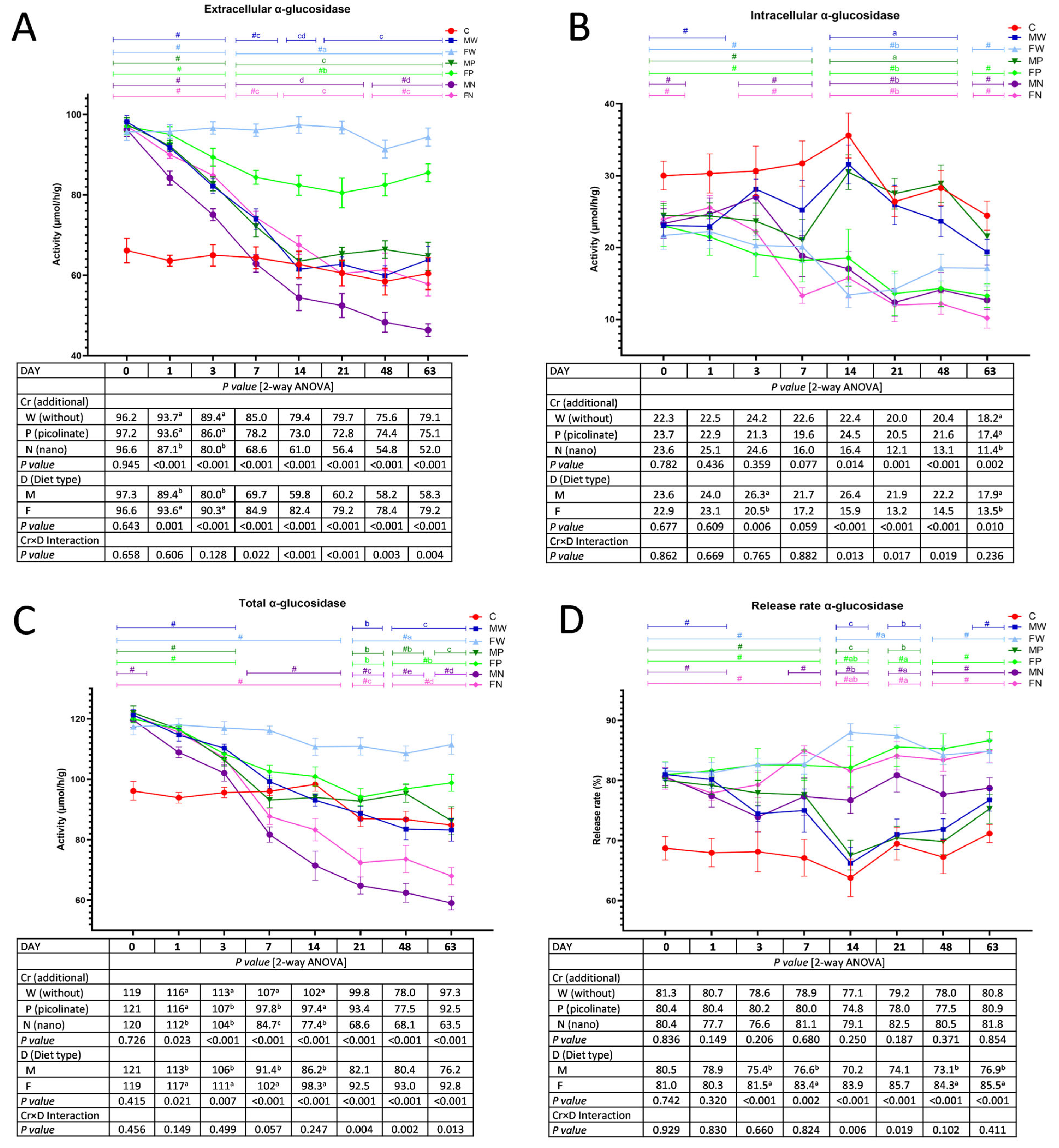
3.1. The Activity of Faecal Bacterial α-Glucosidase
3.2. The Activity of Faecal Bacterial β-Glucosidase
3.3. The Activity of Faecal Bacterial β-Glucuronidase
3.4. The Activity of Faecal Bacterial β-Xylosidase
3.5. Faecal Short-Chain Fatty Acids
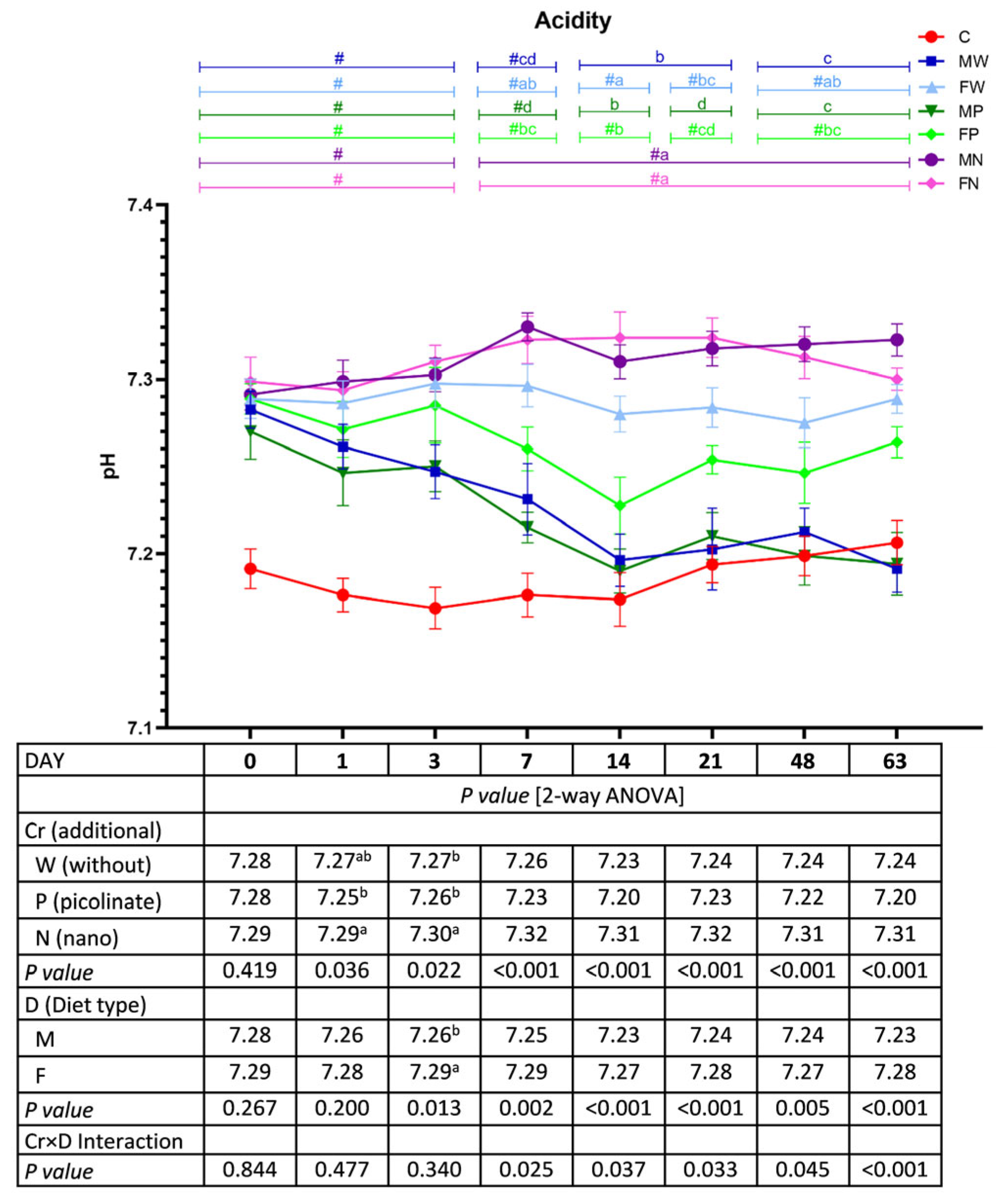
3.6. Faecal pH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baothman, O.A.; Zamzami, M.A.; Abubaker, J.; Abu-Farha, M. The role of gut microbiota in the development of obesity and diabetes. Lipids Health Dis. 2018, 15, 108. [Google Scholar] [CrossRef]
- Bajzer, M.; Seeley, R.J. Physiology: Obesity and gut flora. Nature 2006, 444, 1009–1010. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Nagpal, R.; Wang, S.; Solberg Woods, L.C.; Seshie, O.; Chung, S.T.; Shively, C.A.; Register, T.C.; Craft, S.; McClain, D.A.; Yadav, H. Comparative Microbiome Signatures and Short-Chain Fatty Acids in Mouse, Rat, Non-human Primate, and Human Feces. Front. Microbiol. 2018, 9, 2897. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Ramin, K.I.; Allison, S.D. Bacterial Tradeoffs in Growth Rate and Extracellular Enzymes. Front. Microbiol. 2019, 10, 2956. [Google Scholar] [CrossRef]
- Dashnyam, P.; Mudududdla, R.; Hsieh, T.J.; Lin, T.C.; Lin, H.Y.; Chen, P.Y.; Hsu, C.Y.; Lin, C.H. β-Glucuronidases of opportunistic bacteria are the major contributors to xenobiotic-induced toxicity in the gut. Sci. Rep. 2018, 8, 16372. [Google Scholar] [CrossRef]
- Sui, Y.; Wu, J.; Chen, J. The Role of Gut Microbial β-Glucuronidase in Estrogen Reactivation and Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 631552. [Google Scholar] [CrossRef]
- Awolade, P.; Cele, N.; Kerru, N.; Gummidi, L.; Oluwakemi, E.; Singh, P. Therapeutic significance of β-glucuronidase activity and its inhibitors: A review. Eur. J. Med. Chem. 2020, 187, 111921. [Google Scholar] [CrossRef]
- Zierer, J.; Jackson, M.A.; Kastenmüller, G.; Mangino, M.; Long, T.; Telenti, A.; Mohney, R.P.; Small, K.S.; Bell, J.T.; Steves, C.J.; et al. The fecal metabolome as a functional readout of the gut microbiome. Nat. Genet. 2018, 50, 790–795. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature 2023. [Google Scholar] [CrossRef]
- Tan, K.; Tesar, C.; Wilton, R.; Keigher, L.; Babnigg, G.; Joachimiak, A. Novel α-glucosidase from human gut microbiome: Substrate specificities and their switch. FASEB J. 2010, 24, 3939–3949. [Google Scholar] [CrossRef]
