Adherence to the 2015–2020 Dietary Guidelines for Americans Compared with the Mediterranean Diet in Relation to Risk of Prediabetes: Results from NHANES 2007–2016
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Assessment and Calculation of Diet Quality Scores
2.3. Ascertainment of Prediabetes
2.4. Assessment of Covariates
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
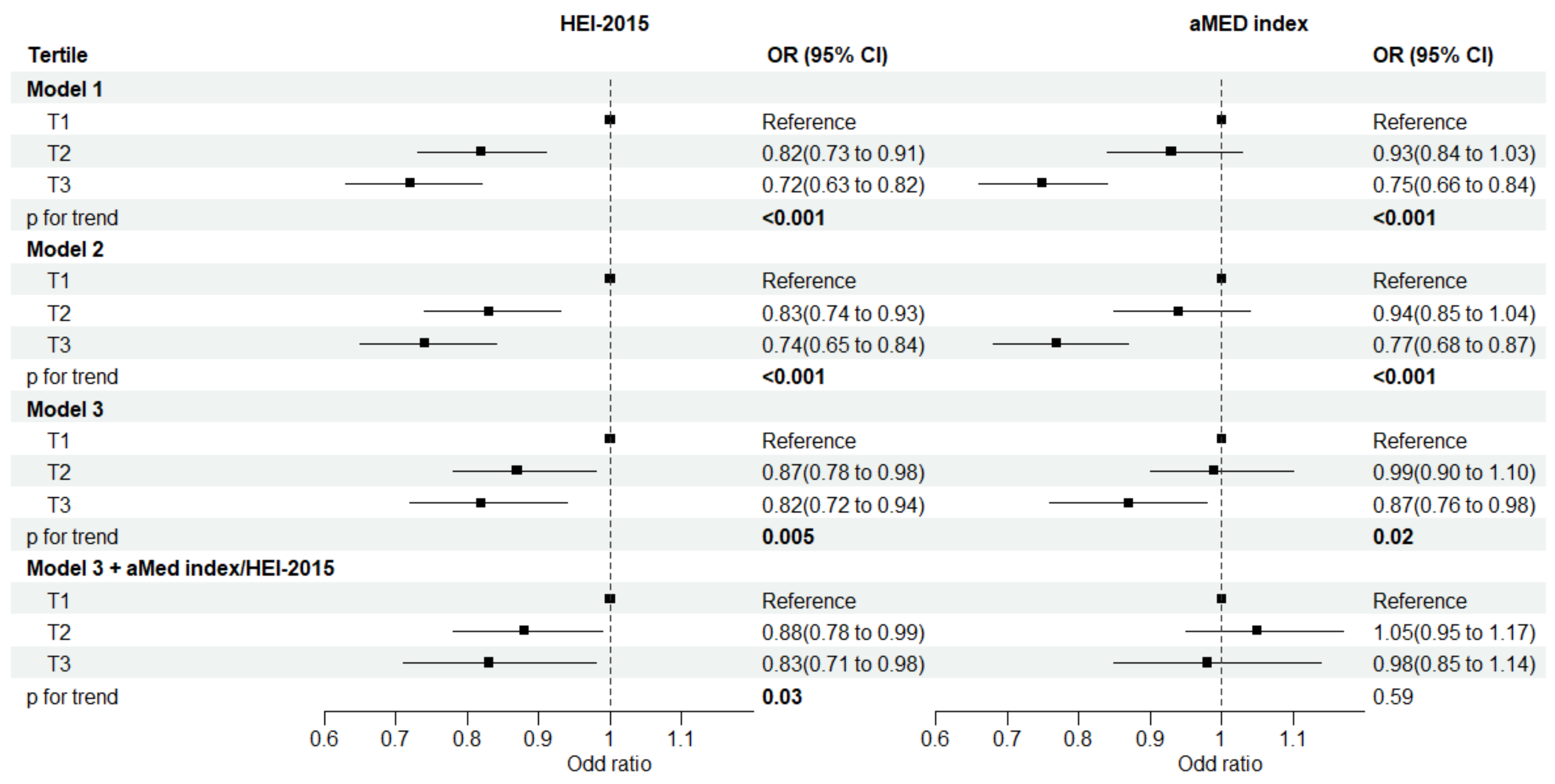
3.2. Diet Quality Scores and Risk of Prediabetes
3.3. Individual Food and Its Components and the Risk of Prediabetes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pour, O.R.; Dagogo-Jack, S. Prediabetes as a therapeutic target. Clin. Chem. 2011, 57, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Tabak, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimaki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Brannick, B.; Dagogo-Jack, S. Prediabetes and Cardiovascular Disease: Pathophysiology and Interventions for Prevention and Risk Reduction. Endocrinol. Metab. Clin. N. Am. 2018, 47, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhang, Y.; Li, M.; Wu, J.H.; Mai, L.; Li, J.; Yang, Y.; Hu, Y.; Huang, Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: Updated meta-analysis. BMJ 2020, 370, m2297. [Google Scholar] [CrossRef]
- Schlesinger, S.; Neuenschwander, M.; Barbaresko, J.; Lang, A.; Maalmi, H.; Rathmann, W.; Roden, M.; Herder, C. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: Umbrella review of meta-analyses of prospective studies. Diabetologia 2022, 65, 275–285. [Google Scholar] [CrossRef]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Wang, L.; Peng, W.; Zhao, Z.; Zhang, M.; Shi, Z.; Song, Z.; Zhang, X.; Li, C.; Huang, Z.; Sun, X.; et al. Prevalence and Treatment of Diabetes in China, 2013–2018. JAMA 2021, 326, 2498–2506. [Google Scholar] [CrossRef]
