Coffee Consumption and Incidence of Cardiovascular and Microvascular Diseases in Never-Smoking Adults with Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Coffee Consumption
2.3. Incident Cardiovascular and Microvascular Outcomes
2.4. Assessment of Other Covariates
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Associations of Coffee Consumption with Cardiovascular and Microvascular Diseases
3.3. Subgroup Analysis
3.4. Coffee Consumption, Coffee Type, and Cardio- and Microvascular Diseases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Boulton, A.J.; MacLeod, A.F.; Williams, D.R.; Sonksen, P.H. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993, 36, 150–154. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Sattar, N.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; Rosengren, A.; et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. Bmj 2017, 359, j5024. [Google Scholar] [CrossRef]
- Yang, J.; Tobias, D.K.; Li, S.; Bhupathiraju, S.N.; Ley, S.H.; Hinkle, S.N.; Qian, F.; Chen, Z.; Zhu, Y.; Bao, W.; et al. Habitual coffee consumption and subsequent risk of type 2 diabetes in individuals with a history of gestational diabetes—A prospective study. Am. J. Clin. Nutr. 2022, 116, 1693–1703. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef]
- Srithongkul, T.; Ungprasert, P. Coffee Consumption is Associated with a Decreased Risk of Incident Chronic Kidney Disease: A Systematic Review and Meta-analysis of Cohort Studies. Eur. J. Intern. Med. 2020, 77, 111–116. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, J.I.; Kwon, S.O.; Hwang, D.D. Coffee consumption and diabetic retinopathy in adults with diabetes mellitus. Sci. Rep. 2022, 12, 3547. [Google Scholar] [CrossRef]
- Komorita, Y.; Ohkuma, T.; Iwase, M.; Fujii, H.; Ide, H.; Oku, Y.; Higashi, T.; Oshiro, A.; Sakamoto, W.; Yoshinari, M.; et al. Relationship of coffee consumption with a decline in kidney function among patients with type 2 diabetes: The Fukuoka Diabetes Registry. J. Diabetes Investig. 2022, 13, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Shahinfar, H.; Jayedi, A.; Khan, T.A.; Shab-Bidar, S. Coffee consumption and cardiovascular diseases and mortality in patients with type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2526–2538. [Google Scholar] [CrossRef]
- Bjørngaard, J.H.; Nordestgaard, A.T.; Taylor, A.E.; Treur, J.L.; Gabrielsen, M.E.; Munafò, M.R.; Nordestgaard, B.G.; Åsvold, B.O.; Romundstad, P.; Davey Smith, G. Heavier smoking increases coffee consumption: Findings from a Mendelian randomization analysis. Int. J. Epidemiol. 2017, 46, 1958–1967. [Google Scholar] [CrossRef] [PubMed]