- Flores, R.; Shi, J.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Association of fecal microbial diversity and taxonomy with selected enzymatic functions. PLoS ONE 2012, 7, e39745. [Google Scholar] [CrossRef]
- Li, Q.; Wu, T.; Qi, Z.; Zhao, L.; Pei, J.; Tang, F. Characterization of a novel thermostable and xylose-tolerant GH 39 β-xylosidase from Dictyoglomus thermophilum. BMC Biotechnol. 2018, 18, 29. [Google Scholar] [CrossRef]
- Pala, R.; Sari, M.A.; Erten, F.; Er, B.; Tuzcu, M.; Orhan, C.; Deeh, P.B.D.; Sahin, N.; Cinar, V.; Komorowski, J.R.; et al. The effects of chromium picolinate on glucose and lipid metabolism in running rats. J. Trace Elem. Med. Biol. 2020, 58, 126434. [Google Scholar] [CrossRef]
- Niehoff, N.M.; Keil, A.P.; O’Brien, K.M.; Jackson, B.P.; Karagas, M.R.; Weinberg, C.R.; White, A.J. Metals and trace elements in relation to body mass index in a prospective study of US women. Environ. Res. 2020, 184, 109396. [Google Scholar] [CrossRef]
- Kooshki, F.; Tutunchi, H.; Vajdi, M.; Karimi, A.; Niazkar, H.R.; Shoorei, H.; Pourghassem Gargari, B. A Comprehensive insight into the effect of chromium supplementation on oxidative stress indices in diabetes mellitus: A systematic review. Clin. Exp. Pharm. Physiol. 2021, 48, 291–309. [Google Scholar] [CrossRef]
- Sahin, K.; Tuzcu, M.; Orhan, C.; Sahin, N.; Kucuk, O.; Ozercan, I.H.; Juturu, V.; Komorowski, J.R. Anti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocin. Br. J. Nutr. 2013, 110, 197–205. [Google Scholar] [CrossRef]
- Piotrowska, A.; Pilch, W.; Czerwińska-Ledwig, O.; Zuziak, R.; Siwek, A.; Wolak, M.; Nowak, G. The Possibilities of Using Chromium Salts as an Agent Supporting Treatment of Polycystic Ovary Syndrome. Biol. Trace Elem. Res. 2019, 192, 91–97. [Google Scholar] [CrossRef]
- Akhtar, A.; Dhaliwal, J.; Saroj, P.; Uniyal, A.; Bishnoi, M.; Sah, S.P. Chromium picolinate attenuates cognitive deficit in ICV-STZ rat paradigm of sporadic Alzheimer’s-like dementia via targeting neuroinflammatory and IRS-1/PI3K/AKT/GSK-3β pathway. Inflammopharmacology 2020, 28, 385–400. [Google Scholar] [CrossRef]
- Stępniowska, A.; Juśkiewicz, J.; Tutaj, K.; Fotschki, J.; Fotschki, B.; Ognik, K. Effects of chromium picolinate and chromium nanoparticles added to low- or high-fat diets on chromium biodistribution and the blood level of selected minerals in rats. Pol. J. Food Nutr. Sci. 2022, 72, 229–238. [Google Scholar] [CrossRef]