- Broz, J.; Malinovska, J.; Nunes, M.A.; Kucera, K.; Rozekova, K.; Zejglicova, K.; Urbanova, J.; Jensovsky, M.; Brabec, M.; Lustigova, M. Prevalence of diabetes and prediabetes and its risk factors in adults aged 25–64 in the Czech Republic: A cross-sectional study. Diabetes Res. Clin. Pract. 2020, 170, 108470. [Google Scholar] [CrossRef]
- Chen, Z.; Franco, O.H.; Lamballais, S.; Ikram, M.A.; Schoufour, J.D.; Muka, T.; Voortman, T. Associations of specific dietary protein with longitudinal insulin resistance, prediabetes and type 2 diabetes: The Rotterdam Study. Clin. Nutr. 2020, 39, 242–249. [Google Scholar] [CrossRef]
- Li, L.; Wan, Z.; Geng, T.; Lu, Q.; Zhu, K.; Qiu, Z.; Zhang, X.; Liu, Y.; Tian, Q.; Liu, L.; et al. Associations of healthy dietary patterns with mortality among people with prediabetes. Eur. J. Nutr. 2023, 62, 1377–1387. [Google Scholar] [CrossRef]
- Reedy, J.; Lerman, J.L.; Krebs-Smith, S.M.; Kirkpatrick, S.I.; Pannucci, T.E.; Wilson, M.M.; Subar, A.F.; Kahle, L.L.; Tooze, J.A. Evaluation of the Healthy Eating Index-2015. J. Acad. Nutr. Diet. 2018, 118, 1622–1633. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.A.; Steffen, L.M.; Coresh, J.; Appel, L.J.; Rebholz, C.M. Adherence to the Healthy Eating Index-2015 and Other Dietary Patterns May Reduce Risk of Cardiovascular Disease, Cardiovascular Mortality, and All-Cause Mortality. J. Nutr. 2020, 150, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Jayanama, K.; Theou, O.; Godin, J.; Cahill, L.; Shivappa, N.; Hebert, J.R.; Wirth, M.D.; Park, Y.M.; Fung, T.T.; Rockwood, K. Relationship between diet quality scores and the risk of frailty and mortality in adults across a wide age spectrum. BMC Med. 2021, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 15 June 2023).
