The Dark Side of Energy Drinks: A Comprehensive Review of Their Impact on the Human Body
Abstract
1. Introduction
| Energy Drink | Caffeine (mg) | Sugar (g) | Other Ingredients |
|---|---|---|---|
| Red Bull | 160 | 54 | Taurine (2000 mg), gluconolactone (1200 mg), inositol N/S [not specified] and vitamins B3, B5, B6 and B12 N/S |
| Monster | 160 | 54 | Taurine (2000 mg), gluconolactone N/S, carnitine N/S, inositol and guarana N/S, ginseng (400 mg) and vitamins B2, B3, B6 and B12 (40 mg) |
| Rockstar | 160 | 62 | Taurine (2000 mg), carnitine (50 mg), inositol (50 mg) and guarana (50 mg), ginseng (50 mg), gingko biloba (300 mg), milk thistle (40 mg) and vitamins B2, B3, B5, B6 and B12 (50 mg) |
| Mountain Dew | 72 | 61 | Carbonated water, high-fructose corn syrup, concentrated orange juice, citric acid, natural flavour, sodium benzoate, caffeine, sodium citrate, gum arabic, erythorbic acid, calcium disodium, yellow 5 |
| Race | 160 | 0 | N/S |
| Sting | 290 | 50 | Taurine (148 mg), inositol (21 mg), ginseng (9.7 mg), vitamin B3 (10.2 mg) |
| Magnus Omnilife | N/S | N/S | N/S |
| Demon Energy Shot (sold in 60 mL) | 1600 (200 mg per 60 mL) | N/S | Taurine (10 g), glucuronolactone (N/S), inositol (N/S), B3 (N/S), guarana (60 mg), B5 (N/S), B6 (N/S), B12 (N/S) |
| Full Throttle | 141 | 57 | Taurine (N/S), guarana (N/S), B3 (N/S), B6 (N/S), B8 (N/S), carnitine (N/S) |
| Lucozade | 60.5 | 23 | Carbonated water (N/S), citric acid (N/S), sodium gluconate (N/S), potassium sorbate (N/S), aspartame (N/S), acesulfame K (N/S), flavourings (N/S), sunset yellow (N/S), ponceau 4R (N/S), ascorbic acid (N/S). |
2. Materials and Methods
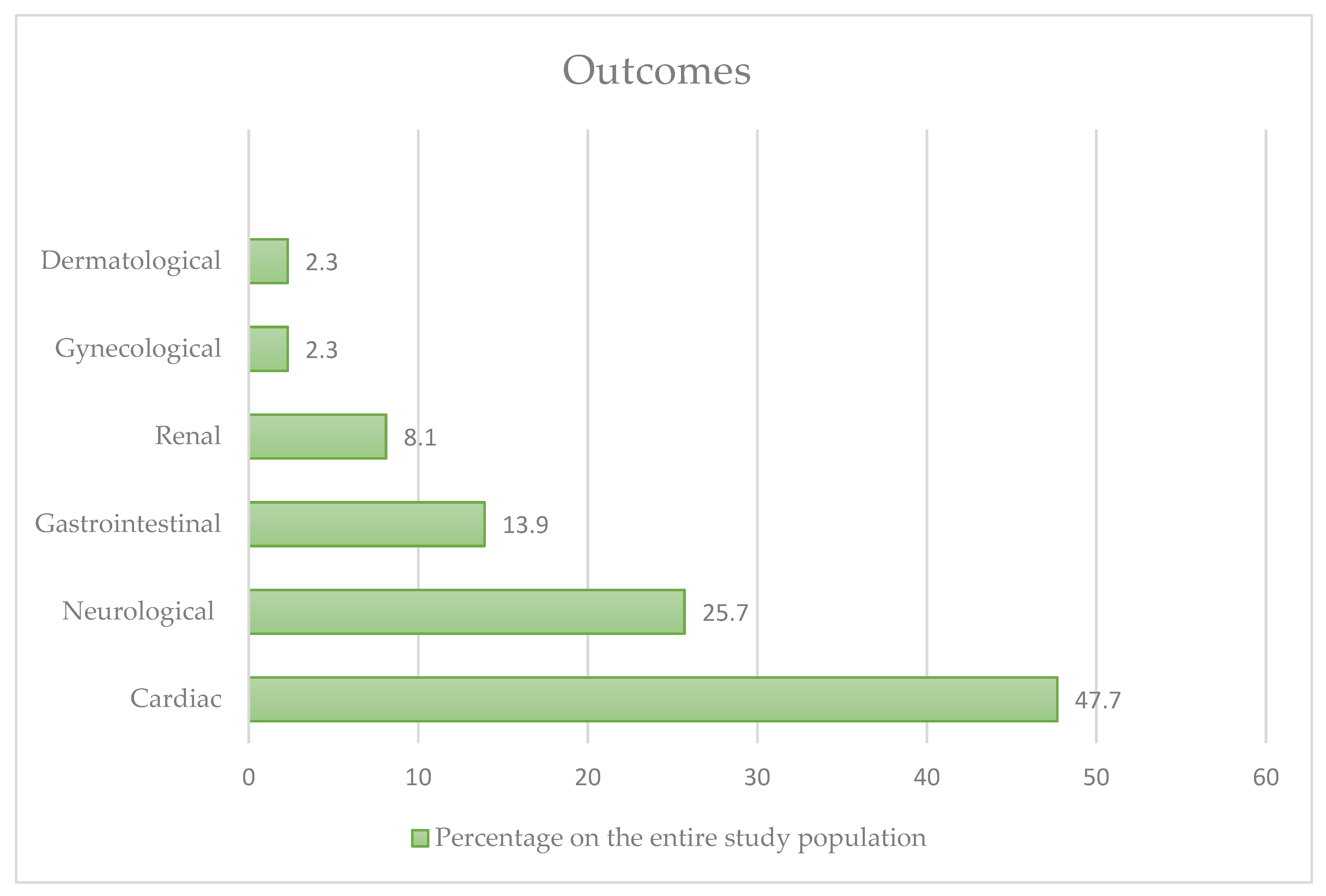
3. Results
4. Discussion
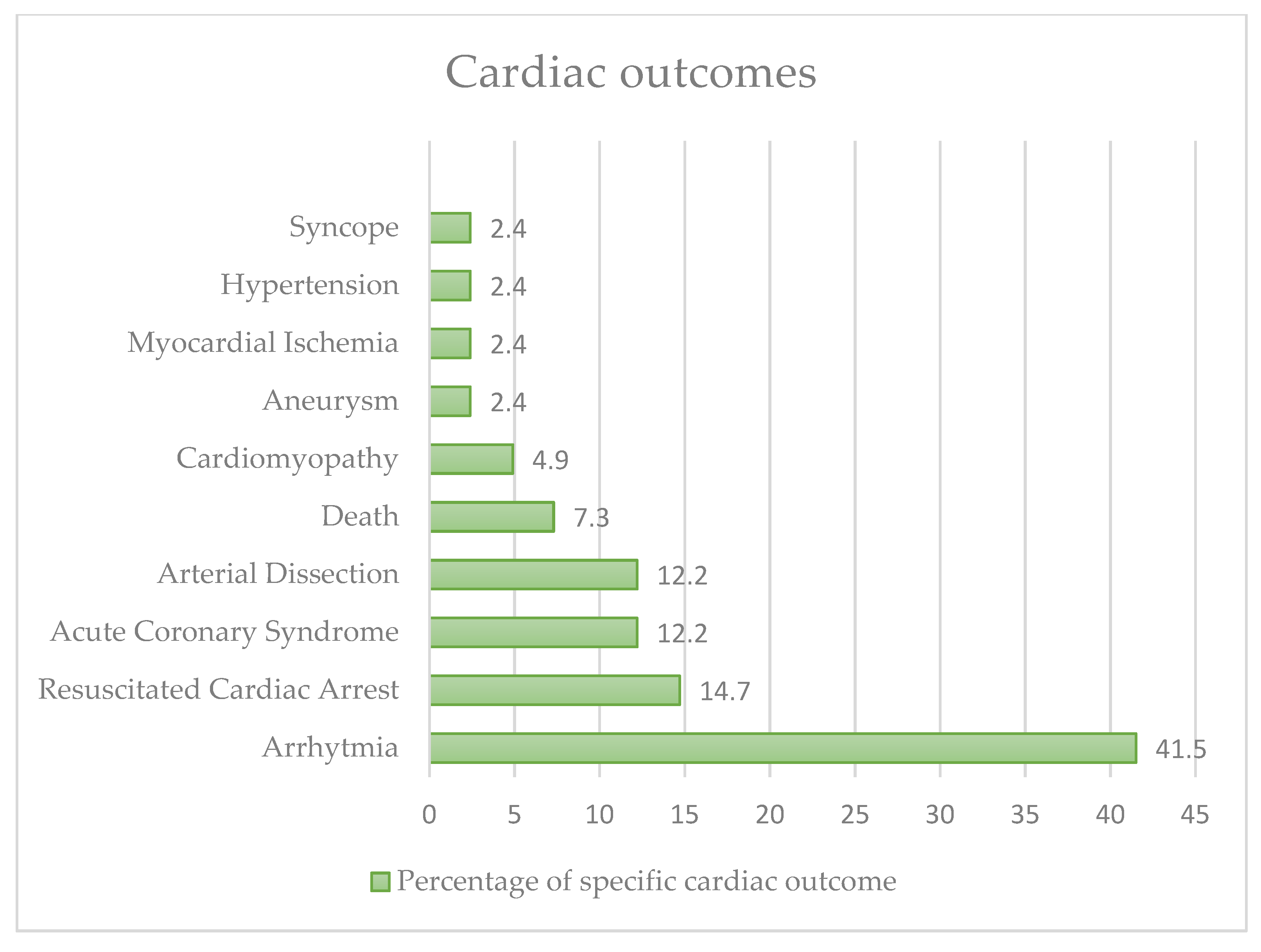
4.1. Effects on the Cardiovascular System
4.2. Effects on the Neurological System
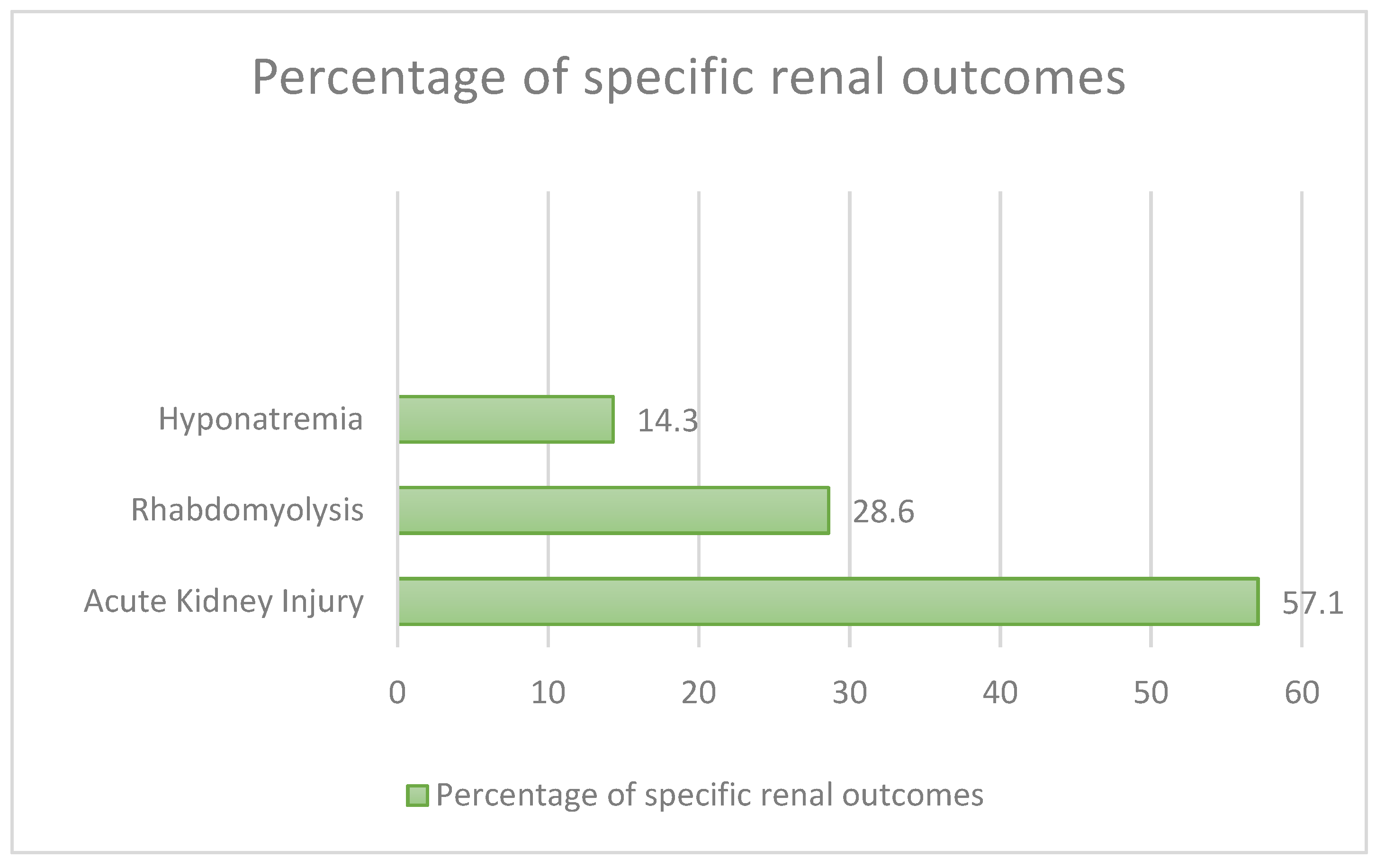
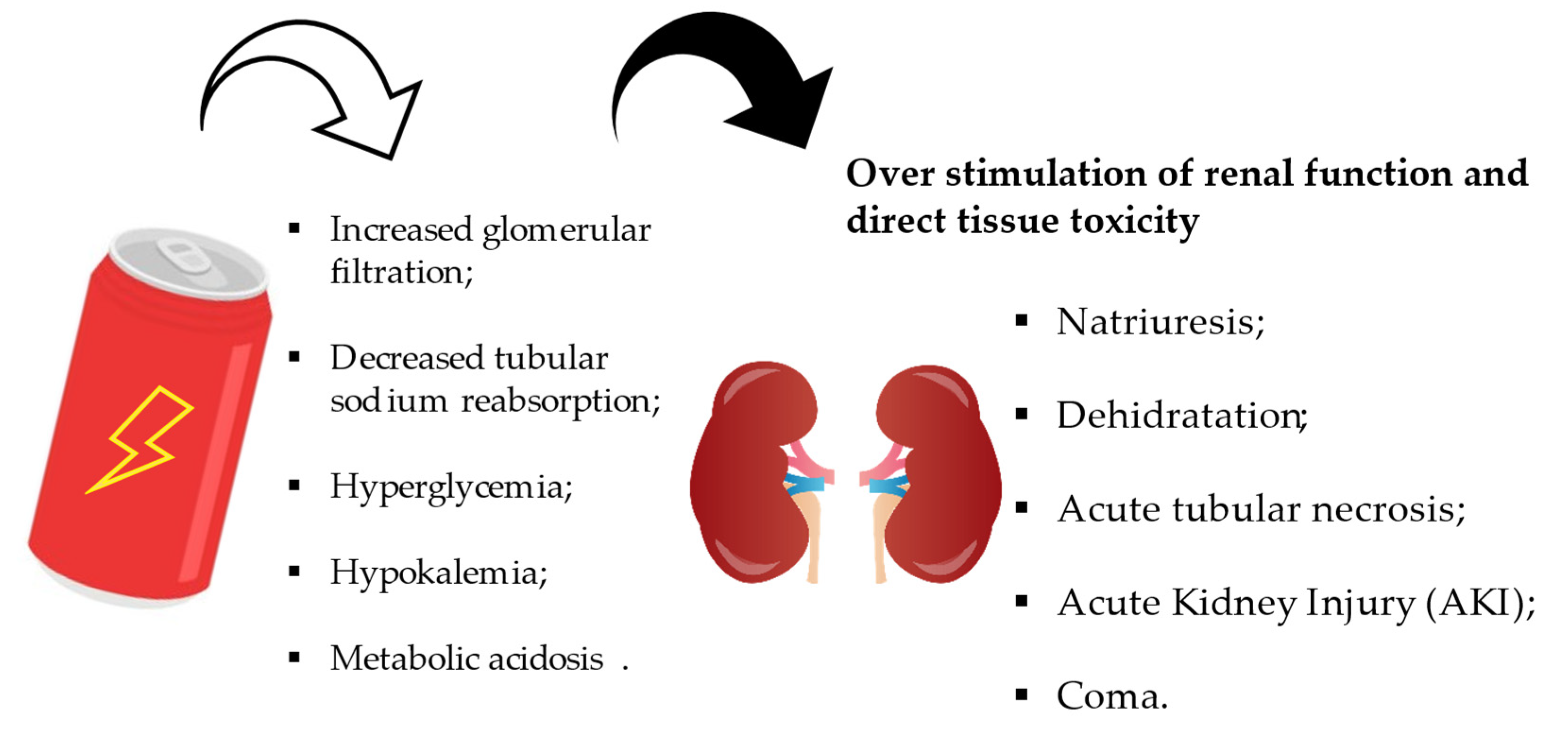
4.3. Effects on the Gastrointestinal and Renal System
4.4. Other Effects
4.5. Experimental Studies on Animal Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seifert, S.M.; Schaechter, J.L.; Hershorin, E.R.; Lipshultz, S.E. Health Effects of Energy Drinks on Children, Adolescents, and Young Adults. Pediatrics 2011, 127, 511–528. [Google Scholar] [CrossRef]
- Heckman, M.; Sherry, K.; De Mejia, E.G. Energy Drinks: An Assessment of Their Market Size, Consumer Demographics, Ingredient Profile, Functionality, and Regulations in the United States. Compr. Rev. Food Sci. Food Saf. 2010, 9, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Reissig, C.J.; Strain, E.C.; Griffiths, R.R. Caffeinated energy drinks—A growing problem. Drug Alcohol Depend. 2009, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rath, M. Energy drinks: What is all the hype? The dangers of energy drink consumption. J. Am. Acad. Nurse Pract. 2012, 24, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Rehm, C.D. Sources of Caffeine in Diets of US Children and Adults: Trends by Beverage Type and Purchase Location. Nutrients 2016, 8, 154. [Google Scholar] [CrossRef]
