Abstract
Modifiable factors associated with cognitive decline (CD) require more attention, particularly dietary patterns. This study aimed to investigate the link between cognitive decline and associated factors, particularly dietary patterns (DPs), in community-dwelling older Lebanese of modest economic status. Our cross-sectional national study included 352 participants above 60 years old, from the medico-social centers of the ministry of social affairs all over the country. CD was screened based on literacy. Nutritional and dietary data were collected through a validated food frequency questionnaire. DPs were extracted by the K-mean cluster analysis. CD was found in 32.7% and 61.5% of literate and illiterate groups, respectively. Identified DPs included a Westernized type and Mediterranean type, with high and moderate food intakes. In the context of literacy, independent factors associated with CD were age above 80 years, living in Beirut, frailty, and adopting a Westernized (OR = 3.08, 95% CI: 1.22–7.8) and a high-intake Mediterranean DP (OR = 2.11, 95% CI: 1.05–4.22). In the context of illiteracy, the same factors were associated with CD, but not DP nor frailty, with an age cut-off at 78 years. In a Lebanese sample of older adults, factors associated with CD depend on the level of literacy, with DP only associated with CD in the context of literacy.
1. Introduction
Life expectancy is increasing in all countries around the world, leading to a rise in the proportion and number of older people. According to the United Nations, the proportion of people over age 65 is expected to be 16% by 2050, compared to 9% in 2019. This increase will accelerate in the coming decades, particularly in developing countries [1,2,3].
With this global trend, the search for successful aging has become an important matter to societies, especially with the rise of age-related diseases. Cognitive impairment (CI) is considered one of the most common geriatric syndromes. Its presence poses major risks to the older population including greater disability, reduced quality of life, and higher morbimortality [4].
The brain undergoes neural development until approximately the age of 30 years, after which a slow process of atrophy takes place, with clinical signs of neurodegeneration occurring at an older age [5]. Normal CD occurs at a certain pace, and some factors, whether pathological or non-pathological, affect the progression of this decline, leading to a more rapid deterioration of functions, CI, and earlier onset of dementia. The trajectory of CD to dementia is affected by the interaction of genetic, endogenous, and environmental factors [6]. Rapid CD has often been linked with frailty, and the role of nutrition in CD has also been retained by researchers as a modifiable risk factor [4,6,7,8,9,10]. Healthy lifestyles may decrease the rate of CD and improve neuroplasticity. Modifiable factors include physical activity, healthy diet, mental stimulation, avoiding excessive exposure to neurotoxins (e.g., alcohol), treating depression and managing stress, and controlling common medical conditions such as hypertension, diabetes, and obstructive sleep apnea [11].
The Lancet Commission estimates that approximately 40% of dementia cases are attributable to a combination of 12 potentially modifiable risk factors, among which are low educational level (LEL), midlife hypertension and obesity, late-life depression (with bidirectional association), hearing loss, diabetes, physical inactivity, social isolation, smoking, and diet [12]. It has been reported that skeletal muscles, by secreting neurotrophic factors, affect brain function and its motor units [13].
Specific nutrients and phytonutrients in food have been found to have a protective role in brain aging [14,15,16,17,18,19]. Indeed, low nutritional status of vitamins (B6, B9, B12) have been found to be associated with higher rates of dementia, cardiovascular disease, and stroke [15,20]. The use of vitamin K antagonists has been associated with CI and lower gray matter volume in the hippocampus in geriatric patients [16]. The antioxidant vitamins C and E, the amino acids tyrosine and tryptophan, necessary for the synthesis of dopamine and serotonin, are also involved [21], as well as long-chain ω-3 fatty acids, which are essential components of neuronal membranes [22,23,24,25,26,27,28]. Existing evidence suggests that food ingredients, in particular specific phytonutrients found in berries, coffee, tea, olive oil, and ω-3 fatty acid-rich foods, have the potential to beneficially affect cognitive function. On the other hand, trans fats and saturated fats have a detrimental effect [28,29,30,31,32,33,34].
The most promising studies focus on the link between types of diets rather than specific nutrients and the risk of CD [14,35,36]. This whole-diet approach has been described as the most optimal preventive approach and is based on the synergistic interaction between several nutrients, as suggested by findings from the Three City observational study [22]. The Mediterranean diet and its correlates, like the MIND diet, characterized by a high intake of whole cereals and legumes, fruits, vegetables, and fish, are among the best described diets, with shown protective effects among several populations [36,37,38,39,40,41,42].