- Marriott, B.P.; Hunt, K.J.; Malek, A.M.; Newman, J.C. Trends in Intake of Energy and Total Sugar from Sugar-Sweetened Beverages in the United States among Children and Adults, NHANES 2003–2016. Nutrients 2019, 11, 2004. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, T.; Wan, Z.; Lu, Q.; Zhang, X.; Qiu, Z.; Li, L.; Zhu, K.; Liu, L.; Pan, A.; et al. Associations of Serum Folate and Vitamin B12 Levels with Cardiovascular Disease Mortality Among Patients with Type 2 Diabetes. JAMA Netw. Open 2022, 5, e2146124. [Google Scholar] [CrossRef]
- Asfar, T.; Perez, A.; Shipman, P.; Carrico, A.W.; Lee, D.J.; Alcaide, M.L.; Jones, D.L.; Brewer, J.; Koru-Sengul, T. National Estimates of Prevalence, Time-Trend, and Correlates of Smoking in US People Living with HIV (NHANES 1999–2016). Nicotine Tob. Res. 2021, 23, 1308–1317. [Google Scholar] [CrossRef]
- Butler, L.; Popkin, B.M.; Poti, J.M. Associations of Alcoholic Beverage Consumption with Dietary Intake, Waist Circumference, and Body Mass Index in US Adults: National Health and Nutrition Examination Survey 2003–2012. J. Acad. Nutr. Diet. 2018, 118, 409–420.e403. [Google Scholar] [CrossRef]
- Murray, J.M.; Coleman, H.G.; Hunter, R.F. Physical activity and cancer risk: Findings from the UK Biobank, a large prospective cohort study. Cancer Epidemiol. 2020, 68, 101780. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S, discussion 1229S–1231S. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Arthur, R.; Mossavar-Rahmani, Y.; Xue, X.; Haring, B.; Shadyab, A.H.; Allison, M.A.; Liu, S.; Tinker, L.F.; Saquib, N.; et al. Adherence to Recommended Eating Patterns Is Associated with Lower Risk of Peripheral Arterial Disease: Results from the women’s health initiative. Hypertension 2021, 78, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Ming-Wei, L.; Qi-Qiang, H.; Larsson, S.C. Egg, cholesterol and protein intake and incident type 2 diabetes mellitus: Results of repeated measurements from a prospective cohort study. Clin. Nutr. 2021, 40, 4180–4186. [Google Scholar] [CrossRef]
- Reedy, J.; Krebs-Smith, S.M.; Miller, P.E.; Liese, A.D.; Kahle, L.L.; Park, Y.; Subar, A.F. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J. Nutr. 2014, 144, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Haring, B.; Crandall, C.J.; Wu, C.; LeBlanc, E.S.; Shikany, J.M.; Carbone, L.; Orchard, T.; Thomas, F.; Wactawaski-Wende, J.; Li, W.; et al. Dietary Patterns and Fractures in Postmenopausal Women: Results from the Women’s Health Initiative. JAMA Intern. Med. 2016, 176, 645–652. [Google Scholar] [CrossRef]