- Torres-Collado, L.; García-de la Hera, M.; Navarrete-Muñoz, E.M.; Compañ-Gabucio, L.M.; Gonzalez-Palacios, S.; Vioque, J. Coffee Drinking and Associated Factors in an Elderly Population in Spain. Int. J. Environ. Res. Public Health 2018, 15, 1661. [Google Scholar] [CrossRef] [PubMed]
- Inouye, M.; Abraham, G.; Nelson, C.P.; Wood, A.M.; Sweeting, M.J.; Dudbridge, F.; Lai, F.Y.; Kaptoge, S.; Brozynska, M.; Wang, T.; et al. Genomic Risk Prediction of Coronary Artery Disease in 480,000 Adults: Implications for Primary Prevention. J. Am. Coll. Cardiol. 2018, 72, 1883–1893. [Google Scholar] [CrossRef]
- Han, H.; Cao, Y.; Feng, C.; Zheng, Y.; Dhana, K.; Zhu, S.; Shang, C.; Yuan, C.; Zong, G. Association of a Healthy Lifestyle With All-Cause and Cause-Specific Mortality Among Individuals With Type 2 Diabetes: A Prospective Study in UK Biobank. Diabetes Care 2022, 45, 319–329. [Google Scholar] [CrossRef]
- Chieng, D.; Canovas, R.; Segan, L.; Sugumar, H.; Voskoboinik, A.; Prabhu, S.; Ling, L.H.; Lee, G.; Morton, J.B.; Kaye, D.M.; et al. The impact of coffee subtypes on incident cardiovascular disease, arrhythmias, and mortality: Long-term outcomes from the UK Biobank. Eur. J. Prev. Cardiol. 2022, 29, 2240–2249. [Google Scholar] [CrossRef]
- Li, F.R.; Hukportie, D.N.; Yang, J.; Yang, H.H.; Chen, G.C.; Wu, X.B. Microvascular Burden and Incident Heart Failure Among Middle-Aged and Older Adults With Type 1 or Type 2 Diabetes. Diabetes Care 2022, 45, 2999–3006. [Google Scholar] [CrossRef]
- Fan, M.; Sun, D.; Zhou, T.; Heianza, Y.; Lv, J.; Li, L.; Qi, L. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: A prospective study of 385 292 UK biobank participants. Eur. Heart J. 2020, 41, 1182–1189. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, F.R.; Jia, Y.; Li, Y.; Guo, D.; Chen, J.; Tian, H.; Yang, J.; Yang, H.H.; Chen, L.H.; et al. Association of Lifestyle With Incidence of Heart Failure According to Metabolic and Genetic Risk Status: A Population-Based Prospective Study. Circ. Heart Fail. 2022, 15, e009592. [Google Scholar] [CrossRef]
- de Jong, M.; Woodward, M.; Peters, S.A.E. Duration of diabetes and the risk of major cardiovascular events in women and men: A prospective cohort study of UK Biobank participants. Diabetes Res. Clin. Pract. 2022, 188, 109899. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hu, Y.; Alperet, D.J.; Liu, G.; Malik, V.; Manson, J.E.; Rimm, E.B.; Hu, F.B.; Sun, Q. Beverage consumption and mortality among adults with type 2 diabetes: Prospective cohort study. Bmj 2023, 381, e073406. [Google Scholar] [CrossRef] [PubMed]