- Król, E.; Krejpcio, Z.; Iwanik, K. Supplementary chromium(III) propionate complex does not protect against insulin resistance in high-fat-fed rats. Biol. Trace Elem. Res. 2014, 157, 147–155. [Google Scholar] [CrossRef]
- Vincent, J.B. Is chromium pharmacologically relevant? J. Trace Elem. Med. Biol. 2014, 28, 397–405. [Google Scholar] [CrossRef]
- Talab, A.T.; Abdollahzad, H.; Nachvak, S.M.; Pasdar, Y.; Eghtesadi, S.; Izadi, A.; Aghdashi, M.A.; Mohammad Hossseini Azar, M.R.; Moradi, S.; Mehaki, B.; et al. Effects of Chromium Picolinate Supplementation on Cardiometabolic Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Clinical Trial. Clin. Nutr. Res. 2020, 9, 97–106. [Google Scholar] [CrossRef]
- Moreira, L.D.P.D.; Gomes, J.V.P.; Mattar, J.B.; Chaves, L.O.; Martino, H.S.D. Potential of trace elements as supplements for the metabolic control of Type 2 Diabetes Mellitus: A systematic review. J. Funct. Foods 2019, 57, 317–327. [Google Scholar] [CrossRef]
- Majewski, M.; Gromadziński, L.; Cholewińska, E.; Ognik, K.; Fotschki, B.; Juśkiewicz, J. Dietary effects of chromium picolinate and chromium nanoparticles in Wistar rats fed with a high-fat, low-fibre diet: The role of fat normalization. Nutrients 2022, 14, 5138. [Google Scholar] [CrossRef]
- Frőhlich, E.E.; Frőhlich, E. Cytotoxicity of nanoparticles contained in food on intestinal cells and the gut microbiota. Int. J. Mol. Sci. 2016, 17, 509. [Google Scholar] [CrossRef]
- Fotschki, B.; Ognik, K.; Fotschki, J.; Napiórkowska, D.; Cholewińska, E.; Krauze, M.; Juśkiewicz, J. Chromium nanoparticles together with a switch away from high-fat/low-fibre dietary habits enhances the pro-healthy regulation of liver lipid metabolism and inflammation in obese rats. Int. J. Mol. Sci. 2023, 24, 2940. [Google Scholar] [CrossRef]
- Lien, T.F.; Yeh, H.S.; Lu, F.Y.; Fu, C.M. Nanoparticles of chromium picolinate enhance chromium digestibility and absorption. J. Sci. Food Agric. 2009, 89, 1164–1167. [Google Scholar] [CrossRef]
- Dworzański, W.; Cholewińska, E.; Fotschki, B.; Juśkiewicz, J.; Ognik, K. Oxidative, epigenetic changes and fermentation processes in the intestine of rats fed high-fat diets supplemented with various chromium forms. Sci. Rep. 2022, 12, 9817. [Google Scholar] [CrossRef]
- Wadden, T.A.; Webb, V.L.; Moran, C.H.; Bailer, B.A. Lifestyle modification for obesity: New developments in diet, physical activity, and behavior therapy. Circulation 2012, 125, 1157–1170. [Google Scholar] [CrossRef]