- Koloverou, E.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Georgousopoulou, E.N.; Grekas, A.; Christou, A.; Chatzigeorgiou, M.; Skoumas, I.; Tousoulis, D.; et al. Adherence to Mediterranean diet and 10-year incidence (2002–2012) of diabetes: Correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes/Metab. Res. Rev. 2016, 32, 73–81. [Google Scholar] [CrossRef]
- Koloverou, E.; Esposito, K.; Giugliano, D.; Panagiotakos, D. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: A meta-analysis of 10 prospective studies and 136,846 participants. Metabolism 2014, 63, 903–911. [Google Scholar] [CrossRef]
- Ojo, O.; Ojo, O.O.; Adebowale, F.; Wang, X.H. The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018, 10, 373. [Google Scholar] [CrossRef]
- Papamichou, D.; Panagiotakos, D.B.; Itsiopoulos, C. Dietary patterns and management of type 2 diabetes: A systematic review of randomised clinical trials. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 531–543. [Google Scholar] [CrossRef]
- Jannasch, F.; Kroger, J.; Schulze, M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017, 147, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Falguera, M.; Castelblanco, E.; Rojo-Lopez, M.I.; Vilanova, M.B.; Real, J.; Alcubierre, N.; Miro, N.; Mollo, A.; Mata-Cases, M.; Franch-Nadal, J.; et al. Mediterranean Diet and Healthy Eating in Subjects with Prediabetes from the Mollerussa Prospective Observational Cohort Study. Nutrients 2021, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Pestoni, G.; Riedl, A.; Breuninger, T.A.; Wawro, N.; Krieger, J.P.; Meisinger, C.; Rathmann, W.; Thorand, B.; Harris, C.; Peters, A.; et al. Association between dietary patterns and prediabetes, undetected diabetes or clinically diagnosed diabetes: Results from the KORA FF4 study. Eur. J. Nutr. 2021, 60, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Fretts, A.M.; Follis, J.L.; Nettleton, J.A.; Lemaitre, R.N.; Ngwa, J.S.; Wojczynski, M.K.; Kalafati, I.P.; Varga, T.V.; Frazier-Wood, A.C.; Houston, D.K.; et al. Consumption of meat is associated with higher fasting glucose and insulin concentrations regardless of glucose and insulin genetic risk scores: A meta-analysis of 50,345 Caucasians. Am. J. Clin. Nutr. 2015, 102, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.; Clifton, P. A review of potential metabolic etiologies of the observed association between red meat consumption and development of type 2 diabetes mellitus. Metabolism 2015, 64, 768–779. [Google Scholar] [CrossRef]
- Abshirini, M.; Mahaki, B.; Bagheri, F.; Siassi, F.; Koohdani, F.; Qorbani, M.; Yavari, P.; Sotoudeh, G. Dietary Fat Quality and Pre-diabetes: A Case-control Study. Int. J. Prev. Med. 2020, 11, 160. [Google Scholar]