- Loftfield, E.; Freedman, N.D.; Inoue-Choi, M.; Graubard, B.I.; Sinha, R. A Prospective Investigation of Coffee Drinking and Bladder Cancer Incidence in the United States. Epidemiology 2017, 28, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; Galeone, C.; Buys, S.S.; Gren, L.; Boffetta, P.; Zhang, Z.F.; La Vecchia, C. Coffee, tea, caffeine intake, and the risk of cancer in the PLCO cohort. Br. J. Cancer 2015, 113, 809–816. [Google Scholar] [CrossRef]
- Śliwińska-Mossoń, M.; Milnerowicz, H. The impact of smoking on the development of diabetes and its complications. Diabetes Vasc. Dis. Res. 2017, 14, 265–276. [Google Scholar] [CrossRef]
- Kennedy, O.J.; Pirastu, N.; Poole, R.; Fallowfield, J.A.; Hayes, P.C.; Grzeszkowiak, E.J.; Taal, M.W.; Wilson, J.F.; Parkes, J.; Roderick, P.J. Coffee Consumption and Kidney Function: A Mendelian Randomization Study. Am. J. Kidney Dis. 2020, 75, 753–761. [Google Scholar] [CrossRef] [PubMed]
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef]
- Drucker, D.J. Glucagon-like peptides. Diabetes 1998, 47, 159–169. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef]
- Williams, C.J.; Fargnoli, J.L.; Hwang, J.J.; van Dam, R.M.; Blackburn, G.L.; Hu, F.B.; Mantzoros, C.S. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: A prospective cohort study. Diabetes Care 2008, 31, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sundvall, J.; Bidel, S.; Kuha, S.; et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010, 91, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Hang, D.; Kværner, A.S.; Ma, W.; Hu, Y.; Tabung, F.K.; Nan, H.; Hu, Z.; Shen, H.; Mucci, L.A.; Chan, A.T.; et al. Coffee consumption and plasma biomarkers of metabolic and inflammatory pathways in US health professionals. Am. J. Clin. Nutr. 2019, 109, 635–647. [Google Scholar] [CrossRef] [PubMed]
- He, W.J.; Chen, J.; Razavi, A.C.; Hu, E.A.; Grams, M.E.; Yu, B.; Parikh, C.R.; Boerwinkle, E.; Bazzano, L.; Qi, L.; et al. Metabolites Associated with Coffee Consumption and Incident Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2021, 16, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Nurminen, M.L.; Niittynen, L.; Korpela, R.; Vapaatalo, H. Coffee, caffeine and blood pressure: A critical review. Eur. J. Clin. Nutr. 1999, 53, 831–839. [Google Scholar] [CrossRef]