- Kalita, H.; Hazarika, A.; Devi, R. Withdrawal of High-Carbohydrate, High-Fat Diet Alters Status of Trace Elements to Ameliorate Metabolic Syndrome in Rats With Type 2 Diabetes Mellitus. Can. J. Diabetes 2020, 44, 317–326.e1. [Google Scholar] [CrossRef]
- Fotschki, B.; Wiczkowski, W.; Sawicki, T.; Sójka, M.; Myszczyński, K.; Ognik, K.; Juśkiewicz, J. Stimulation of the intestinal microbiota with prebiotics enhances hepatic levels of dietary polyphenolic compounds, lipid metabolism and antioxidant status in healthy rats. Food Res. Int. 2022, 160, 111754. [Google Scholar] [CrossRef]
- Żary-Sikorska, E.; Fotschki, B.; Kosmala, M.; Milala, J.; Matusevicius, P.; Rawicka, A.; Juśkiewicz, J. Strawberry polyphenol-rich fractions can mitigate disorders in gastrointestinal tract and liver functions caused by a high-fructose diet in experimental rats. Pol. J. Food Nutr. Sci. 2021, 71, 423–440. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Cao, W.; Chin, Y.; Chen, X.; Mi, Y.; Xue, C.; Wang, Y.; Tang, Q. The role of gut microbiota in the resistance to obesity in mice fed a high fat diet. Int. J. Food Sci. Nutr. 2020, 71, 453–463. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Panek, M.; Čipčić Paljetak, H.; Barešić, A.; Perić, M.; Matijašić, M.; Lojkić, I.; Vranešić Bender, D.; Krznarić, Ź.; Verbanac, D. Methodology challenges in studying human gut microbiota—Effects of collection, storage, DNA extraction and next generation sequencing technologies. Sci. Rep. 2018, 8, 5143. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef]
- Grzelak-Błaszczyk, K.; Milala, J.; Kołodziejczyk, K.; Sójka, M.; Czarnecki, A.; Kosmala, M.; Klewicki, R.; Fotschki, B.; Jurgoński, A.; Juśkiewicz, J. Protocatechuic acid and quercetin glucosides in onions attenuate changes induced by high fat diet in rats. Food Funct. 2020, 11, 3585–3597. [Google Scholar] [CrossRef]
- Michlmayr, H.; Kneifel, W. β-Glucosidase activities of lactic acid bacteria: Mechanisms, impact on fermented food and human health. FEMS Microbiol. Lett. 2014, 352, 1–10. [Google Scholar] [CrossRef]
- Ermini, M.L.; Voliani, V. Antimicrobial Nano-Agents: The Copper Age. ACS Nano 2021, 15, 6008–6029. [Google Scholar] [CrossRef]
- Utembe, W.; Tlotleng, N.; Kamng’ona, A.W. A systematic review on the effects of nanomaterials on gut microbiota. Curr. Res. Microb. Sci. 2022, 3, 100118. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.-L.; Sun, Y.-Y.; Cha, Q.-Q.; Li, C.-Y.; Zhao, D.-L.; Song, X.-Y.; Wang, M.; McMinn, A.; Chen, X.-L.; et al. Extracellular enzyme activity and its implications for organic matter cycling in Notthern Chinese Marginal Sea. Front. Microbiol. 2019, 10, 2137. [Google Scholar] [CrossRef]