- Nguyen, C.Q.; Pham, T.T.P.; Fukunaga, A.; Hoang, D.V.; Phan, T.V.; Phan, D.C.; Huynh, D.V.; Hachiya, M.; Le, H.X.; Do, H.T.; et al. Red meat consumption is associated with prediabetes and diabetes in rural Vietnam: A cross-sectional study. Public Health Nutr. 2022, 26, 1006–1013. [Google Scholar] [CrossRef]
- Kolahdouz-Mohammadi, R.; Soltani, S.; Clayton, Z.S.; Salehi-Abargouei, A. Sodium status is associated with type 2 diabetes mellitus: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2021, 60, 3543–3565. [Google Scholar] [CrossRef]
- Hao, G.; Liu, K.; Halbert, J.D.; Chen, H.; Wu, J.; Jing, C. Dietary sodium and potassium and risk of diabetes: A prospective study using data from the China Health and Nutrition Survey. Diabetes Metab. 2020, 46, 377–383. [Google Scholar] [CrossRef]
- Von Frankenberg, A.D.; Marina, A.; Song, X.; Callahan, H.S.; Kratz, M.; Utzschneider, K.M. A high-fat, high-saturated fat diet decreases insulin sensitivity without changing intra-abdominal fat in weight-stable overweight and obese adults. Eur. J. Nutr. 2017, 56, 431–443. [Google Scholar] [CrossRef]
- Cullmann, M.; Hilding, A.; Ostenson, C.G. Alcohol consumption and risk of pre-diabetes and type 2 diabetes development in a Swedish population. Diabet. Med. 2012, 29, 441–452. [Google Scholar] [CrossRef] [PubMed]
| HEI-2015 | aMed Index | |||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | |
| Age, years (mean (SD)) | 42.26 (16.01) | 45.62 (16.41) | 48.86 (16.81) | 42.57 (16.08) | 45.92 (16.67) | 48.71 (16.61) |
| Sex = male (%) | 52.2 | 49.6 | 43.2 | 51.7 | 49.1 | 43.1 |
| Ethnicity (%) | ||||||
| Non-Hispanic White | 67.1 | 67.0 | 70.6 | 68.3 | 66.3 | 71.4 |
| Non-Hispanic Black | 12.9 | 11.3 | 7.7 | 12.7 | 11.0 | 7.6 |
| Mexican American | 8.8 | 9.1 | 6.9 | 8.3 | 9.1 | 7.0 |
| other ethnicities | 11.2 | 12.6 | 14.7 | 10.8 | 13.6 | 14.1 |
| Education (%) | ||||||
| Less than high school | 18.9 | 15.5 | 11.7 | 20.3 | 15.3 | 9.4 |
| High school or equivalent | 27.2 | 22.4 | 15.8 | 27.6 | 22.4 | 13.7 |
| College or above | 53.8 | 62.1 | 72.6 | 52.1 | 62.2 | 76.9 |
| Marital status (%) | ||||||
| Married | 58.1 | 61.2 | 66.5 | 57.5 | 61.6 | 67.8 |
| Previously married | 17.1 | 19.0 | 15.7 | 17.9 | 18.5 | 14.6 |
| Never married | 24.8 | 19.8 | 17.8 | 24.6 | 19.9 | 17.6 |
| PIR (%) | ||||||
| ≤1.0 | 18.4 | 14.4 | 10.0 | 19.0 | 14.1 | 8.7 |
| 1.1–3.0 | 36.0 | 32.9 | 28.9 | 36.3 | 33.5 | 26.5 |
| >3.0 | 45.6 | 52.7 | 61.2 | 44.7 | 52.3 | 64.8 |
| Smoking status (%) | ||||||
| Former | 20.0 | 23.4 | 25.5 | 19.4 | 23.4 | 26.6 |
| Current | 30.2 | 20.9 | 12.1 | 32.3 | 19.2 | 10.4 |
| Never | 49.9 | 55.6 | 62.4 | 48.3 | 57.4 | 63.0 |
| Drinking status (%) | ||||||
| Nondrinkers | 11.6 | 12.6 | 12.8 | 11.0 | 13.7 | 11.7 |
| Former drinkers | 14.6 | 12.3 | 10.7 | 13.4 | 13.1 | 10.5 |