- Mesas, A.E.; Leon-Muñoz, L.M.; Rodriguez-Artalejo, F.; Lopez-Garcia, E. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1113–1126. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.H.; Shen, D.; Zhang, P.D.; Song, W.Q.; Zhang, W.T.; Huang, Q.M.; Chen, P.L.; Zhang, X.R.; Mao, C. Association of Sugar-Sweetened, Artificially Sweetened, and Unsweetened Coffee Consumption with All-Cause and Cause-Specific Mortality: A Large Prospective Cohort Study. Ann. Intern. Med. 2022, 175, 909–917. [Google Scholar] [CrossRef]

| Total | Coffee Consumption (cups/day) | ||||
|---|---|---|---|---|---|
| N = 9964 | None (n = 2859) | 0.5–1 (n = 2779) | 2–4 (n = 3449) | ≥5 (n = 877) | |
| Age, y | 57.9 ± 7.6 | 56.9 ± 7.8 | 58.3 ± 7.5 | 58.4 ± 7.4 | 57.9 ± 7.5 |
| Men | 4948 (49.7) | 1269 (44.4) | 1381 (49.7) | 1785 (51.8) | 513 (58.5) |
| White | 7889 (79.2) | 1889 (66.1) | 2122 (76.4) | 3041 (88.2) | 837 (95.4) |
| Townsend deprivation index a | −0.55 ± 3.4 | 0.09 ± 3.5 | −0.52 ± 3.4 | −0.98 ± 3.3 | −1.07 ± 3.1 |
| BMI, kg/m2 | |||||
| <25 | 1239 (12.5) | 358 (12.5) | 404 (14.5) | 398 (11.5) | 79 (9.0) |
| 25 to <30 | 3437 (34.5) | 1008 (35.3) | 972 (35.0) | 1185 (34.4) | 272 (31.0) |
| ≥30 | 5288 (53.0) | 1493 (52.2) | 1403 (50.5) | 1866 (54.1) | 526 (60.0) |
| Physical activity, MET-h/week | 38.4 ± 37.7 | 38.8 ± 37.7 | 38.9 ± 38.6 | 38.1 ± 37.0 | 36.9 ± 37.4 |
| Alcohol consumption | |||||
| Never | 1476 (14.8) | 743 (26.0) | 353 (12.7) | 311 (9.0) | 69 (7.9) |
| Former | 539 (5.4) | 214 (7.5) | 120 (4.3) | 162 (4.7) | 43 (4.9) |
| Current: <1 drinks/week | 3333 (33.5) | 951 (33.3) | 991 (35.7) | 1097 (31.8) | 294 (33.5) |
| Current: 1–2 drinks/week | 2265 (22.7) | 521 (18.2) | 654 (23.5) | 877 (25.4) | 213 (24.3) |
| Current: ≥3 drinks/week | 2339 (23.5) | 423 (14.8) | 659 (23.7) | 1000 (29.0) | 257 (29.3) |
| Diet score ≥3 points | 5550 (55.7) | 1612 (56.4) | 1596 (57.4) | 1900 (56.1) | 442 (50.4) |
| Hypertension | 7824 (78.5) | 2192 (76.7) | 2205 (79.4) | 2747 (79.7) | 680 (77.5) |
| Hyperlipidemia | 6224 (62.5) | 1670 (58.4) | 1725 (62.1) | 2227 (64.6) | 602 (68.6) |
| HbA1c, mmol/L | 52.7 ± 14.6 | 52.7 ± 15.0 | 52.7 ± 14.2 | 52.4 ± 14.4 | 53.8 ± 15.5 |
| Diabetes duration, y | |||||
| 0–<5 | 5746 (57.7) | 1701 (59.5) | 1617 (58.2) | 1984 (57.5) | 444 (50.6) |
| 5–<10 | 2181 (21.9) | 572 (20.0) | 604 (21.7) | 788 (22.9) | 217 (24.7) |
| ≥10 | 2037 (20.4) | 586 (20.5) | 558 (20.1) | 677 (19.6) | 216 (24.6) |
| Retinopathy | 1652 (16.6) | 492 (17.2) | 474 (17.1) | 553 (16.0) | 133 (15.2) |
| Peripheral neuropathy | 92 (0.90) | 28 (0.98) | 22 (0.79) | 30 (0.87) | 12 (1.37) |