- Agans, R.T.; Gordon, A.; Hussain, S.; Paliy, O. Titanium dioxide nanoparticles elicit lower direct inhibitory effect on human gut microbiota than silver nanoparticles. Toxicol. Sci. 2019, 172, 411–416. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, R.; Wang, B.; Cai, C.; Zheng, L.; Wang, H.; Wang, M.; Ouyang, H.; Zhou, X.; Chai, Z. The effects of orally administered Ag, TiO2 and SiO2 nanoparticles on gut microbiota composition and colitis induction in mice. NanoImpact 2017, 8, 80–88. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Q.-L.; O’Connor, P.; Sheng, G.D. Does soil CuO nanoparticles pollution alter the gut microbiota and resistome of Enchytraeus crypticus? Environ. Pollut. 2020, 256, 113463. [Google Scholar] [CrossRef]
- Wu, J.; Li, C.; Zhang, J.; Menzies, N.W.; Bertsch, P.M.; Wang, P.; Kopittke, P.M. Release of silver from nanoparticle-based filter paper and the impacts to mouse gut microbiota. Environ. Sci. Nano 2020, 7, 1554–1565. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Y.; Qian, K.; Zhang, W.; Wu, D.; Wang, C. Effects of chromium-enriched Bacillus subtilis KT260179 supplementation on growth performance, caecal microbiology, tissue chromium level, insulin receptor expression and plasma biochemical profile of mice under heat stress. Br. J. Nutr. 2016, 115, 774–781. [Google Scholar] [CrossRef]
- Guo, W.L.; Chen, M.; Pan, W.L.; Zhang, Q.; Xu, J.X.; Lin, Y.C.; Li, L.; Liu, B.; Bai, W.D.; Zhang, Y.Y.; et al. Hypoglycemic and hypolipidemic mechanism of organic chromium derived from chelation of Grifola frondosa polysaccharide-chromium (III) and its modulation of intestinal microflora in high fat-diet and STZ-induced diabetic mice. Int. J. Biol. Macromol. 2020, 145, 1208–1218. [Google Scholar] [CrossRef]
- Fotschki, B.; Ognik, K.; Cholewińska, E.; Grzelak-Błaszczyk, K.; Myszczyński, K.; Krauze, M.; Juśkiewicz, J. Effect of Chromium Nanoparticles and Switching from a High-Fat to a Low-Fat Diet on the Cecal Microenvironment in Obese Rats. Nutrients 2023, 15, 3118. [Google Scholar] [CrossRef]