- Higgins, J.P.; Tuttle, T.D.; Higgins, C.L. Energy beverages: Content and safety. Mayo Clin. Proc. 2010, 85, 1033–1041. [Google Scholar] [CrossRef]
- Eley, D.W.; Lake, N.; Eter Keurs, H. Taurine depletion and excitation-contraction coupling in rat myocardium. Circ. Res. 1994, 74, 1210–1219. [Google Scholar] [CrossRef]
- Wassef, B.; Kohansieh, M.; Makaryus, A.N. Effects of energy drinks on the cardiovascular system. World J. Cardiol. 2017, 9, 796–806. [Google Scholar] [CrossRef]
- Timbrell, J.A.; Seabra, V.; Waterfield, C.J. The in vivo and in vitro protective properties of taurine. Gen. Pharmacol. Vasc. Syst. 1995, 26, 453–462. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Shimada, K.; Jong, C.J.; Ito, T.; Azuma, J.; Takahashi, K. Effect of taurine and potential interactions with caffeine on cardiovascular function. Amino Acids 2014, 46, 1147–1157. [Google Scholar] [CrossRef]
- Lake, N.; de Roode, M.; Nattel, S. Effects of taurine depletion on rat cardiac electrophysiology: In vivo and in vitro studies. Life Sci. 1987, 40, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H. Inhibition of the Fast Na+ Current by Taurine in Guinea Pig Ventricular Myocytes. Gen. Pharmacol. Vasc. Syst. 1998, 31, 155–157. [Google Scholar] [CrossRef]
- Chepkova, A.N.; Doreulee, N.; Yanovsky, Y.; Mukhopadhyay, D.; Haas, H.L.; Sergeeva, O.A. Long-term enhancement of corticostriatal neurotransmission by taurine. Eur. J. Neurosci. 2002, 16, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Seidl, R.; Peyrl, A.; Nicham, R.; Hauser, E. A taurine and caffeine-containing drink stimulates cognitive performance and well-being. Amino Acids 2000, 19, 635–642. [Google Scholar] [CrossRef]
- Kim, W. Debunking the Effects of Taurine in Red Bull Energy Drink. Nutr. Bytes 2003, 9, 1. [Google Scholar]
- Tamura, S.; Tsutsumi, S.; Ito, H.; Nakai, K.; Masuda, M. Effects of glucuronolactone and the other carbohydrates on the biochemicalchanges produced in the living body of rats by hard exercise. Jpn. J. Pharmacol. 1968, 18, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Caballero, B.; Cousins, R.J.; Tucker, K.L.; Ziegler, T.R. Carnitine. In Modern Nutrition in Health and Disease, 11th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2014; pp. 252–253. [Google Scholar]
- Ferreira, G.C.; McKenna, M.C. l-Carnitine and Acetyl-l-carnitine Roles and Neuroprotection in Developing Brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef]
- National Institute of Child Health and Human Development; National Center for Complementary and Alternative Medicine; National Institute of Mental Health; Office of Dietary Supplements; National Institutes of Health. Carnitine: The Science Behind a Conditionally Essential Nutrient; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Belay, B.; Esteban-Cruciani, N.; Walsh, C.A.; Kaskel, F.J. The use of levo-carnitine in children with renal disease: A review and a call for future studies. Pediatr. Nephrol. 2006, 21, 308–317. [Google Scholar] [CrossRef]
- NIH—National Institutes of Health-Carnitine Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Carnitine-HealthProfessional/ (accessed on 17 April 2023).