Healthy diet alone or diet combined with physical activity and cognitive training are also considered beneficial cumulative lifestyle modifications that have promising results in the prevention of CD; they have the potential to improve cognitive performance and delay neurodegeneration [43].
Among countries in the Arab world, Lebanon reported in 2015 one of the highest proportion of people aged 65 years and above, and this proportion is expected to become, by 2030 and 2050, 14.0 and 23.3%, respectively [44]. In a cultural context where the family is the primary provider of both financial and social care, the age dependency ratio is expected to shift in the near future from providing for children to supporting older people [44,45,46,47,48]. With more than 50% of older adults already considered in 2015 as economically deprived, decades of political and economic turmoil, aggravated by a sharp financial decline since October 2019, the Beirut port blast, and the Covid-19 pandemic, resulted in further limiting resources for older people [44,49] and making this age group rank higher in terms of priority of healthcare for the most vulnerable.
In Lebanon, the age-standardized prevalence of dementia is 9% in individuals above 65 years of age. Such figures make dementia prevalence in this country rank high within the global range of estimates [50]. Studies exploring associated factors of CD in Lebanon are scarce [50,51,52,53] and few investigated its association with nutrition-related parameters [54]. Determining the impact of risk factors and diet on CD is therefore of high importance.
The causal link between CD and diet remains to this day inconclusive, hence the interest of this trial, given that Lebanon is one of the countries where the Mediterranean diet is part of the food heritage [14,55].
The main goals of this study are therefore to explore the association of nutritional, dietary, and other modifiable factors on CD as well as evaluate the dietary habits and nutritional intake of a national sample of older Lebanese and estimate the prevalence of CD among this age category, contributing to a better understanding of this health issue.
2. Materials and Methods
2.1. Study Design and Participants
The national, cross-sectional study was carried out from October 2017 to October 2019, in seven of the eight governorates of Lebanon, in collaboration with the Ministry of Social Affairs (MOSA) through 77 medico-social centers serving low- to middle-income families. A sample of 600 individuals was initially targeted, including individuals aged 60 years and above who were community-dwelling and attending the MOSA socio-medical centers for medical care and social assistance. Data were finally retained for 401 participants, of whom 352 (88%) had a valid Food Frequency Questionnaire (FFQ). Details of the study, recruitment of participants, and data collection are described elsewhere [56].
2.2. Cognitive Status Screening Tools
Screening and diagnosis of cognitive impairment were conducted using 2 instruments, the Adapted Arabic versions of Mini-Mental State Examination (A-MMSE) and Test des Neufs Images (A-TNI-93) [57,58]; these tools were administered to the literate and illiterate participants, respectively.
The MMSE is one of the most frequently used screening instruments for dementia. The MMSE is an 11-item assessment tool used to evaluate global cognitive performance. It has well-documented reliability and validity. Trained investigators administered the MMSE to participants, measuring 5 domains: orientation, memory, attention, verbal skills, and visuospatial reasoning for a maximum score of 30 points. It is easy to administer and takes 5 to 10 min. In our study, we used the Lebanese population-specific cut-offs for the MMSE suggested by El Hayeck et al., based on gender, age, and educational level [57].
The TNI93 is a test designed to evaluate episodic memory in illiterate population samples. Subjects are asked to learn images using the principle of encoding specificity. The TNI93 does not require the use of written language or the ability to read and can therefore be administered in any language. The duration of the test is around 10–15 min. Participants having scored at or below the population-specific cut-offs, below or equal to 6 for the TNI Free Recall or a TNI Total Recall score below or equal to 8, were considered as having low cognitive status [58,59]. Since, to our knowledge, no previous research showed interchangeable use between MMSE and TNI, and with education being an important component of cognitive reserve preservation and determinant of cognitive decline rates in elderly [50,60], participants were split into 2 groups for analysis, and data concerning CD were dichotomized based on literacy and analyzed separately.
2.3. Sociodemographic-, Health-, and Nutrition-Related Data
The 7 governorates of Lebanon were divided into 5 regions: Beirut and suburbs, Mount Lebanon, North, South, and Bekaa regions.