| Non-excessive drinkers | 25.8 | 28.9 | 35.9 | 23.7 | 30.1 | 38.3 |
| Excessive drinkers | 47.9 | 46.2 | 40.7 | 51.9 | 43.2 | 39.4 |
| Physical activity (%) | ||||||
| Low | 37.3 | 36.5 | 29.9 | 37.4 | 35.4 | 29.8 |
| Moderate | 13.1 | 14.5 | 18.0 | 13.2 | 14.9 | 18.2 |
| High | 49.6 | 48.9 | 52.1 | 49.4 | 49.7 | 52.1 |
| Total energy intake, kcal/d (mean (SD)) | 2254.32 (1066.44) | 2201.82 (991.77) | 2058.64 (864.22) | 2125.27 (1018.77) | 2187.63 (1001.79) | 2203.02 (894.91) |
| BMI, kg/m2 (mean (SD)) | 29.22 (7.07) | 28.41 (6.39) | 27.32 (5.74) | 29.20 (7.01) | 28.42 (6.34) | 27.08 (5.76) |
| HEI-2015 | aMed Index | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | Pinteraction | T1 | T2 | T3 | Pinteraction | ||
| Age, years | 0.134 | 0.832 | |||||||
| <45 | 2669/10,121 | 1 | 1.09 (0.92, 1.30) | 0.81 (0.66, 0.98) | 1 | 1.12 (0.99, 1.28) | 0.86 (0.70, 1.05) | ||
| ≥45 | 5852/10,723 | 1 | 0.78 (0.66, 0.92) | 0.91 (0.77, 1.06) | 1 | 0.94 (0.81, 1.09) | 0.94 (0.80, 1.12) | ||
| Sex | 0.204 | 0.467 | |||||||
| Male | 4430/10,158 | 1 | 0.97 (0.83, 1.14) | 0.81 (0.68, 0.97) | 1 | 0.91 (0.78, 1.07) | 0.89 (0.75, 1.06) | ||
| Female | 4091/10,686 | 1 | 0.78 (0.65, 0.93) | 0.82 (0.70, 0.96) | 1 | 1.05 (0.92, 1.20) | 0.82 (0.69, 0.98) | ||
| Ethnicity | 0.060 | 0.169 | |||||||
| Non-Hispanic White | 3560/9252 | 1 | 0.81 (0.78, 0.84) | 0.78 (0.73, 0.83) | 1 | 0.94 (0.82, 1.06) | 0.82 (0.78, 0.86) | ||
| Other | 4961/11,592 | 1 | 1.01 (0.88, 1.16) | 0.91 (0.77, 1.06) | 1 | 1.06 (0.92, 1.22) | 0.97 (0.83, 1.14) | ||
| PIR | 0.326 | 0.783 | |||||||
| <1.0 | 1635/4156 | 1 | 0.87 (0.76, 1.00) | 0.82 (0.71, 0.95) | 1 | 1.12 (0.90, 1.39) | 0.82 (0.62, 1.08) | ||
| ≥1.0 | 6886/16,688 | 1 | 0.88 (0.71, 1.09) | 0.84 (0.65, 1.09) | 1 | 1.00 (0.89, 1.12) | 0.84 (0.72, 0.97) | ||
| Smoking status | 0.271 | 0.171 | |||||||
| Current | 1803/4527 | 1 | 1.04 (0.86, 1.27) | 0.78 (0.60, 1.03) | 1 | 1.05 (0.87, 1.27) | 0.99 (0.71, 1.38) | ||
| Never/former | 6718/16,317 | 1 | 0.83 (0.72, 0.96) | 0.82 (0.71, 0.95) | 1 | 0.99 (0.88, 1.11) | 0.89 (0.77, 1.01) | ||
| Drinking status | 0.787 | ||||||||
| Current | 5571/14,605 | 1 | 0.86 (0.75, 1.00) | 0.79 (0.68, 0.92) | |||||
| Never/former | 2950/6239 | 1 | 0.91 (0.77, 1.08) | 0.93 (0.77, 1.21) | |||||
| Physical activity | 0.884 | 0.972 | |||||||
| ≤600 MET-min/wk | 3654/8044 | 1 | 0.86 (0.74, 1.00) | 0.88 (0.73, 1.04) | 1 | 0.99 (0.84, 1.16) | 0.95 (0.79, 1.14) | ||
| >600 MET-min/wk | 4867/12,800 | 1 | 0.87 (0.76, 1.03) | 0.80 (0.67, 0.97) | 1 | 0.98 (0.86, 1.12) | 0.83 (0.70, 0.99) | ||
| BMI | 0.076 | 0.167 | |||||||
| <25 kg/m2 | 1980/6726 | 1 | 0.97 (0.79, 1.19) | 0.92 (0.73, 1.16) | 1 | 0.94 (0.75, 1.17) | 0.95 (0.75, 1.21) | ||