- Schimpl, F.C.; da Silva, J.F.; Gonçalves, J.F.d.C.; Mazzafera, P. Guarana: Revisiting a highly caffeinated plant from the Amazon. J. Ethnopharmacol. 2013, 150, 14–31. [Google Scholar] [CrossRef]
- Moustakas, D.; Mezzio, M.; Rodriguez, B.R.; Constable, M.A.; Mulligan, M.E.; Voura, E.B. Guarana Provides Additional Stimulation over Caffeine Alone in the Planarian Model. PLoS ONE 2015, 10, e0123310. [Google Scholar] [CrossRef]
- D’Angelo, S.; Ascione, A. Guarana and physical performance: A myth or reality? J. Hum. Sport Exerc. 2020, 15, S539–S551. [Google Scholar]
- Williams, M.H. Dietary Supplements and Sports Performance: Introduction and Vitamins. J. Int. Soc. Sports Nutr. 2004, 1, 1–6. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Argano, C.; Colomba, D.; Di Chiara, T.; La Rocca, E. Take the wind out your salis: Relationship among energy drink abuse, hypertension, and break-up of cerebral aneurysm. Intern. Emerg. Med. 2012, 7, 9–10. [Google Scholar] [CrossRef][Green Version]
- Avcı, S.; Sarıkaya, R.; Büyükcam, F. Death of a young man after overuse of energy drink. Am. J. Emerg. Med. 2013, 31, 1624.e3–1624.e4. [Google Scholar] [CrossRef]
- Berger, A.J.; Alford, K. Cardiac Arrest in a Young Man Following Excess Consumption of Caffeinated “Energy Drinks”. Med. J. Aust. 2009, 190, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Benjo, A.M.; Pineda, A.M.; Nascimento, F.O.; Zamora, C.; Lamas, G.A.; Escolar, E. Left Main Coronary Artery Acute Thrombosis Related to Energy Drink Intake. Circulation 2012, 125, 1447–1448. [Google Scholar] [CrossRef]
- Cannon, M.E.; Cooke, C.T.; McCarthy, J.S. Caffeine-induced cardiac arrhythmia: An unrecognized danger of health food products. Med. J. Aust. 2001, 174, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, T.R.; Abdool, M.A.; Galasko, G. Energy drinks give you wings but also an abnormal exercise test. BMJ Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, J.R.; During, A.; Morelli, P.J.; Heyden, M.; Biancaniello, T.A. Atrial fibrillation in healthy adolescents after highly caffeinated beverage consumption: Two case reports. J. Med. Case Rep. 2011, 5, 18. [Google Scholar] [CrossRef]
- Dufendach, K.A.; Horner, J.M.; Cannon, B.C.; Ackerman, M.J. Congenital type 1 long QT syndrome unmasked by a highly caffeinated energy drink. Heart Rhythm. 2012, 9, 285–288. [Google Scholar] [CrossRef]
- Enriquez, A.; Frankel, D.S. Arrhythmogenic Effects of Energy Drinks. J. Cardiovasc. Electrophysiol. 2017, 28, 711–717. [Google Scholar] [CrossRef]
- Gharacholou, S.M.; Ijioma, N.; Banwart, E.; Munoz, F.D.C. ST-Segment Elevation Myocardial Infarction and Normal Coronary Arteries after Consuming Energy Drinks. Case Rep. Cardiol. 2017, 2017, 4061205. [Google Scholar] [CrossRef]
- Hanif, M.; Saleem, S.; Naz, S.; Sundas, F. Energy Drinks and Atrial Fibrillation: An Unusual Case of Caution. Cureus 2020, 12, e10807. [Google Scholar] [CrossRef] [PubMed]
- Israelit, S.H.; Strizevsky, A.; Raviv, B. ST elevation myocardial infarction in a young patient after ingestion of caffeinated energy drink and ecstasy. World J. Emerg. Med. 2012, 3, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Jonjev, Z.S.; Bala, G. High-energy drinks may provoke aortic dissection. Coll. Antropol. 2013, 37, 227–229. [Google Scholar] [PubMed]
- Kaoukis, A.; Panagopoulou, V.; Mojibian, H.R.; Jacoby, D. Reverse Takotsubo Cardiomyopathy Associated With the Consumption of an Energy Drink. Circulation 2012, 125, 1584–1585. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Osman, M.; Zafar, S.; Sen, S. Energy Drink Induced Ventricular Fibrillation and Cardiac Arrest: A Successful Outcome. J. Med. Cases 2015, 6, 409–412. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Pennella, S.; Farinetti, A.; Manenti, A. Energy Drinks and atrial fibrillation in young adults. Clin. Nutr. 2018, 37, 1073–1074. [Google Scholar] [CrossRef]
- Nagajothi, N.; Khraisat, A.; Velazquez-Cecena, J.-L.E.; Arora, R.; Raghunathan, K.; Patel, R.; Parajuli, R. Energy Drink-related Supraventricular Tachycardia. Am. J. Med. 2008, 121, e3–e4. [Google Scholar] [CrossRef]
- Osman, H.; Tabatabai, S.; Korashy, M.; Hussein, M. Caffeinated Energy Drink Induced Ventricular Fibrillation: The Price for Overexcitement. Cureus 2019, 11, e6358. [Google Scholar] [CrossRef]
- Peake, S.T.C.; Mehta, P.A.; Dubrey, S.W. Atrial fibrillation-related cardiomyopathy: A case report. J. Med. Case Rep. 2007, 1, 111–112. [Google Scholar] [CrossRef]
- Polat, N. Spontaneous coronary artery dissection in a healthy adolescent following consumption of caffeinated “energy drinks”. Turk Kardiyol. Dern. Ars.-Arch. Turk. Soc. Cardiol. 2013, 41, 738–742. [Google Scholar] [CrossRef]
- Rottlaender, D.; Motloch, L.; Reda, S.; Larbig, R.; Hoppe, U. Cardiac arrest due to long QT syndrome associated with excessive consumption of energy drinks. Int. J. Cardiol. 2012, 158, e51–e52. [Google Scholar] [CrossRef]
- Rutledge, M.; Witthed, A.; Khouzam, R.N. It took a Redbull to unmask Brugada syndrome. Int. J. Cardiol. 2012, 161, e14–e15. [Google Scholar] [CrossRef]
- Sattari, M.; Sattari, A.; Kazory, A. Energy Drink Consumption and Cardiac Complications: A Case for Caution. J. Addict. Med. 2016, 10, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.J.; El-Hassan, M.; Khan, A.A. Myocardial infarction in a young adult following the consumption of a caffeinated energy drink. BMJ Case Rep. 2011, 2011, bcr0220113854. [Google Scholar] [CrossRef] [PubMed]
- Solomin, D.; Borron, S.W.; Watts, S.H. STEMI Associated with Overuse of Energy Drinks. Case Rep. Emerg. Med. 2015, 2015, 537689. [Google Scholar] [CrossRef] [PubMed]
- Ten Bos, L.M.; Veenstra, T.C.; Westerhof, B.D.; Bosch, F.H. A case of extreme hypokalaemia. Neth. J. Med. 2016, 74, 9. [Google Scholar]
- Terlizzi, R.; Rocchi, C.; Serra, M.; Solieri, L.; Cortelli, P. Reversible postural tachycardia syndrome due to inadvertent overuse of Red Bull®. Clin. Auton. Res. 2008, 18, 221–223. [Google Scholar] [CrossRef]
- Torbey, E.; Rafeh, N.A.; Khoueiry, G.; Kowalski, M.; Bekheit, S. Ginseng: A potential cause of long QT. J. Electrocardiol. 2011, 44, 357–358. [Google Scholar] [CrossRef]
- Ullah, M.W.; Lakhani, S.; Siddiq, W.; Handa, A.; Kahlon, Y.; Siddiqui, T. Energy Drinks and Myocardial Infarction. Cureus 2018, 10, e2658. [Google Scholar] [CrossRef]
- Ünal, S.; Şensoy, B.; Yilmaz, S.; Ünal, G.G.; Süleymanoğlu, M.; Şen, F.; Açar, B.; Balcı, M.M. Left main coronary artery thrombosis and acute anterior myocardial infarction related to energy drink. Int. J. Cardiol. 2015, 179, 66–67. [Google Scholar] [CrossRef]
- Usman, A.; Jawaid, A. Hypertension in a young boy: An energy drink effect. BMC Res. Notes 2012, 5, 591. [Google Scholar] [CrossRef] [PubMed]
- Uyanik, M.; Gedikli, O.; Yildirim, U. Energy Drink-Associated Cardiomyopathy after Excessive Consumption: A Case Report. J. Tehran Univ. Heart Cent. 2021, 16, 119–122. [Google Scholar] [CrossRef]
- Ward, A.E.; Lipshultz, S.E.; Fisher, S.D. Energy Drink–Induced Near-Fatal Ventricular Arrhythmia Prevented by an Intracardiac Defibrillator Decades After Operative “Repair” of Tetralogy of Fallot. Am. J. Cardiol. 2014, 114, 1124–1125. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.E.; Kado, H.S.; Samson, R.; Miller, A.B. A Case of Caffeine-Induced Coronary Artery Vasospasm of a 17-Year-Old Male. Cardiovasc. Toxicol. 2012, 12, 175–179. [Google Scholar] [CrossRef]
- Zacher, J.; May, E.; Horlitz, M.; Pingel, S. Binge drinking alcohol with caffeinated “energy drinks”, prolonged emesis and spontaneous coronary artery dissection: A case report, review of the literature and postulation of a pathomechanism. Clin. Res. Cardiol. 2018, 107, 975–979. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Ali, M.; Al-Bast, B.; Bhandari, B.; Kulkarni, A. Energy drink consumption resulting in severe hypertriglyceridemia, hyperglycemia, and acute pancreatitis. J. Am. Coll. Cardiol. 2019, 73, 2752. [Google Scholar] [CrossRef]
- Al Yacoub, R.; Luczkiewicz, D.; Kerr, C. Acute kidney injury and hepatitis associated with energy drink consumption: A case report. J. Med. Case Rep. 2020, 14, 23. [Google Scholar] [CrossRef]
- Al-Tamimi, A.; Kassim, T.; Chandra, S. Recurrent Acute Pancreatitis With Energy Drinks Consumption: Rare but Definite Association: 1442. Am. J. Gastroenterol. 2018, 113, S828. [Google Scholar] [CrossRef]
- Apestegui, C.A.; Julliard, O.; Ciccarelli, O.; Duc, D.K.; Lerut, J. Energy drinks: Another red flag for the liver allograft. Liver Transpl. 2011, 17, 1117–1118. [Google Scholar] [CrossRef]
- Garg, A.; Rodriguez, A.; Lewis, J.T.; Bansal, R.; Brahmbhatt, B. Energy Drinks: A Reversible Risk Factor for Atrophic Gastritis and Gastric Intestinal Metaplasia. Cureus 2020, 12, e12298. [Google Scholar] [CrossRef]
- Harb, J.N.; Taylor, Z.A.; Khullar, V.; Sattari, M. Rare cause of acute hepatitis: A common energy drink. BMJ Case Rep. 2016, 2016, bcr2016216612. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Kunkel, D.; El Kabany, M. Acute Liver Failure Following One Year of Daily Consumption of a Sugar-Free Energy Drink. ACG Case Rep. J. 2014, 1, 214–216. [Google Scholar] [CrossRef]