Literacy was evaluated based on the capacity of participants to read or write. Educational level was classified into several categories based on the obtainment of official degrees: elementary, complementary, secondary, and university diplomas. Depressive tendency was evaluated using the Arabic version of the Geriatric depression scale, the A-GDS5 [61,62]. Use of anti-depressant and anti-anxiolytic medications was reported. Data were then transformed into 2 categories. The first category included people with a tendency for depression; they either had a score of GDS5 ≥ 2 or were taking medication to treat anxiety and/or depression. The second category included people with GDS5 < 2 who were not taking any medication to treat depression or anxiety.
Other data collected are described in a previous publication [56].
Nutritional status was estimated using the Mini-Nutritional Assessment Short Form (MNA-SF) [63,64], classifying individuals into three levels: normal nutritional status (score above 12), at risk of malnutrition (scores 8–11), and malnutrition (scores below 8). Participants were then classified into two categories. The first category, called “poor nutritional status”, included participants that were considered malnourished and at risk of malnutrition by the MNA-SF, and the second category, called “normal nutritional status”, included participants who had a normal nutritional status according to MNA-SF.
Nutrient intake and dietary patterns were extracted from the validated FFQ [64]. Calculation of the Nutrient Adequacy ratio (NAR) of 17 selected nutrients was computed. Nutrients included vitamins A, D, E, K, C, B1, B2, B3, B6, B9, B12, and minerals such as calcium, phosphorus, magnesium, iron, selenium, and zinc. The NAR was calculated by dividing the estimated nutrient intake of individuals by the age and sex-specific recommended dietary allowance (RDA) for these nutrients, according to the established dietary reference intake (DRI) recommendations [64,65]. For all nutrients, RDA values were used, except for vitamin K, where adequate intake (AI) was used as the DRI value for comparison.
To extract dietary patterns, the 90 foods listed in the FFQ were then grouped into 20 predefined categories based on similarities in nutrient composition and consumption characteristics. After running the cluster analysis, three dietary patterns were identified in the total sample. The first, called the Westernized-type dietary pattern (WDP), was characterized by the highest caloric intake, consumption of refined flour products, sugar and sweets, dairy products, as well as processed and saturated fats and the lowest olive, seed, and oleaginous fruit and whole cereal product intake. The second pattern, called the high intake/Mediterranean-type dietary pattern (HIMEDDP), was characterized by a relatively high caloric intake, a higher consumption of vegetables, fruits, legumes, than the other 2 DPs, and the highest consumption of foods rich in monounsaturated fats. Median consumption of olive, seeds, and oleaginous fruits in the HI-MEDDP group was above nine teaspoons of oil equivalent per day. This pattern also had the lowest consumption of refined flour products, and the highest consumption of whole cereal products. Finally, the third pattern, called the moderate intake/Mediterranean-type dietary pattern (MOD-MEDDP), was characterized by a diversified and balanced DP. Consumption of most foods in this pattern was either intermediate or lower compared to the other two patterns, with the lowest consumption of sweets and sugar among the three patterns [56].
2.4. Statistical Analysis
Statistical analysis was performed using the SPSS program version 21.0. For numerical variables, data were expressed as mean ± standard deviation and median (interquartile ranges), and as numbers and percentages, for categorical variables.
Differences between cognitive status characteristics for sociodemographic, nutritional, dietary, health-related, and anthropometric data were compared using the non-parametric tests, Mann–Whitney and Kruskal–Wallis for numeric variables that are not normally distributed, and chi-square test for categorical variables. K-means clustering was used to regroup participants with similar dietary patterns. Differences in food intake between dietary patterns were evaluated by Kruskal–Wallis test.
Cut-offs for age, polypharmacy, multimorbidity, age-related conditions, and waist-to-height ratio (WTHR) were determined through a classification tree and used for subsequent multivariate analysis. Age cut-offs at 80 years and 78 years were determined, respectively, for the literate and illiterate groups for a specificity of >80%. In the multivariate analyses, we proceeded to several models of binary logistic regression using the dichotomized CS variable as the dependent variable. Odds ratios (ORs) with 95% confidence intervals were calculated. The main explanatory variables reaching significance level identified in each model were added to the following model. In the multivariate analysis, age above 80 years and 78 years for the literate and illiterate groups, respectively, level of education (more than 13 years) (only for the literate group), female gender, living conditions, marital status, income, and living in Beirut were entered first (model 1). From this model, we retained covariates significantly associated with CD and then added anthropometric and health-related variables, including multimorbidity, polypharmacy, frailty, presence of age-related conditions, health perception, poor mental health, BMI categories, WTHR above 0.718, malnutrition, and level of physical activity (model 2). From model 2, we retained all covariates significantly associated with CD and then added the 3 dietary patterns (model 3).