| CKD | 643 (6.5) | 209 (7.3) | 171 (6.2) | 215 (6.2) | 48 (5.5) |
| Coffee Consumption (cups/day) | P for Trend | P for Nonlinearity | ||||
|---|---|---|---|---|---|---|
| None | 0.5–1 | 2–4 | ≥5 | |||
| Total CVD | ||||||
| Events/N | 548/2859 | 528/2779 | 600/3449 | 184/877 | ||
| Model 1 | 1.00 (Ref.) | 0.92 (0.82, 1.04) | 0.83 (0.74, 0.93) | 1.01 (0.85, 1.19) | 0.252 | |
| Model 2 | 1.00 (Ref.) | 0.95 (0.84, 1.07) | 0.83 (0.74, 0.94) | 0.96 (0.80, 1.14) | 0.061 | |
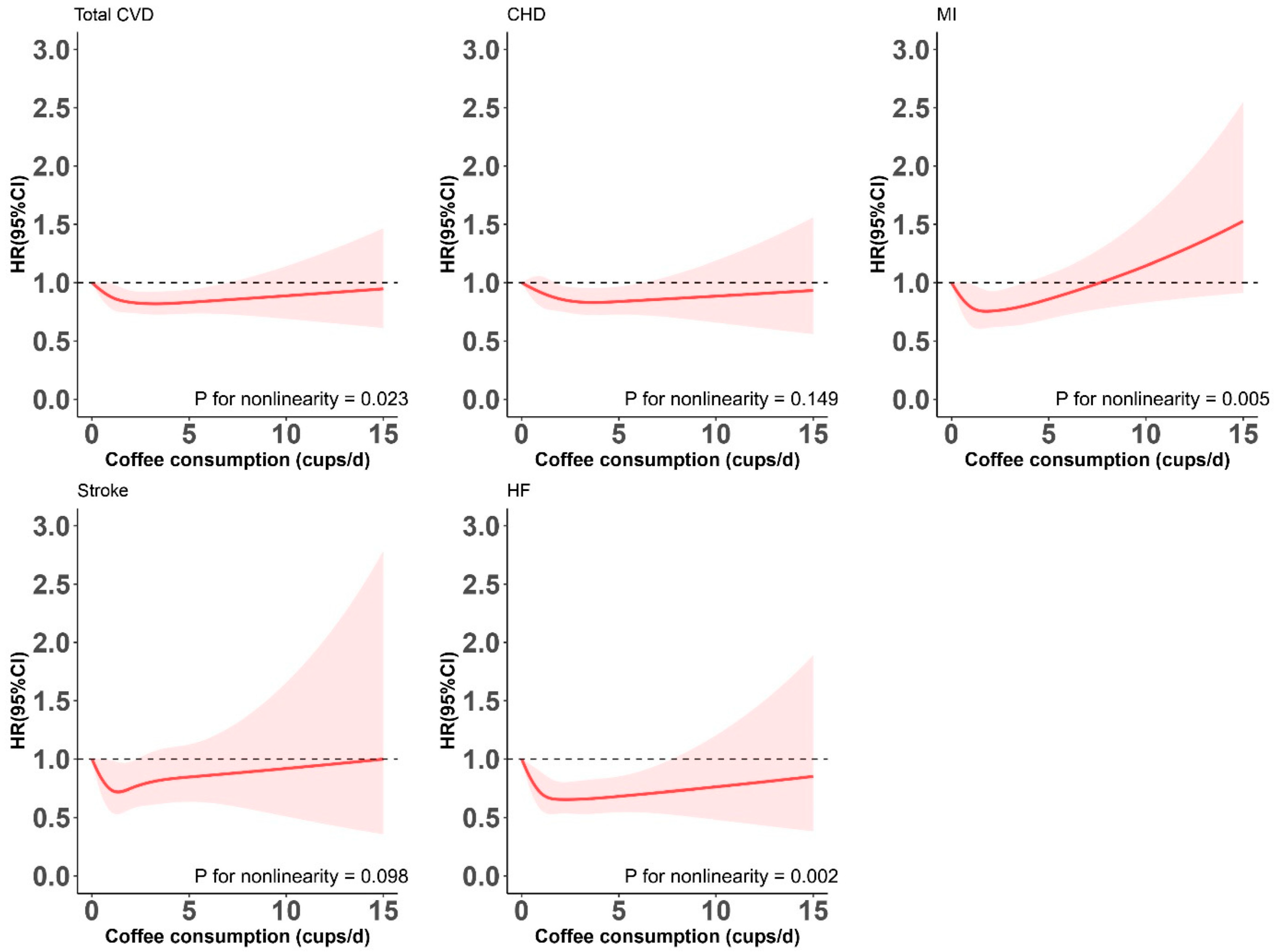
| Model 3 | 1.00 (Ref.) | 0.91 (0.80, 1.04) | 0.82 (0.73, 0.93) | 0.89 (0.74, 1.07) | 0.037 | 0.023 |
| CHD | ||||||
| Events/N | 412/2859 | 410/2779 | 454/3449 | 135/877 | ||
| Model 1 | 1.00 (Ref.) | 0.96 (0.84, 1.10) | 0.84 (0.74, 0.96) | 0.98 (0.81, 1.19) | 0.232 | |
| Model 2 | 1.00 (Ref.) | 0.98 (0.85, 1.13) | 0.85 (0.74, 0.97) | 0.94 (0.76, 1.15) | 0.162 | |
| Model 3 | 1.00 (Ref.) | 0.93 (0.80, 1.07) | 0.84 (0.73, 0.97) | 0.85 (0.69, 1.06) | 0.052 | 0.149 |
| MI | ||||||
| Events/N | 174/2859 | 151/2779 | 162/3449 | 65/877 | ||
| Model 1 | 1.00 (Ref.) | 0.83 (0.67, 1.03) | 0.71 (0.57, 0.88) | 1.10 (0.83, 1.47) | 0.752 | |
| Model 2 | 1.00 (Ref.) | 0.85 (0.68, 1.08) | 0.73 (0.58, 0.91) | 1.06 (0.78, 1.43) | 0.551 | |
| Model 3 | 1.00 (Ref.) | 0.82 (0.64, 1.04) | 0.73 (0.57, 0.92) | 1.06 (0.78, 1.45) | 0.735 | 0.005 |
| Stroke | ||||||
| Events/N | 100/2859 | 90/2779 | 102/3449 | 37/877 | ||
| Model 1 | 1.00 (Ref.) | 0.86 (0.64, 1.14) | 0.78 (0.59, 1.03) | 1.14 (0.78, 1.66) | 0.857 | |
| Model 2 | 1.00 (Ref.) | 0.94 (0.70, 1.27) | 0.82 (0.61, 1.10) | 1.16 (0.78, 1.72) | 0.819 | |
| Model 3 | 1.00 (Ref.) | 0.82 (0.61, 1.10) | 0.76 (0.57, 1.02) | 0.98 (0.65, 1.48) | 0.378 | 0.098 |
| HF | ||||||
| Events/N | 177/2859 | 144/2779 | 166/3449 | 52/877 | ||
| Model 1 | 1.00 (Ref.) | 0.77 (0.62, 0.96) | 0.71 (0.57, 0.88) | 0.89 (0.65, 1.21) | 0.182 | |
| Model 2 | 1.00 (Ref.) | 0.76 (0.61, 0.96) | 0.66 (0.53, 0.82) | 0.75 (0.54, 1.03) | 0.054 | |
| Model 3 | 1.00 (Ref.) | 0.80 (0.64, 1.01) | 0.68 (0.55, 0.85) | 0.75 (0.54, 1.05) | 0.022 | 0.002 |
| Coffee Consumption (cups/day) | P for Trend | P for Nonlinearity | ||||
|---|---|---|---|---|---|---|
| None | 0.5–1 | 2–4 | ≥5 | |||
| Total MVD | ||||||
| Events/N | 426/2191 | 393/2177 | 463/2716 | 121/697 | ||
| Model 1 | 1.00 (Ref.) | 0.86 (0.75, 0.98) | 0.81 (0.71, 0.92) | 0.84 (0.68, 1.03) | 0.075 | |
| Model 2 | 1.00 (Ref.) | 0.87 (0.76, 0.99) | 0.80 (0.70, 0.92) | 0.82 (0.67, 1.02) | 0.053 | |
| Model 3 | 1.00 (Ref.) | 0.89 (0.77, 1.03) | 0.81 (0.70, 0.94) | 0.81 (0.65, 0.99) | 0.045 | 0.005 |