- Randhawa, N.; Shah, M.; Spyratos, T. The Pain of Staying Alert: A Case Report and Literature Review on Energy Drink–Induced Acute Pancreatitis. J. Investig. Med. High Impact Case Rep. 2022, 10, 23247096221104468. [Google Scholar] [CrossRef] [PubMed]
- Shmelev, A.; Abdo, A.; Sachdev, S.; Shah, U.; Kowdley, G.C.; Cunningham, S.C. Energetic etiologies of acute pancreatitis: A report of five case. World J. Gastrointest Pathophysiol. 2015, 6, 243–248. [Google Scholar] [CrossRef]
- Takahashi, K.; Tsukamoto, S.; Kakizaki, Y.; Saito, K.; Ohkohchi, N.; Hirayama, K. Hypercobalaminemia Induced by an Energy Drink after Total Gastrectomy: A Case Report. J. Rural Med. 2013, 8, 181–185. [Google Scholar] [CrossRef]
- Uwaifo, G.I. Beware Energy Drinks: A Case of a Toxic Triad Syndrome in a Diabetic Patient With Nonalcoholic Fatty Liver Disease. Am. J. Med. Sci. 2019, 358, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandarajah, A.; Ni, S.; Waked, A. Acute hepatitis in a woman following excessive ingestion of an energy drink: A case report. J. Med. Case Rep. 2011, 5, 227. [Google Scholar] [CrossRef]
- Casas-Gomez, C.; Muñoz-Molero, M.J.; Guerrero-Sánchez, R.; Martínez-León, F. Mania and energy drinks. Actas Esp. Psiquiatr. 2018, 46, 156–158. [Google Scholar]
- Calabrò, R.S.; Italiano, D.; Gervasi, G.; Bramanti, P. Single tonic–clonic seizure after energy drink abuse. Epilepsy Behav. 2012, 23, 384–385. [Google Scholar] [CrossRef]
- Cerimele, J.M.; Stern, A.P.; Jutras-Aswad, D. Psychosis Following Excessive Ingestion of Energy Drinks in a Patient With Schizophrenia. Am. J. Psychiatry 2010, 167, 353-353. [Google Scholar] [CrossRef] [PubMed]
- Cruzado, L.; Sánchez-Fernández, M.; Cortez-Vergara, C.; Rojas-Rojas, G. Mania induced by high content caffeinated energy drinks. Actas Esp. Psiquiatr. 2014, 42, 259–262. [Google Scholar]
- Görgülü, Y.; Taşdelen, Ö.; Sönmez, M.B.; Köse Çinar, R. A Case of Acute Psychosis Following Energy Drink Consumption. Noro. Psikiyatr. Ars. 2014, 51, 79–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grant, R.A.; Cord, B.J.; Kuzomunhu, L.; Sheth, K.; Gilmore, E.; Matouk, C.C. Aneurysmal subarachnoid hemorrhage and severe, catheter-induced vasospasm associated with excessive consumption of a caffeinated energy drink. Interv. Neuroradiol. 2016, 22, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Padidam, S.; Tewari, A. Acute macular neuroretinopathy (AMN) related to energy drink consumption. BMJ Case Rep. 2019, 12, e232144. [Google Scholar] [CrossRef]
- Hernandez-Huerta, D.; Martin-Larregola, M.; Gomez-Arnau, J.; Correas-Lauffer, J.; Dolengevich-Segal, H. Psychopathology Related to Energy Drinks: A Psychosis Case Report. Case Rep. Psychiatry 2017, 2017, 5094608. [Google Scholar] [CrossRef]
- Iyadurai, S.J.P.; Chung, S.S. New-onset seizures in adults: Possible association with consumption of popular energy drinks. Epilepsy Behav. 2007, 10, 504–508. [Google Scholar] [CrossRef]
- Kelsey, D.; Berry, A.J.; Swain, R.A.; Lorenz, S. A Case of Psychosis and Renal Failure Associated with Excessive Energy Drink Consumption. Case Rep. Psychiatry 2019, 2019, 3954161. [Google Scholar] [CrossRef]
- Butragueño Laiseca, L.; Toledo del Castillo, B.; Miranda Herrero, M.C. Bebidas enérgicas como desencadenante de crisis convulsivas en pacientes pediátricos: A propósito de un caso. Neurología 2019, 34, 343–345. [Google Scholar] [CrossRef]
- Menkes, D.B. Transient Psychotic Relapse Temporally Related to Ingestion of an “Energy Drink”. Med. J. Aust. 2011, 194, 206. [Google Scholar] [CrossRef]
- Norelli, L.J.; Xu, C. Manic Psychosis Associated With Ginseng: A Report of Two Cases and Discussion of the Literature. J. Diet. Suppl. 2014, 12, 119–125. [Google Scholar] [CrossRef]
- Pagano, C.W.; Wu, M.; Wu, L. Acute visual loss and intraretinal hemorrhages associated to energy drink consumption. Int. Ophthalmol. 2017, 37, 1349–1351. [Google Scholar] [CrossRef]
- Sharma, V. Red Bull and Mania. Ger. J. Psychiatry 2010, 13, 178–180. [Google Scholar]
- Staikoglou, N.; Polanagnostaki, A.; Lamprou, V.; Chartampilas, E.; Pavlou, E.; Tegos, T.; Finitsis, S. Posterior cerebral artery dissection after excessive caffeine consumption in a teenager. Radiol. Case Rep. 2022, 17, 2081–2084. [Google Scholar] [CrossRef]
- Trabulo, D.; Marques, S.; Pedroso, E. Caffeinated energy drink intoxication. BMJ Case Rep. 2011, 2011, bcr0920103322. [Google Scholar] [CrossRef]
- Yartsev, A.; Peisah, C. Caffeine-clozapine interaction associated with severe toxicity and multiorgan system failure: A case report. BMC Psychiatry 2021, 21, 192. [Google Scholar] [CrossRef]
- Greene, E.; Oman, K.; Lefler, K. Energy Drink–Induced Acute Kidney Injury. Ann. Pharmacother. 2014, 48, 1366–1370. [Google Scholar] [CrossRef]
- Icin, T.; Medic-Stojanoska, M.; Ilic, T.; Kuzmanovic, V.; Vukovic, B.; Percic, I.; Kovacev-Zavisic, B. Multiple Causes of Hyponatremia: A Case Report. Med. Princ. Pract. 2017, 26, 292–295. [Google Scholar] [CrossRef]
- Iyer, P.S.; Rishitha Yelisetti, R.; Miriyala, V.; Siddiqui, W.; Kaji, A. Remarkable Case of Rhabdomyolysis Associated with Ingestion of Energy Drink ‘Neon Volt’. J. Community Hosp. Intern. Med. Perspect. 2016, 6, 32528. [Google Scholar] [CrossRef]
- Schöffl, I.; Kothmann, J.F.; Schöffl, V.; Rupprecht, H.D.; Rupprecht, T. “Vodka Energy”: Too Much for the Adolescent Nephron? Pediatrics 2011, 128, e227–e231. [Google Scholar] [CrossRef]
- Tinawi, M. Severe Rhabdomyolysis Due to Strenuous Exercise With a Potential Role of a High-Caffeine Energy Drink. Cureus 2022, 14, e20867. [Google Scholar] [CrossRef]
- West, N.J.; Thorpe, M. Neonatal hyperinsulinism secondary to maternal intake of high-sugar drinks. BMJ Case Rep. 2011, 2011, bcr0320113990. [Google Scholar] [CrossRef]
- Zekavat, O.R.; Fathpour, G.; Haghpanah, S.; Dehghani, S.J.; Zekavat, M.; Shakibazad, N. Acquired Vitamin K Deficiency as Unusual Cause of Bleeding Tendency in Adults: A Case Report of a Nonhospitalized Student Presenting with Severe Menorrhagia. Case Rep. Obstet. Gynecol. 2017, 2017, 4239148. [Google Scholar] [CrossRef]
- Gerqari, A.M.B.; Ferizi, M.; Halimi, S.; Daka, A.; Hapciu, S.; Begolli, I.M.; Begolli, M.; Gerqari, I.H. Erythema exsudativum multiforme induced by a taurine-containing energy drink. Acta Dermatovenerol. Alp. Pannonica Adriat. 2016, 25, 83–84. [Google Scholar] [CrossRef]
- Seung-Eun, L.; Suh-Young Eun-Jung, J.; Mi-Young, K.; Min-Suk, Y.; Yoon-Seok, C.; Sae-Hoon, K. A Case of Taurine-Containing Drink Induced anaphylaxis. Asia Pac. Aller. 2013, 3, 70. [Google Scholar]
- Wolk, B.J.; Ganetsky, M.; Babu, K.M. Toxicity of energy drinks. Curr. Opin. Pediatr. 2012, 24, 243–251. [Google Scholar] [CrossRef]
- Gutiérrez-Hellín, J.; Varillas-Delgado, D. Energy Drinks and Sports Performance, Cardiovascular Risk, and Genetic Associations; Future Prospects. Nutrients 2021, 13, 715. [Google Scholar] [CrossRef]
- Schüttler, D.; Rudi, W.-S.; Bauer, A.; Hamm, W.; Brunner, S. Impact of energy drink versus coffee consumption on periodic repolarization dynamics: An interventional study. Eur. J. Nutr. 2022, 61, 2847–2851. [Google Scholar] [CrossRef]
- Lévy, S.; Santini, L.; Capucci, A.; Oto, A.; Santomauro, M.; Riganti, C.; Raviele, A.; Cappato, R. European Cardiac Arrhythmia Society Statement on the cardiovascular events associated with the use or abuse of energy drinks. J. Interv. Card. Electrophysiol. 2019, 56, 99–115. [Google Scholar] [CrossRef]
- Ehlers, A.; Marakis, G.; Lampen, A.; Hirsch-Ernst, K.I. Risk assessment of energy drinks with focus on cardiovascular parameters and energy drink consumption in Europe. Food Chem. Toxicol. 2019, 130, 109–121. [Google Scholar] [CrossRef]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberger, J.; Lieberman, H.R.; O’Brien, C.; Peck, J.; et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem. Toxicol. 2017, 109 Pt 1, 585–648. [Google Scholar] [CrossRef] [PubMed]
- Mangi, M.A.; Rehman, H.; Rafique, M.; Illovsky, M. Energy Drinks and the Risk of Cardiovascular Disease: A Review of Current Literature. Cureus 2017, 9, e1322. [Google Scholar] [CrossRef]
- Protano, C.; Valeriani, F.; De Giorgi, A.; Marotta, D.; Ubaldi, F.; Napoli, C.; Liguori, G.; Spica, V.R.; Vitali, M.; Gallè, F. Consumption patterns of energy drinks in university students: A systematic review and meta-analysis. Nutrition 2023, 107, 111904. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, S.; Piacentino, D.; Fineschi, V.; Frati, P.; Cipolloni, L.; Aromatario, M. Caffeine-Related Deaths: Manner of Deaths and Categories at Risk. Nutrients 2018, 10, 611. [Google Scholar] [CrossRef] [PubMed]
- Frost, L.; Vestergaard, P.; Mosekilde, L. Hyperthyroidism and risk of atrial fibrillation or flutter: A population-based study. ACC Curr. J. Rev. 2004, 13, 13. [Google Scholar] [CrossRef]
- Lorist, M.M.; Tops, M. Caffeine, fatigue, and cognition. Brain Cogn. 2003, 53, 82–94. [Google Scholar] [CrossRef]
- Morgan, J.C.; Sethi, K.D. Drug-induced tremors. Lancet Neurol. 2005, 4, 866–876. [Google Scholar] [CrossRef]
- Epilepsy Foundation. Energy Drinks May Increase the Risk of Seizures According to a New Study. Available online: https://www.epilepsy.com/stories/energy-drinks-may-increase-risk-seizures-according-new-study (accessed on 17 April 2023).