The level of significance was fixed at p = 0.05, unless otherwise noted, for all analyses.
3. Results
3.1. Description of the Total Sample
Characteristics of the total sample population were published elsewhere [56].
Three types of dietary patterns were identified in the total sample: a Westernized-type dietary pattern (WDP), adopted by 11.9% of the participants, a high intake/Mediterranean-type dietary pattern (HI-MEDDP), followed by 23% of participants, and a third pattern, called the moderate intake/Mediterranean-type dietary pattern (MOD-MEDDP), representing the highest proportion of our sample (65.1%). Details of DPs are published elsewhere [56].
3.2. Cognitive Status and Characteristics of the Sample Based on Literacy
The total number of participants scoring low on either cognitive test (MMSE and TNI) was 145 (41.2%). The illiterate group accounted for 29.5% of the sample population and the literate group for 70.5%. In the total sample, the group with low cognitive results, compared to the one with good cognitive results, had more than double the proportion of people aged 80 and above, with 29% and 12.1% respectively, a higher proportion of dependency (24.1% vs. 10.2%), malnourishment (9.7% vs. 2.4%), and frailty (23.4% vs. 9.2%), and a higher proportion of participants with more than one age-related condition (60.7% vs. 48.8%), and a lower proportion of good health perception (31.3% vs. 50.2%). No gender difference was observed in the prevalence of CI (Supplementary Table S1).
The characteristics of literate and illiterate participants reflect almost the same characteristics as the total sample. The characteristics of the participants according to their cognitive test results are presented based on literacy level in Table 1 and Table 2.

Table 1.
Socio-demographic characteristics of participants based on cognitive status, for the literate and illiterate groups.

Table 2.
Health characteristics of participants based on cognitive status, for the literate and illiterate groups.
Low CS was found in 32.7% and 61.5% of the literate and illiterate groups, respectively.
When only participants with low CS were compared based on literacy (Supplementary Table S2), illiterate participants with CI were older, with a mean age of 76.5 and 72 years, had a higher proportion of women (62.5% vs. 40.7% p = 0.009) and of participants considering their income insufficient income (70.3% vs. 53.1% p = 0.035), a higher proportion of high WHTR (>0.718) (34.9% vs. 10.7%), and poorer mental status (71.55 vs. 55% p = 0.038). The illiterate group with CI also had a higher proportion having more than one disease, age-related conditions, and poor self-rated health.
The characteristics of participants living in the capital vs. the rest of the regions are found in Supplementary Tables S3–S6. The regional distribution of cognitive status is shown in Figure 1.
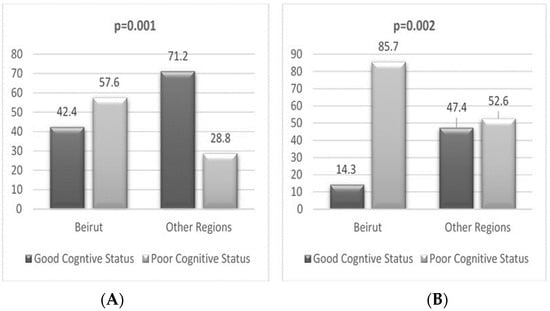
Figure 1.
Regional distribution of participants based on cognitive status for the literate (A) and illiterate (B) groups.
3.3. Cognitive Status and Nutrient Adequacy
In the total sample as well as in the literate and illiterate groups analyzed separately, NARs were found to be inadequate for Ca, vit D, and ω3-FA, for both levels of CS. No difference was found in the prevalence of inadequacy between the two levels of CS except for vitamin K and fiber in the group of illiterate, where the group with low CS had a significantly higher proportion of inadequacy than the group with good CS, with 19% vs. 2.5% and 51.6% vs. 27.5%, respectively, for vitamin K and fiber intakes, respectively.
3.4. Cognitive Status and Food Intake
In Supplementary Table S7, when comparing the two levels of CS, in the literate group, results were similar to those described in the total sample regarding the food intakes, showing a significant difference in low-fat sweet intake and sugars and jam portions. In the illiterate group, no difference was found in food intake between participants with low and good CS.