- Chassard, C.; Goumy, V.; Leclerc, M.; Del’homme, C.; Bernalier-Donadille, A. Characterization of the xylan-degrading microbial community from human faeces. FEMS Microbiol. Ecol. 2007, 61, 121–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Mortimer, M.; Guo, L.-H. Interplay between engineered nanomaterials and microbiota. Environ. Sci. Nano 2020, 7, 2454–2485. [Google Scholar] [CrossRef]
- Ruiz, A.; Cerdó, T.; Jáuregui, R.; Pieper, D.H.; Marcos, A.; Clemente, A.; García, F.; Margolles, A.; Ferrer, M.; Campoy, C.; et al. One-year calorie restriction impacts gut microbial composition but not its metabolic performance in obese adolescents. Environ. Microbiol. 2017, 19, 1536–1551. [Google Scholar] [CrossRef]




| Group | Control C | MW | FW | MP | FP | MN | FN |
|---|---|---|---|---|---|---|---|
| Introductory period (1–9 weeks) | Diet C | Diet F | |||||
| Cr dietary addition | Without Cr | ||||||
| Experimental period (10–18 weeks) | Diet C | Diet F | Diet C | Diet F | Diet C | Diet F | |
| Cr dietary addition * | Without Cr | Cr-Pic | Cr-NP | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juśkiewicz, J.; Ognik, K.; Fotschki, J.; Napiórkowska, D.; Cholewińska, E.; Grzelak-Błaszczyk, K.; Krauze, M.; Fotschki, B. The Effects of Dietary Chromium Supplementation along with Discontinuing a High-Fat Diet on the Microbial Enzymatic Activity and the Production of SCFAs in the Faeces of Rats. Nutrients 2023, 15, 3962. https://doi.org/10.3390/nu15183962
Juśkiewicz J, Ognik K, Fotschki J, Napiórkowska D, Cholewińska E, Grzelak-Błaszczyk K, Krauze M, Fotschki B. The Effects of Dietary Chromium Supplementation along with Discontinuing a High-Fat Diet on the Microbial Enzymatic Activity and the Production of SCFAs in the Faeces of Rats. Nutrients. 2023; 15(18):3962. https://doi.org/10.3390/nu15183962
Chicago/Turabian StyleJuśkiewicz, Jerzy, Katarzyna Ognik, Joanna Fotschki, Dorota Napiórkowska, Ewelina Cholewińska, Katarzyna Grzelak-Błaszczyk, Magdalena Krauze, and Bartosz Fotschki. 2023. "The Effects of Dietary Chromium Supplementation along with Discontinuing a High-Fat Diet on the Microbial Enzymatic Activity and the Production of SCFAs in the Faeces of Rats" Nutrients 15, no. 18: 3962. https://doi.org/10.3390/nu15183962
APA StyleJuśkiewicz, J., Ognik, K., Fotschki, J., Napiórkowska, D., Cholewińska, E., Grzelak-Błaszczyk, K., Krauze, M., & Fotschki, B. (2023). The Effects of Dietary Chromium Supplementation along with Discontinuing a High-Fat Diet on the Microbial Enzymatic Activity and the Production of SCFAs in the Faeces of Rats. Nutrients, 15(18), 3962. https://doi.org/10.3390/nu15183962