- Rossi, S.; De Chiara, V.; Musella, A.; Mataluni, G.; Sacchetti, L.; Siracusano, A.; Bernardi, G.; Usiello, A.; Centonze, D. Effects of caffeine on striatal neurotransmission: Focus on cannabinoid CB1 receptors. Mol. Nutr. Food Res. 2010, 54, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Herrick, J.; Shecterle, L.M.; St Cyr, J.A. D-ribose--an additive with caffeine. Med. Hypotheses. 2009, 72, 499–500. [Google Scholar] [CrossRef]
- Persad, L.A.B. Energy Drinks and the Neurophysiological Impact of Caffeine. Front. Neurosci. 2011, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.; Smith, A.P.; Distelberg, B.J.; Staack, A.; Elsen, K.D.; Sabaté, J. A Review of Energy Drinks and Mental Health, with a Focus on Stress, Anxiety, and Depression. J. Caffeine Res. 2016, 6, 49–63. [Google Scholar] [CrossRef]
- Rizkallah, G.S.; Mertens, M.K.; Brown, M.L. Should liver enzymes be checked in a patient taking niacin? J. Fam. Pract. 2005, 54, 265–282. [Google Scholar]
- Huang, K.-H.; Chang, C.-C.; Ho, J.-D.; Lu, R.-H.; Tsai, L.H. Role of taurine on acid secretion in the rat stomach. J. Biomed. Sci. 2011, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.-J.; Li, Y.; Zhou, Z.-G. Anaphylactic shock and lethal anaphylaxis caused by compound amino acid solution, a nutritional treatment widely used in China. Amino Acids 2012, 42, 2501–2505. [Google Scholar] [CrossRef]
- Salih, N.A.; Abdul-Sadaand, I.H.; Abdulrahman, N.R. Histopathological effect of energy drinks (Red Bull) on Brain, Liver, Kidney, and Heart in Rabbits. Med. J. Babylon 2018, 15, 16. [Google Scholar] [CrossRef]
- Nieradko-Iwanicka, B.; Pietraszek, D.; Pośnik, K. Prolonged consumption of energy drinks does not affect the processes of memory, and increases the activity of transaminases, and cholesterol concentration—Animal model study results. Fam. Med. Prim. Care Rev. 2016, 3, 313–316. [Google Scholar] [CrossRef]
- Sadowska, J. Evaluation of the effect of consuming an energy drink on the concentration of glucose and triacylglycerols and on fatty tissue deposition. A model study. Acta Sci. Pol. Technol. Aliment. 2012, 11, 311–318. [Google Scholar]
- Rasheed, A.; Aslam, I.; Tafweez, R.; Kiani, M.R.B.; Ameer, M.K.; Saeed, A.A. Histological consequences of consumption of energy drink on renal tubules. Pak. Armed Forces Med. J. 2021, 71, 1736–1740. [Google Scholar] [CrossRef]
- Abonar, M.; Aboraya, A.; Elbakary, N.; Elwan, W. Effect of Energy Drink on the Pancreas of Adult Male Albino Rat and the Possible Protective Role of Avocado Oil. Histological and Immunohistochemical Study. Egypt. J. Histol. 2021, 42, 386–403. [Google Scholar] [CrossRef]
- Rehman, F.; Islam, Z.U.; Haq, S.U.; Faisal, L.; Rehman, S.; Kumar, S. Morphometric Study on the Energy Drink Induced Pancreatitis on Wistar Albino Rats. J. Dow Univ. Health Sci. 2020, 14, 102–106. [Google Scholar] [CrossRef]
- Haroun, H.; Mohamed, E.; El Shahat, A.E.R.; Labib, H.; Atef, M. Adverse effects of energy drink on rat pancreas and the therapeutic role of each of bone marrow mesenchymal stem cells and Nigella Sativa oil. Folia Morphol. 2020, 79, 272–279. [Google Scholar] [CrossRef]
- Kassab, A.A.; Tawfik, S. Effect of a caffeinated energy drink and its withdrawal on the submandibular salivary gland of adult male albino rats: A histological and immunohistochemical study. Egypt. J. Histol. 2018, 41, 11–26. [Google Scholar] [CrossRef]
- Ellermann, C.; Hakenes, T.; Wolfes, J.; Wegner, F.K.; Willy, K.; Leitz, P.; Rath, B.; Eckardt, L.; Frommeyer, G. Cardiovascular risk of energy drinks: Caffeine and taurine facilitate ventricular arrhythmias in a sensitive whole-heart model. J. Cardiovasc. Electrophysiol. 2022, 33, 1290–1297. [Google Scholar] [CrossRef]
- Demirel, A.; Başgöze, S.; Çakıllı, K.; Aydın, U.; Şentürk, G.E.; Diker, V.O.; Ertürk, M. Histopathological Changes in the Myocardium Caused by Energy Drinks and Alcohol in the Mid-term and Their Effects on Skeletal Muscle Following Ischemia-reperfusion in a Rat Model. Anatol. J. Cardiol. 2023, 27, 12–18. [Google Scholar] [CrossRef]
- Díaz, A.; Treviño, S.; Guevara, J.; Muñoz-Arenas, G.; Brambila, E.; Espinosa, B.; Moreno-Rodríguez, A.; Lopez-Lopez, G.; Peña-Rosas, U.; Venegas, B.; et al. Energy Drink Administration in Combination with Alcohol Causes an Inflammatory Response and Oxidative Stress in the Hippocampus and Temporal Cortex of Rats. Oxidative Med. Cell. Longev. 2016, 2016, 8725354. [Google Scholar] [CrossRef]
- Ulenius, L.; Adermark, L.; Söderpalm, B.; Ericson, M. Energy drink constituents (caffeine and taurine) selectively potentiate ethanol-induced locomotion in mice. Pharmacol. Biochem. Behav. 2019, 187, 172795. [Google Scholar] [CrossRef]
- Ugwuja, E. Biochemical Effects of Energy Drinks Alone or in Combination with Alcohol in Normal Albino Rats. Adv. Pharm. Bull. 2014, 4, 69–74. [Google Scholar] [CrossRef]
- Krahe, T.E.; Filgueiras, C.C.; Quaresma, R.d.S.; Schibuola, H.G.; Abreu-Villaça, Y.; Manhães, A.C.; Ribeiro-Carvalho, A. Energy drink enhances the behavioral effects of alcohol in adolescent mice. Neurosci. Lett. 2017, 651, 102–108. [Google Scholar] [CrossRef]
- Reis, R.; Charehsaz, M.; Sipahi, H.; Ekici, A.I.; Macit, Ç.; Akkaya, H.; Aydın, A. Energy Drink Induced Lipid Peroxidation and Oxidative Damage in Rat Liver and Brain When Used Alone or Combined with Alcohol. J. Food Sci. 2017, 82, 1037–1043. [Google Scholar] [CrossRef]
- Nasi, M.; De Gaetano, A.; Carnevale, G.; Bertoni, L.; Selleri, V.; Zanini, G.; Pisciotta, A.; Caramaschi, S.; Reggiani Bonetti, L.; Farinetti, A.; et al. Effects of Energy Drink Acute Consumption in Gastrointestinal Tract of Rats. Nutrients 2022, 14, 1928. [Google Scholar] [CrossRef]
- Elçi, E.; Elçi, G.G.; Çim, N.; Aras, I.; Sayan, S.; Yıldızhan, R. Energy drinks may affect the ovarian reserve and serum anti-mullerian hormone levels in a rat model. J. Turk. Soc. Obstet. Gynecol. 2021, 18, 23–29. [Google Scholar] [CrossRef]
- Oyelowo, T.O.; Awosika, O.I.; Adesina, T.H. Comparative assessment of artificial and natural energy drinks in the epididymal and testicular milieu. Stud. Univ. Babeş-Bolyai Biol. 2022, 67, 15–33. [Google Scholar] [CrossRef]
- Al-Basher, G.I.; Aljabal, H.; Almeer, R.S.; Allam, A.A.; Mahmoud, A.M. Perinatal exposure to energy drink induces oxidative damage in the liver, kidney and brain, and behavioral alterations in mice offspring. BioMedicine 2018, 102, 798–811. [Google Scholar] [CrossRef]
- Posokhov, Y.O.; Tkachenko, A.S.; Nakonechna, O.A.; Onishchenko, A.I.; Korniyenko, Y.M.; Tkachenko, M.O. Experimental study of erythrocyte membranes in rats orally exposed to caffeinated energy drinks by fluorescent probe technique. Stud. Univ. Vasile Goldiş 2019, 29, 129–133. [Google Scholar]
- Turillazzi, E.; Di Paolo, M.; Neri, M.; Riezzo, I.; Fineschi, V. A theoretical timeline for myocardial infarction: Immunohistochemical evaluation and western blot quantification for Interleukin-15 and Monocyte chemotactic protein-1 as very early markers. J. Transl. Med. 2014, 12, 188. [Google Scholar] [CrossRef]