3.5. Dietary Patterns and Associated Factors
The multivariate logistic regression analysis performed in the total sample included 352 individuals with a complete set of data each and fulfilling all criteria of inclusion (Table 3). In the first model, the only socio-demographic factors associated with low cognitive status (LCS) included age above 80 years and 78 years, for the literate and illiterate participants, respectively, and living in Beirut. In the second model, in addition to previous factors, anthropometric and health-related factors associated with CD were frailty and bad health perception. In the final model, by adding dietary patterns, independent factors associated with CD were advanced age, living in Beirut, bad health perception, and adopting a HI-MEDDP as compared to MOD-MEDDP pattern.

Table 3.
Binary logistic regression models for low cognitive status vs. good cognitive status in the total sample (N = 352).
The same regression analysis was performed in the literate and illiterate sub-samples including 248 and 104 participants, respectively (Table 4 and Table 5).

Table 4.
Binary logistic regression models for low cognitive status vs. good cognitive status in the literate sub-sample (N = 248).

Table 5.
Binary logistic regression models for low cognitive status vs. good cognitive status in the illiterate sub-sample (N = 104).
Within the literate sample (Table 4), in the first model, factors associated with low cognitive status (LCS) included age above 80 years and living in Beirut. In the second model, the only anthropometric and health-related parameters that were found to be associated with low cognitive status were frailty and BMI above 32 kg/m2. In the final model, by adding dietary patterns, independent factors associated with CD were age above 80 years, living in Beirut, frailty, as well as adopting WDP and HI-MEDDP patterns, as compared to the MOD-MEDDP pattern.
In the illiterate group, the same binary regression analysis performed (Table 5) showed in the first model that factors associated with LCS included age above 78 years, living in Beirut, and living conditions. In the second model, no anthropometric nor health-related parameters were found to be associated with LCS. In the final model, by adding dietary patterns, the factors associated with CD remained: above 78 years, living in Beirut, and living alone as compared to living with partners, with no association with dietary patterns adopted.
4. Discussion
This study showed no gender difference regarding cognitive impairment. In the total sample, factors associated with low cognitive test results were advanced age, living in Beirut, living alone compared with living with a partner, and bad health perception. Only adopting a HI-MEDDP was found associated with LCS. In the literate group, in addition to being 80 years old and above and living in Beirut, being frail and following a WDP and HI-MEDDP were also found to be independent factors associated with CD. In the illiterate group, identified independent factors associated with CD were being 78 years old and above, living in the capital, and living alone compared to living with partners.
This study suggests that age remains the strongest factor related to CD. Our results pointed out that age, above 80 years for the literate and 78 years for the illiterate, remains one of the most important non-modifiable risk factors related to CD, as already previously established [11,66].
The study findings did not show any difference in prevalence of CD between gender. With a higher proportion of illiteracy in women, the TNI test used in this study for illiterate participants only measures periodic memory and does not evaluate other domains of cognition like the MMSE, possibly underestimating CD in this category of participants [59,66]. According to the literature, female gender is another prominent risk factor described to be related to CD. Most studies reported that women are more prone to CD than men, mostly because they live longer and have lower educational and social attainment levels [12,50]. Furthermore, a meta-analysis pinpointed that reporting gender difference in the prevalence of CD must be taken with caution, and to better understand such a difference, especially in the early stages, a better knowledge of the type of CI should be made [57,67].
Differences existed between the literate and illiterate groups, suggesting that in the context of low cognitive reserve, risk factors might be different. Having a higher cognitive reserve, as observed previously with higher educational attainments, could be considered a protective factor for cognitive status and slow the age-related cognitive decline observed with aging, by possibly delaying the decline by 2 years [11,66]. Furthermore, DP does not impact CS like social status and social interactions, and particularly age. The latter overrides the impact of other factors, particularly DP [57,68,69]; in the illiterate group, DPs were not found to be associated with CD. In the literate group, although educational level did not stand as an independent associated factor with CD, some modifiable factors like frailty and dietary patterns were found to be associated with CD. This result could imply that in the context of literacy, i.e., with a higher cognitive reserve, possible modifiable factors such as frailty and diet can be worked upon to possibly change the course of CD. Nevertheless, further research should be tailored to better understand the role of education as a mediating factor for these modifiable factors and clarify the role of education, using a unified detection tool and different study design. Previous data in Lebanon, describing the importance of education and occupational attainment on cognitive reserve, suggested that reaching higher levels of education, engaging in complex occupations, and participating in more cognitively stimulating leisure activities enhance the cognitive reserve and allow older adults who attained high educational levels to be 7.1 times more likely to have better global cognitive function than others who attained lower education [50]. Cognitive reserve, characterized by neuro-elasticity, is the result of lifetime intellectual activities and environmental factors, allowing individuals with high cognitive reserve levels to better cope with the same amount of brain damage as opposed to individuals with lower levels [69]. Living alone compared to living with partners showed to be associated with CD in the illiterate group. Previous findings pointed out the importance of social interaction on the prevalence of CI [70,71].