| Retinopathy | ||||||
| Events/N | 230/2191 | 225/2177 | 269/2716 | 79/697 | ||
| Model 1 | 1.00 (Ref.) | 0.94 (0.78, 1.13) | 0.90 (0.75, 1.08) | 1.07 (0.82, 1.38) | 0.733 | |
| Model 2 | 1.00 (Ref.) | 0.96 (0.80, 1.17) | 0.90 (0.75, 1.08) | 1.07 (0.82, 1.39) | 0.751 | |
| Model 3 | 1.00 (Ref.) | 0.98 (0.80, 1.20) | 0.90 (0.73, 1.09) | 1.05 (0.80, 1.38) | 0.782 | 0.179 |
| Peripheral neuropathy | ||||||
| Events/N | 64/2191 | 46/2177 | 67/2716 | 14/697 | ||
| Model 1 | 1.00 (Ref.) | 0.72 (0.49, 1.05) | 0.84 (0.60, 1.19) | 0.68 (0.38, 1.21) | 0.432 | |
| Model 2 | 1.00 (Ref.) | 0.69 (0.47, 1.03) | 0.82 (0.57, 1.17) | 0.63 (0.34, 1.15) | 0.392 | |
| Model 3 | 1.00 (Ref.) | 0.72 (0.47, 1.09) | 0.82 (0.56, 1.20) | 0.65 (0.35, 1.19) | 0.431 | 0.327 |
| CKD | ||||||
| Events/N | 192/2191 | 188/2177 | 205/2716 | 46/697 | ||
| Model 1 | 1.00 (Ref.) | 0.90 (0.73, 1.10) | 0.77 (0.63, 0.94) | 0.69 (0.50, 0.96) | 0.009 | |
| Model 2 | 1.00 (Ref.) | 0.94 (0.76, 1.15) | 0.79 (0.64, 0.98) | 0.68 (0.49, 0.95) | 0.006 | |
| Model 3 | 1.00 (Ref.) | 0.95 (0.76, 1.18) | 0.77 (0.62, 0.96) | 0.64 (0.45, 0.91) | 0.003 | 0.471 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-J.; Miao, M.-Y.; Wang, J.-M.; Tang, Q.; Han, W.-W.; Jia, Y.-P.; Tao, H.-W.; Zheng, Y.; van Dam, R.M.; Qin, L.-Q.; et al. Coffee Consumption and Incidence of Cardiovascular and Microvascular Diseases in Never-Smoking Adults with Type 2 Diabetes Mellitus. Nutrients 2023, 15, 3910. https://doi.org/10.3390/nu15183910
Liu Y-J, Miao M-Y, Wang J-M, Tang Q, Han W-W, Jia Y-P, Tao H-W, Zheng Y, van Dam RM, Qin L-Q, et al. Coffee Consumption and Incidence of Cardiovascular and Microvascular Diseases in Never-Smoking Adults with Type 2 Diabetes Mellitus. Nutrients. 2023; 15(18):3910. https://doi.org/10.3390/nu15183910
Chicago/Turabian StyleLiu, Yu-Jie, Meng-Yuan Miao, Jia-Min Wang, Quan Tang, Wen-Wen Han, Yi-Ping Jia, Hao-Wei Tao, Yan Zheng, Rob M. van Dam, Li-Qiang Qin, and et al. 2023. "Coffee Consumption and Incidence of Cardiovascular and Microvascular Diseases in Never-Smoking Adults with Type 2 Diabetes Mellitus" Nutrients 15, no. 18: 3910. https://doi.org/10.3390/nu15183910
APA StyleLiu, Y.-J., Miao, M.-Y., Wang, J.-M., Tang, Q., Han, W.-W., Jia, Y.-P., Tao, H.-W., Zheng, Y., van Dam, R. M., Qin, L.-Q., & Chen, G.-C. (2023). Coffee Consumption and Incidence of Cardiovascular and Microvascular Diseases in Never-Smoking Adults with Type 2 Diabetes Mellitus. Nutrients, 15(18), 3910. https://doi.org/10.3390/nu15183910