- Aguilar, F.; Charrondiere, U.R.; Dusemund, B.; Galtier, P.; Gilbert, J.; Gott, D.M.; Grilli, S.; Guertler, R.; Kass, G.E.N.; Koenig, J.; et al. Scientific Opinion of the Panel on Food Additives and Nutrient Sources added to Food. The use of taurine and D-glucurono-gamma-lactone as constituents of the so-called “energy” drinks. EFSA J. 2009, 935, 1–31. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on Dietary Reference Values for vitamin A. EFSA J. 2015, 13, 4028. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration Center for Food Safety and Applied Nutrition. Highly Concentrated Caffeine in Dietary Supplements: Guidance for Industry; FDA: Silver Spring, MD, USA, 2018.
- Maiese, A.; La Russa, R.; Del Fante, Z.; Turillazzi, E.; David, M.C.; Frati, P.; Fineschi, V. Massive Œ ≤ 1-Adrenergic Receptor Reaction Explains Irreversible Acute Arrhythmia in a Fatal Case of Acute Pure Caffeine Intoxication. Cardiovasc. Toxicol. 2021, 21, 88–92. [Google Scholar] [CrossRef]


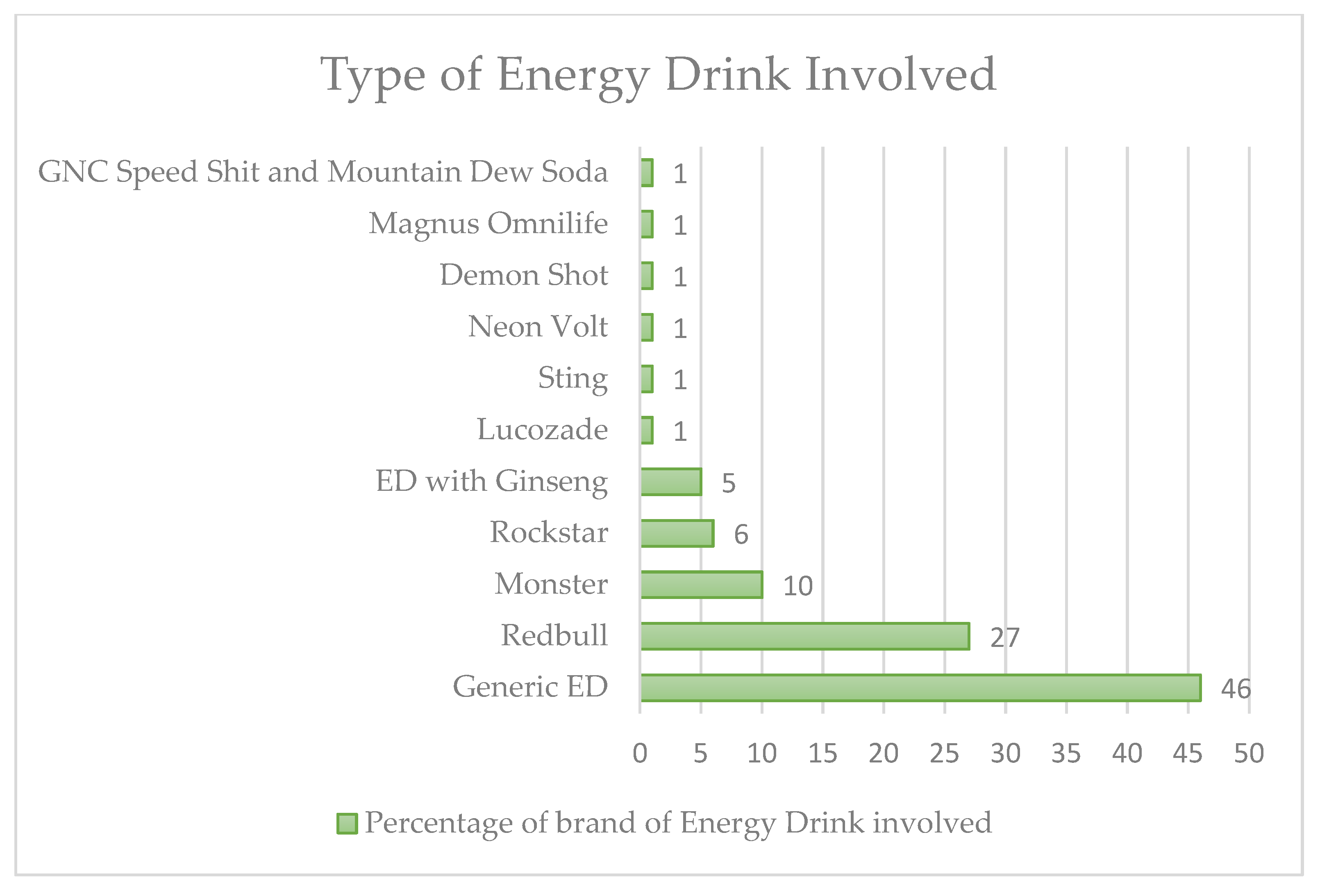
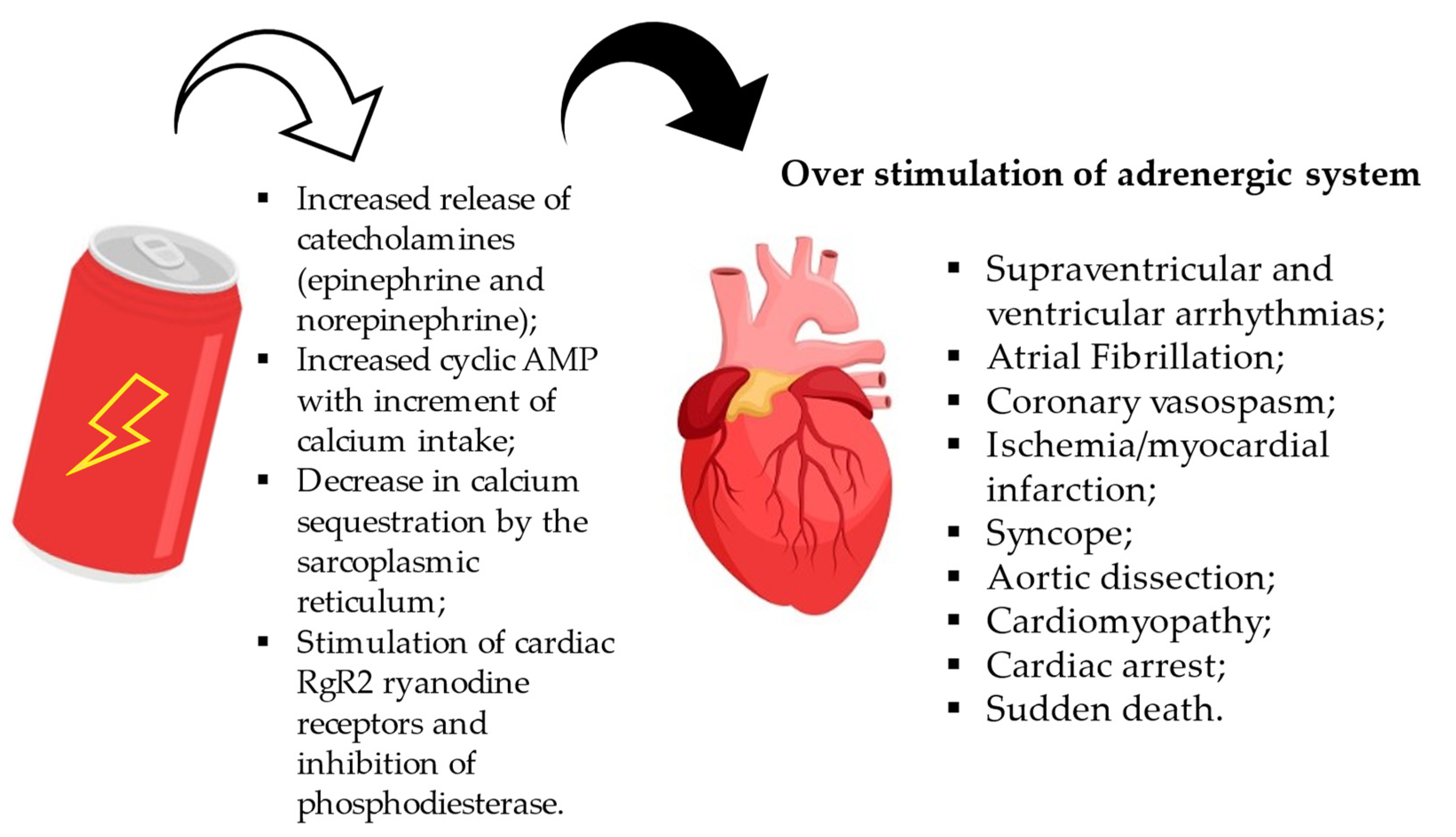
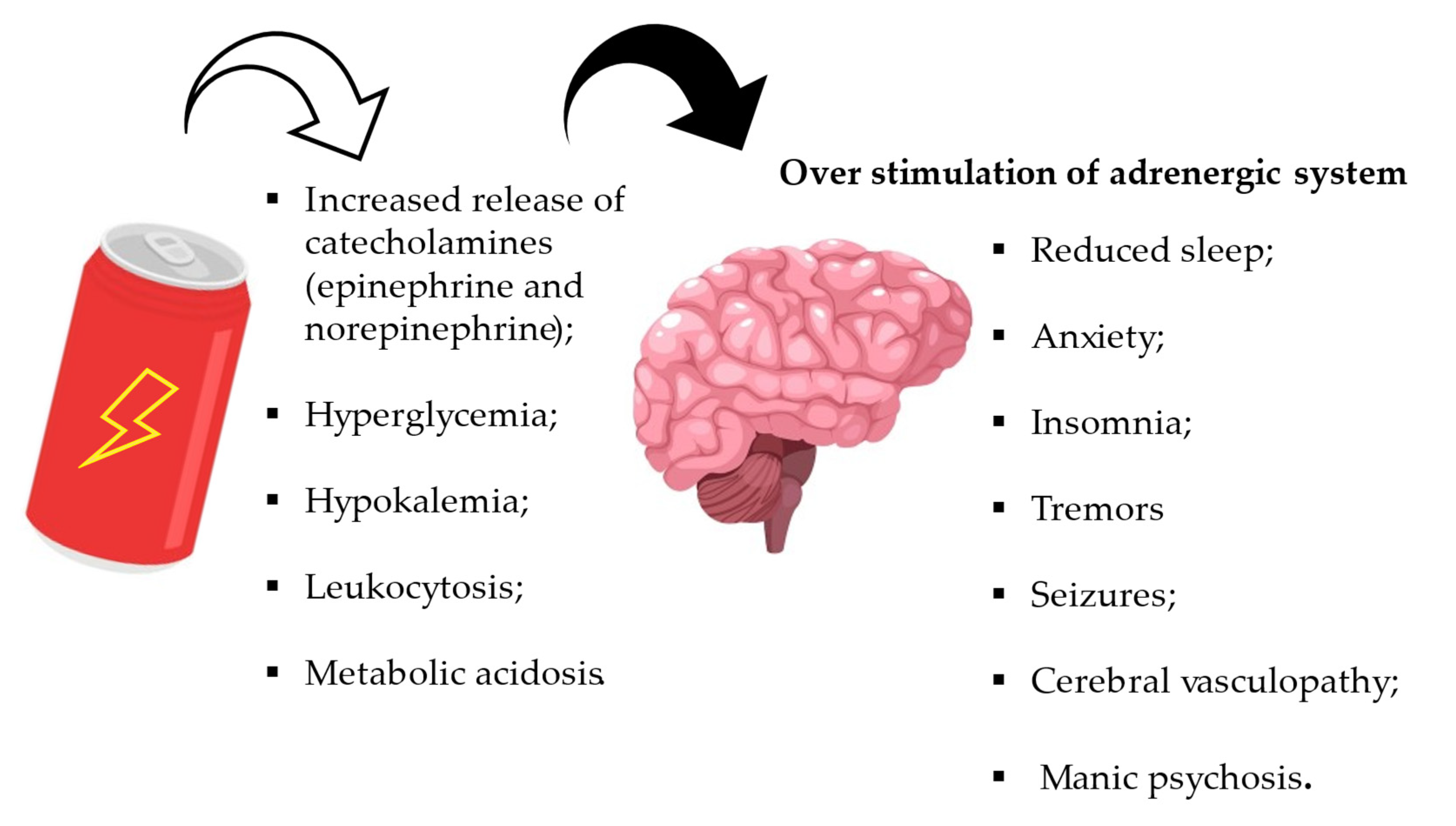
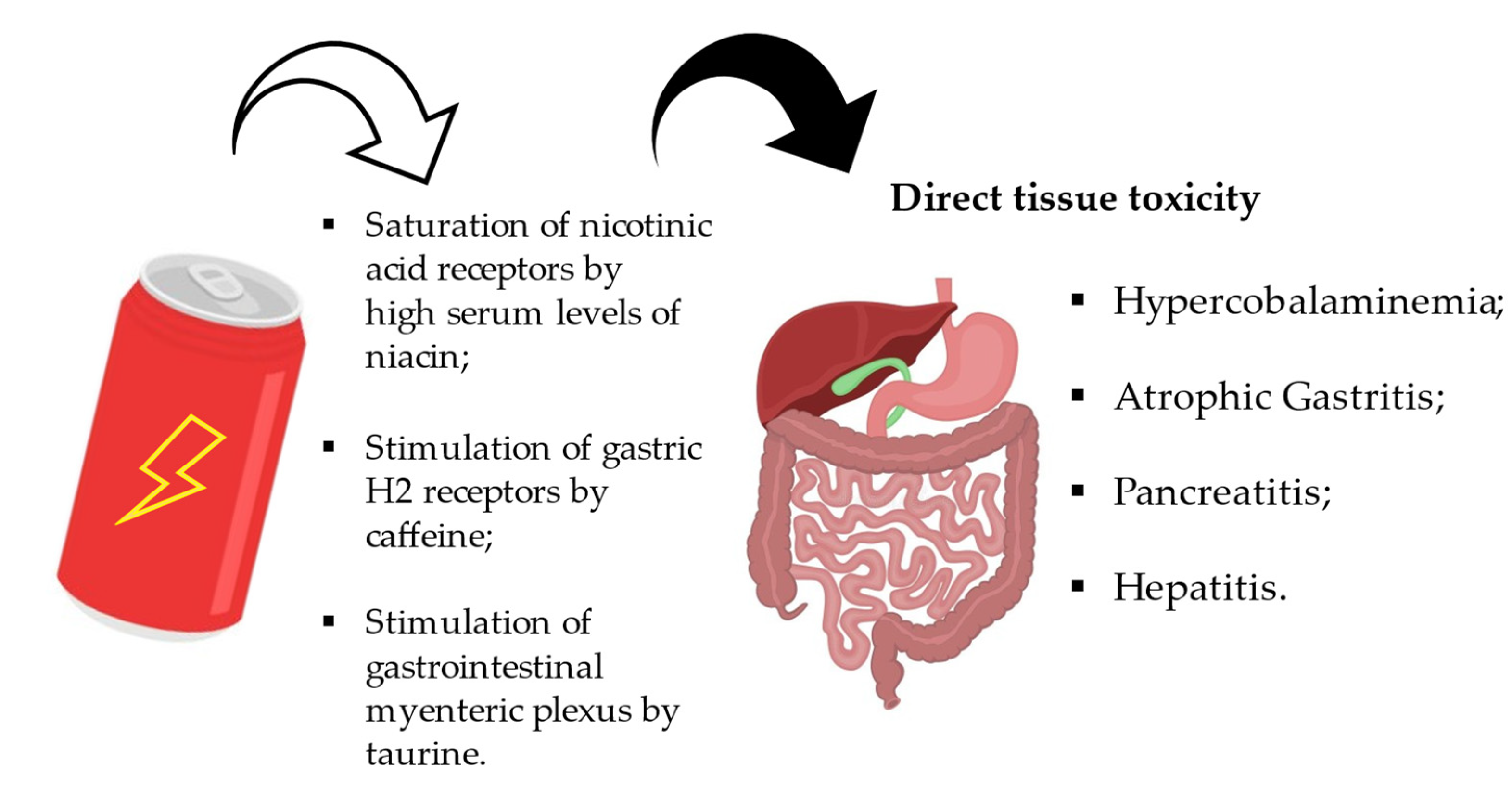
| References | N° Cases | Age/Sex | Energy Drink (If Applicable) or Substances | Major Pathology | Onset | Adverse Event |
|---|---|---|---|---|---|---|
| Argano et al., 2011 [27] | 1 | 38/M (Male) | Red Bull | N/A [not available] | Consumption of 5 cans of Red Bull per day 4 days before the onset of symptoms | Haemorrhagic aneurysm of the anterior communicating artery |
| Avci et al., 2013 [28] | 1 | 28/M | ED [generic energy drink] | N/A | Consumption of 3 cans of 250 mL energy drink 5 h before a basketball game. Taking one ED a day for 7 months | Death from sudden cardiac arrest. |
| Berger et Alford, 2009 [29] | 1 | 28/M | ED | N/A | Ingestion of 7–8 cans of a caffeinated “energy drink” between 8 am and his collapse 7 h later | Resuscitated cardiac arrest with anteroseptal ST Elevation Myocardial Infarction (STEMI). |
| Benjo et al., 2012 [30] | 1 | 24/M | ED + vodka | N/A | Symptoms started 1 or 2 h after drinking vodka mixed with an energy drink. | Left main coronary artery acute thrombosis. |
| Cannon et al., 2001 [31] | 1 | 25/F (Female) | Race 2005 Energy Blast with Guarana and Ginseng | Mitral valve prolapses | Consumption of a 55 mL shot of “Race 2005 Energy Blast with Guarana and Ginseng” before onset. | Death after ventricular fibrillation. |
| Choudhury et al., 2017 [32] | 1 | 53/M | Red Bull | N/A | Ingestion of 2 cans of Red Bull shortly before the test, thinking it would improve his performance on the treadmill. | Partial right bundle branch block, hypertension. |
| Di Rocco et al., 2016 [33] | 2 | 14/M | Red Bull + caffeinated drink | N/A | Intake of an unknown amount of a highly caffeinated drink the day before a race. He also reported drinking a Red Bull™ energy drink five days prior to enrolment. | Arrhythmia |
| 16/M | Red Bull + vodka | Attention deficit hyperactivity disorder, asthma, and allergies | Ingestion of an unknown quantity of Red Bull™ mixed with vodka at a party. | Atrial tachycardia/atrial Fibrillation (AF) | ||
| Dufendach et al., 2012 [34] | 1 | 13/F | ED | Idiopathic QT prolongation | Consumption of at least one 16 oz (476 mL) can of an energy drink (160 mg caffeine) before onset. | Palpitations and chest pain, associated with a QTc |
| Enriquez et Frankel, 2017 [35] | 2 | 19/F | Monster ED | N/A | Consumption of three 8 fl oz (263 mL) cans of Monster ED within 2 h of onset. | Resuscitated cardiac arrest. |
| 23/F | Red Bull | Peripartum cardiomyopathy, subcutaneous ICD (Implantable Cardioverter Defibrillator), left ventricular ejection (20%) | Ingestion of a can of Red Bull for the first time in her life | Syncope | ||
| Gharacholou et al., 2017 [36] | 1 | 27/M | Rockstar energy | Family history of coronary artery disease. | Ingestion of 4–5 beverages in 12-h. | Acute anterior ST-segment elevation myocardial infarction (STEMI) |
| Hanif et al., 2020 [37] | 1 | 22/M | ED | N/A | Consumption of two energy drinks (111 mg of caffeine and taurine) before onset | Atrial fibrillation |
| Israelit et al., 2012 [38] | 1 | 24/M | ED | Obesity, hypertension. | Ingestion about 20 cans of energy drink (XL) over the previous night | Death after ST elevation myocardial infarction (STEMI) |
| Jonjev et Bala, 2013 [39] | 3 | 54/M | Red Bull | Obesity, hypertension | Consumption of 4–5 high-energy drinks (mostly Red Bull) per night. | Subacute aortic dissection (De Bakey type I) |
| 26/M | ED | Bicuspid aortic valve, dilation of the ascending aorta | Consumption of 5–6 high-energy drinks per night just after a party | Acute aortic dissection (De Bakey type II) | ||
| 48/M | ED | Hypertension, myocardial infarction. | Consumption of high energy drinks before onset. | Acute aortic dissection (De Bakey type I) | ||
| Kaoukis et al., 2012 [40] | 1 | 24/M | ED | N/A | Ingestion of a small amount of an energy drink in little cups one after another. | Reverse Takotsubo Cardiomyopathy |
| Khan et al., 2015 [41] | 1 | 27/M | Red Bull | PVCs (Premature ventricular contractions) | Ingestion of 6 red bull energy drinks per day for over 6–8 months. | Resuscitated cardiac arrest with ventricular fibrillation |
| Mattioli et al., 2018 [42] | 3 | 22/M | ED | N/A | Consumption of 750 mL of ED | Atrial fibrillation (AF) |
| 23/M | ED | N/A | Consumption of 600 mL of ED | Atrial fibrillation (AF) | ||
| 26/M | ED + alcohol | N/A | Consumption of an alcoholic beverage, corresponding to 30 g of alcohol, associated with 600 mL of EDs | Atrial fibrillation (AF) | ||
| Nagajothi et al., 2008 [43] | 1 | 23/F | GNC Speed Shot and Mountain Dew soda drink | N/A | Ingestion of one can of GNC and one of Mountain Dew (containing caffeine) before onset | Supraventricular tachycardia |
| Osman et al., 2019 [44] | 1 | 32/M | ED | N/A | Consumption of 48 cans of 250 mL ED (XXL, containing a mixture of caffeine, vodka, and taurine) over the past three days. | Ventricular fibrillation |
| Peake et al., 2007 [45] | 1 | 58/m | ED | N/A | Ingestion of one bottle (1000 mL) per week of a highly caffeinated (caffeine content 4.04 mg/mL) commercially available energy drink for 6 months | Atrial fibrillation-related cardiomyopathy |
| Polat et al., 2013 [46] | 1 | 13/M | ED | N/A | Consumption of an energy drink for the first time the night before. About 8 h after consuming the energy drink, the patient’s chest pain started. | Spontaneous coronary artery dissection (SCAD) |
| Roettlaender et al., 2012 [47] | 1 | 22/F | ED | N/A | Consumption of six cans of a caffeinated energy drink within 4 h | Resuscitated cardiac arrest due to long QT syndrome. |
| Rutledge et al., 2012 [48] | 1 | 24/M | Red Bull + vodka | Unknown Brugada Syndrome | Consumption of a Red Bull energy drink (80 mg caffeine and 1000 mg taurine) combined with vodka. | Resuscitated cardiac arrest. |
| Sattari et al., 2016 [49] | 1 | 28/M | Monster ED | N/A | Ingestion of two Monster ED, two to three beers, and chewing tobacco for several months. | AF |