| ≥25 kg/m2 | 6541/14,118 | 1 | 0.82 (0.72, 0.94) | 0.74 (0.64, 0.86) | 1 | 0.96 (0.86, 1.08) | 0.78 (0.68, 0.91) | ||
| Components | Tertile for the Components | Ptrend | ||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| Fruit | 1 | 0.98 (0.87, 1.10) | 0.94 (0.84, 1.07) | 0.33 |
| Vegetables | 1 | 1.07 (0.98, 1.18) | 0.99 (0.89, 1.09) | 0.59 |
| Whole grain | 1 | 0.89 (0.80, 0.98) | 0.93 (0.81, 1.08) | 0.48 |
| Refined grain | 1 | 1.10 (0.99, 1.22) | 1.07 (0.95, 1.20) | 0.29 |
| Dairy | 1 | 1.04 (0.93, 1.17) | 1.04 (0.92, 1.17) | 0.55 |
| Nut and seeds | 1 | 0.96 (0.86, 1.07) | 0.92 (0.83, 1.02) | 0.12 |
| Legumes | 1 | 0.99 (0.88, 1.11) | 1.00 (0.86, 1.16) | 0.99 |
| Red and processed meat | 1 | 1.04 (0.94, 1.16) | 1.13 (1.02, 1.26) | 0.016 |
| Fish | 1 | 1.07 (0.92, 1.25) | 1.00 (0.86, 1.16) | 0.91 |
| Fruit juice | 1 | 1.01 (0.91, 1.13) | 0.98 (0.86, 1.13) | 0.78 |
| Alcohol—male | 1 | 0.78 (0.65, 0.94) | 0.74 (0.61, 0.90) | 0.003 |
| Alcohol—female | 1 | 0.74 (0.58, 0.94) | 0.81 (0.65, 1.01) | 0.050 |
| Total saturated fatty acids | 1 | 1.07 (0.96, 1.19) | 1.19 (1.06, 1.33) | 0.005 |
| Total monounsaturated fatty acids | 1 | 1.01 (0.91, 1.13) | 1.10 (0.99, 1.23) | 0.09 |
| Total polyunsaturated fatty acids | 1 | 1.08 (0.97, 1.21) | 0.97 (0.88, 1.08) | 0.46 |
| Sodium | 1 | 1.08 (0.96, 1.21) | 1.14 (1.00, 1.30) | 0.045 |
| Added sugar | 1 | 0.91 (0.82, 1.01) | 1.10 (0.97, 1.24) | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Zhang, L.; Zhao, Y.; Xu, M.; Tang, Q.; Chen, G.-C.; Qin, L. Adherence to the 2015–2020 Dietary Guidelines for Americans Compared with the Mediterranean Diet in Relation to Risk of Prediabetes: Results from NHANES 2007–2016. Nutrients 2023, 15, 3546. https://doi.org/10.3390/nu15163546
Wu P, Zhang L, Zhao Y, Xu M, Tang Q, Chen G-C, Qin L. Adherence to the 2015–2020 Dietary Guidelines for Americans Compared with the Mediterranean Diet in Relation to Risk of Prediabetes: Results from NHANES 2007–2016. Nutrients. 2023; 15(16):3546. https://doi.org/10.3390/nu15163546
Chicago/Turabian StyleWu, Pengcheng, Lili Zhang, Yan Zhao, Miao Xu, Quan Tang, Guo-Chong Chen, and Liqiang Qin. 2023. "Adherence to the 2015–2020 Dietary Guidelines for Americans Compared with the Mediterranean Diet in Relation to Risk of Prediabetes: Results from NHANES 2007–2016" Nutrients 15, no. 16: 3546. https://doi.org/10.3390/nu15163546
APA StyleWu, P., Zhang, L., Zhao, Y., Xu, M., Tang, Q., Chen, G.-C., & Qin, L. (2023). Adherence to the 2015–2020 Dietary Guidelines for Americans Compared with the Mediterranean Diet in Relation to Risk of Prediabetes: Results from NHANES 2007–2016. Nutrients, 15(16), 3546. https://doi.org/10.3390/nu15163546