| Scott et al., 2011 [50] | 1 | 19/M | Red Bull | Gastro-oesophageal reflux disease | Ingestion of around two to three cans of ‘Red Bull’ daily | STEMI |
| Solomin et al., 2015 [51] | 1 | 26/M | Monster ED + Rockstar energy | N/A | Consumption of any kind of energy drink: eight to ten 473 mL drinks per day | STEMI |
| Ten Bos et al., 2016 [52] | 1 | 45/F | Red Bull | N/A | High consumption of Red Bull energy drinks. | Myocardial ischaemia |
| Terlizzi et al., 2008 [53] | 1 | 16/F | Red Bull | N/A | Consumption of four to five cans before onset. | Reversible postural tachycardia syndrome |
| Torbey et al., 2011 [54] | 1 | 43/F | Panax ginseng + ED | N/A | Consumption of at least 70 cc of caffeine and 4 L of Korean Panax ginseng daily for six months. | Long QT syndrome. |
| Ullah et al., 2018 [55] | 1 | 25/M | ED | N/A | Drinking large amounts of caffeinated ED every day for the past week. | Acute coronary syndrome |
| Unal et al., 2014 [56] | 1 | 32/M | ED | N/A | Consumption of 5 bottles of energy drink before onset. | Left main coronary artery thrombosis and acute anterior myocardial infarction |
| Usman et Jawaid, 2012 [57] | 1 | 16/M | “Sting” energy drink | N/A | Consumption of about 80–100 cans in the past 2 weeks, an average of 3 cans per day. | Hypertension. |
| Uyanik et al., 2021 [58] | 1 | 24/M | ED | N/A | Ingestion of 8 to 10 cans of energy drinks per day (3.5–4 L/day) in the past 2 weeks | Cardiomyopathy |
| Ward et al., 2014 [59] | 1 | 45/M | Red Bull | Tetralogy of Fallot | Consumption of 3 Red Bull energy drinks before onset. | Resuscitated cardiac arrest with ventricular tachycardia. |
| Wilson et al., 2012 [60] | 1 | 17/M | Red Bull + Monster | Myopericarditis | Ingestion of caffeinated energy drinks (3–4 Red Bull 80 mg of caffeine/can and 2–3 Monster 160 mg of caffeine/can) before onset. | Coronary artery vasospasm |
| Zacher et al., 2018 [61] | 1 | 25/M | ED + alcohol | N/A | Ingestion of 8 cans, mixed with strong liquor, before onset. | Spontaneous coronary artery dissection |
| References | N° Cases | Age/Sex | Energy Drink (If Applicable) or Substances | Major Pathologies | Onset | Adverse Event |
|---|---|---|---|---|---|---|
| Abdisamad et al., 2019 [62] | 1 | 46/M | ED | Diabetes mellitus | Daily consumption of 3–4 16 oz cans for the 2 weeks before onset. | Acute pancreatitis, hepatomegaly, hypertriglyceridemia. |
| Al Yacoub et al., 2020 [63] | 1 | 62/F | Sugar-free ED | Small-cell left lung carcinoma. | Ingestion of five to six cans of a 16 fluid oz sugar-free ED daily. | Acute hepatitis. |
| Al-Tamini et al., 2018 [64] | 1 | 19/M | Full Throttle + Red Bull | Serine Protease Inhibitor Kazal-type I (SPINK1) gene mutation. | Regular consumption of Full Throttle and Red Bull. | Acute pancreatitis |
| Apestegui et al., 2011 [65] | 1 | 16/M | Red Bull | Biliary tumour; retransplantation | Consumption of 3 cans of Red Bull in 4 h to stay fit for his examinations. The next day, he separately took 2 tablets (800 mg) of the non-steroidal anti-inflammatory drug ibuprofen for a headache. | Acute hepatitis |
| Garg et al., 2020 [66] | 1 | 34/F | Red Bull and Monster Energy | N/A | Regular use of 1–2 ED/day for the past 15 years. | Atrophic gastritis (AG) and Gastric intestinal metaplasia (GIM). |
| Harb et al., 2016 [67] | 1 | 50/M | ED | N/A | Ingestion of 4–5 ED before onset. | Acute hepatitis. |
| Huang et al., 2014 [68] | 1 | 36/M | Rockstar energy | N/A | Consumption of 3 Rockstar energy drinks per day for the past year. | Acute hepatitis. |
| Randhawa et al., 2022 [69] | 1 | 29/M | ED | N/A | Consumed 5 to 6 energy drinks a day. Consumed 7 16-ounce energy drinks the day before hospital admission. | Acute pancreatitis. |
| Shmelev et al., 2015 [70] | 1 | 40/M | Rockstar energy | Acute alcoholic pancreatitis with pseudocyst formation. | Consumption of Rockstar™ followed by unexplained episodes of pancreatitis | Acute pancreatitis. |
| Takahashi et al., 2013 [71] | 1 | 76/M | ED | Hypertension; benign prostatic hypertrophy; total gastrectomy | Drinking half a bottle of energy drink as a dietary supplement after total gastrectomy. | Hypercobalaminemia. |
| Uwaifo, 2019 [72] | 1 | 46/M | Monster energy | Type 2 diabetes, nephrectomy, hyperuricemia. | Daily consumption of 2–3 16 oz cans for 4 months | Toxic triad syndrome of gastritis, hepatitis and pancreatitis. |
| Vivekanandarajah et al., 2011 [73] | 1 | 22/F | ED | N/A | Daily ingestion of 10 cans of an energy drink. | Acute hepatitis. |
| References | N° Cases | Age/Sex | Energy Drink (If Applicable) or Substances | Major Pathologies | Onset | Adverse Event |
|---|---|---|---|---|---|---|
| Casas-Gomez et al., 2018 [74] | 1 | 22/M | ED | N/A | Ingestion of 20 cans of 250 cc of an ED in 24 h because he was worried about flying. | Maniac episode. |
| Calabrò et al., 2012 [75] | 1 | 20/M | Red Bull | N/A | Consumed 4 to 6 cans of Red Bull a day for about 5 months before onset of seizures. | Tonic–clonic seizure. |
| Cerimele et al., 2010 [76] | 1 | 43/M | ED | Schizophrenia, paranoid type, and alcohol dependence in remission | Consumption of energy drinks 8 weeks prior to presentation. After drinking his first can of the drink, he decided to increase his daily consumption to 8 to 10 cans (16 oz per can) a day. | Paranoia, internal preoccupations, constricted affect, and delusional religious beliefs. |
| Cruzado et al., 2014 [77] | 1 | 31/F | Energy drink Magnus Omnilife products (each ration contains 81 mg of caffeine, 202 mg of taurine and 151 mg of glycine). | N/A | Increase consumption of filtered coffee from 2 to 5 cups a day and drink 3 to 4 rations of the Magnus Omnilife Products energy drink a day. After a few days, up to 10 daily portions of the energy drink (in addition to the five cups of filtered coffee per day). | Delusional ideas of grandeur, auditory hallucinations, and lacked awareness of disease manic syndrome. |
| Gorgulu et al., 2014 [78] | 1 | 21/M | ED + vodka | N/A | Regularly consumed one or two energy drinks a day in order to be more energetic during his training, sometimes drank energy drink in combination with a small amount of vodka. | Hallucinations, disorganized behavior, excitation, persecution, mystic and grandious delusions. |
| Grant et al., 2016 [79] | 1 | 44/F | Monster Energy drink | N/A | Consumption of five oversized cans (16 oz/can) of Monster Energy drink (totalling 800 mg of caffeine) | Aneurysmal subarachnoid haemorrhage complicated by severe catheter-induced vasospasm and symptomatic thromboembolism. |
| Gupta et al., 2019 [80] | 1 | 34/F | Multiple ED | N/A | Consumption multiple energy drinks before onset. | Macular neuroretinopathy (AMN) |
| Hernandez-Huerta et al., 2017 [81] | 1 | 18/M | ED + cannabis | Daily use of tobacco, daily use of cannabis. A paternal uncle had an unspecified chronic mental illness. | Consumption of 6 ED cans (80 mg caffeine per can) per day during in the last seven days before onset. | Psychotic disorder |
| Iyadurai et Chung 2007 [82] | 4 | 25/M | Rockstar energy | N/A | Drink two 24 oz bottles of Rockstar energy drink on an empty stomach about 30 to 60 min before the seizure. | Tonic–clonic seizure |
| 19/M | ED | Complex migraine | Ingestion of energy drinks on an empty stomach approximately 2 h prior to each ‘‘seizure’’ episode. | Tonic–clonic seizure | ||
| 28/F | Monster energy + diet pills | Migraine | Regular consumption of an energy drink (Monster). | Tonic–clonic seizure | ||
| 26/M | Monster energy | N/A | Ingestion of more than 2 24 oz cans before onset. | Tonic–clonic seizure | ||
| Kelsey et al., 2019 [83] | 1 | 30/M | Red Bull + alcohol | One episode of low mood 12 years prior, and one deliberate over-dose a year later. | Consumed up to twelve 250 mL cans of Red Bull per day. Consumed 7 units of alcohol on the day of arrest. | Agitation and anxiety |
| Laiseca et al., 2018 [84] | 1 | 8/M | Monster energy | N/A | Ingestion of energy drinks in the previous week (500-mL cans of Monster Energy). | Rolandic epilepsy |
| Menkes, 2011 [85] | 1 | 27/M | Demon Shot energy drink | Obesity (125 kg), schizophrenia | Consumed a 60 mL Demon Shot energy drink and enjoyed an hour-long “buzz”. Repeating the dose did not produce the desired effect. One week later, three shots over 15 min. | Transient psychotic |
| Norelli et Xu, 2014 [86] | 2 | 23/M | Red Ginseng drink | N/A | Consumption of seven times the recommended dose of ginseng every day for months. | Manic psychosis |
| 79/M | Red Ginseng drink | Substance-induced hypomanic episode | Ingestion of Korean red ginseng 3–4 times per day, everyday, during the two months prior the episode | Hypomanic psychosis | ||
| Pagano et al., 2017 [87] | 1 | 48/M | ED | Hypertension | Consumption of three large cans (473 mL each) of an energy drink over 30–40 min before onset. | Deep intraretinal haemorrhages |
| Sharma, 2010 [88] | 1 | 32/M | Red Bull | Occasional ‘mood swings’, alcohol abuse. Family history: post-partum depression and a case of suicide. | Consumption of 6–8 large cans (550 mL per can) a day during the week preceding his hospitalisation. | Substance-induced mood disorder with maniac features. |
| Staikoglou et al., 2022 [89] | 1 | 14/M | ED | N/A | Consumption of 2 L of energy drink during the previous 10 h before onset. | Brain ischemia secondary to dissection of the posterior cerebral artery (PCA) |
| Trabulo et al., 2011 [90] | 1 | 28/M | Red Bull | Heroin and cocaine abuse, chronic hepatitis C, severe mitral insufficiency and post-infectious endocarditis. | Ingestion of several cans (about 6) of Red Bull together with coffee in the 4 h before onset. | Clonic seizures + tonic clonic seizures |
| Yartsev et Peisah, 2021 [91] | 1 | 35/M | Red Bull | Chronic schizophrenia, obesity (142 kg), childhood asthma, gastro-oesophageal reflux disease, hypercholesterolaemia and impaired glucose tolerance | Consumption of energy drinks for weight loss, with daily caffeine consumption varying between 150 and 450 mg/day (between two and six 250 mL cans of Red Bull per day, with a caffeine concentration of 30 mg/100 mL). | Hyperosmolar hyperglycaemic syndrome with diabetic ketoacidosis. |
| References | N° Cases | Age/Sex | Energy Drink (If Applicable) or Substances | Major Pathologies | Onset | Adverse Event |
|---|---|---|---|---|---|---|
| Al Yacoub et al., 2020 [63] | 1 | 62/F | Sugar-free ED | Small-cell left lung carcinoma. | Consumption of five to six cans of a 16 fluid oz sugar-free ED daily. | Acute kidney injury (AKI) |
| Greene et al., 2014 [92] | 1 | 40/M | Red Bull | Diabetes mellitus type 2, hypertension, anxiety, depression, alcohol abuse, posttraumatic stress disorder, chronic obstructive pulmonary disease, obstructive sleep apnoea, gout, and hypertriglyceridemia-induced pancreatitis. | Ingestion of at least 5 to 6 20 oz (590 mL) Red Bull energy drinks daily for several weeks. | AKI |
| Icin et al., 2017 [93] | 1 | 27/M | ED | N/A | Consumption of 5000 mL of beer, 400–500 mL of liquor, and 2000 mL of energy drink (containing 25 mL/100 mL of caffeine and taurine, vitamins, sugar, citric acid, and caramel) during a 6-h period before the onset of symptoms. | Hyponatremia followed by coma |
| Iyer et al., 2016 [94] | 1 | 35/M | “Neon Volt” | N/A | Consumed 2 bottles of Neon Volt and then started training | Rhabdomyolysis |
| Kelsey et al., 2019 [83] | 1 | 30/M | Red Bull + alcohol | The psychiatric history consisted of a depressive episode 12 years earlier and a deliberate overdose one year later. On both occasions, no referral to psychiatric services was made and no treatment was initiated. | Consumption of up to twelve 250 mL cans of Red Bull per day, and he also had consumed 7 units of alcohol on the day of his arrest. | AKI |
| Schoffl et al., 2011 [95] | 1 | 17/M | ED + vodka | N/A | Ingestion of 3 L of ED with 1 L of vodka before onset | AKI |
| References | N° Cases | Age/Sex | Energy Drink (If Applicable) or Substances | Major Pathologies | Onset | Adverse Event |
|---|---|---|---|---|---|---|
| West et Thorpe, 2011 [96] | 1 | 23/F | Lucozade energy | N/A | Consumption of 2 L of “lucozade energy” per day in the last 3 months of pregnancy. | The infant was born with macrosomia because of refractory hyperinsulinism |
| Zekavat et al., 2017 [97] | 1 | 23/F | ED | N/A | Ingestion of high-energy drinks with inadequate dietary intake in the past 6 months. | Severe menorrhagia because of vitamin K deficiency |
| Tinawi, 2022 [98] | 1 | 40/M | ED | N/A | Consumption of 355 mL of ED after exercise | Severe rhabdomyolysis |
| References | N° Cases | Age/Sex | Energy Drink (If Applicable) or Substances | Major Pathologies | Onset | Adverse Event |
|---|---|---|---|---|---|---|
| Gerqari et al., 2016 [99] | 1 | 19/M | ED | N/A | Every time, he drank the same taurine-containing energy drink. | Erythema exudative multiforme |
| Lee et al., 2013 [100] | 1 | 33/F | ED | N/A | After taking ED containing 1000 mg of synthetic taurine. | Erythema, lips angioedema |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantino, A.; Maiese, A.; Lazzari, J.; Casula, C.; Turillazzi, E.; Frati, P.; Fineschi, V. The Dark Side of Energy Drinks: A Comprehensive Review of Their Impact on the Human Body. Nutrients 2023, 15, 3922. https://doi.org/10.3390/nu15183922
Costantino A, Maiese A, Lazzari J, Casula C, Turillazzi E, Frati P, Fineschi V. The Dark Side of Energy Drinks: A Comprehensive Review of Their Impact on the Human Body. Nutrients. 2023; 15(18):3922. https://doi.org/10.3390/nu15183922
Chicago/Turabian StyleCostantino, Andrea, Aniello Maiese, Julia Lazzari, Chiara Casula, Emanuela Turillazzi, Paola Frati, and Vittorio Fineschi. 2023. "The Dark Side of Energy Drinks: A Comprehensive Review of Their Impact on the Human Body" Nutrients 15, no. 18: 3922. https://doi.org/10.3390/nu15183922
APA StyleCostantino, A., Maiese, A., Lazzari, J., Casula, C., Turillazzi, E., Frati, P., & Fineschi, V. (2023). The Dark Side of Energy Drinks: A Comprehensive Review of Their Impact on the Human Body. Nutrients, 15(18), 3922. https://doi.org/10.3390/nu15183922