Living in Beirut was one of the identified independent factors associated with CD in both groups of literacy. In contrast to most studies describing the association of urban vs. rural living in relation to CD, our study showed that living in Beirut increased the odds of CD by 5-fold. Participants living in Beirut were found to have the highest prevalence of multi-morbidity, age-related conditions, malnutrition, and insufficient income. They also had the highest proportion of inadequate nutrient intakes, also previously reported to be related to CD, like vitamin A, E, B9, Mg, and Ca. These parameters could be considered as confounding factors when comparing rural vs. urban prevalence of CD, since each of these factors is described by the literature as a risk factor for CD [7,72,73,74,75,76,77,78]. Some previous findings showed that rates of dementia among urban dwellers tended to be lower than those of rural dwellers. Theories explaining this difference focus on better access to higher-quality education, as well as public and health services among urban dwellers [51,70,79], but contextualization of the findings to Lebanon where older adults lack health coverage and retirement plans [48] would allow a better understanding of results. These findings are an opportunity to highlight the importance of directing the attention of healthcare not only to rural areas but also to urban areas with socio-economic difficulties.
Our study failed to find an association between nutrient intake and CD. Previous reports have documented the role of specific vitamins and nutrients as having a protective effect on CD. Vitamins B6, B9, B12, Mg, vitamin D, and W3-FA are among the studied nutrients [78,80,81,82].
Our study results pinpointed the difference in the prevalence of inadequate intakes of vitamins in the illiterate group with CI compared to the group with GCS, showing a possible role of sub-optimal intake of vitamin K in the development of CI. Vitamin K has been previously shown to be involved in the synthesis of sphingolipids, a major constituent of the myelin sheath, and in neuronal survival thanks to vitamin K-dependent proteins (VKDP proteins). The use of vitamin K antagonists has been associated with CI and lower gray matter volume in the hippocampus in geriatric patients [16].
Regarding food intake, in the literate group, in addition to intakes of low-fat sweets, a significant difference was observed with intakes of sugars and jams, as absolute quantity or relative per 1000 Cal. These findings were confirmed by the association of the WDP characterized by higher intakes of sugars. Mitigated results regarding the impact of sugar-sweetened foods and lower CS were reported in the literature. In a study using data from NHANES, high consumption of added sugar, above the 75th percentile of the sample population intake, was found to be associated with low CS (OR = 1.509, 95% CI: 1.109–2.052) [83]. Another study linked intake of total sugar, sugar-sweetened beverages, but not sugar-sweetened foods, to higher cognitive decline [84]. In the context of high BMI and high WTHR ranges, a high intake of sugar could be implicated in the physiopathology of development of CD, especially given the fact that sugar intake is described to be related to metabolic syndrome and cardiovascular diseases, described to have an association with CD [72]. Our study has paved the way to defining a possible threshold for sugar intake in relation to CD, making added sugar intake a new target for future studies as an associated factor for CD in a Mediterranean context.
Our main findings in the literate group reported that the adoption of a WDP is associated with 3-fold the odds of CD and a HI-MEDDP is associated with almost 2-fold the odds of CD, when compared to MOD-MEDDP. Some specific food components and specific nutrients were previously implicated and affect the course of development of CD. The underlying mechanisms by which they attenuate CI can be attributed to modulation of gut microbiota, to antioxidant and anti-inflammatory components, as well as the synergistic effect of food components in these DPs [35,38,41,85]. Several food combinations in specific dietary patterns have been studied in the context of cognitive decline, from MCI to dementia, in various settings, with some exhibiting favorable results and others showing no evidence of such effects. Previous studies associated specific dietary patterns like the Mediterranean diet (MedDiet) [37,86,87], dietary approaches to stop hypertension (DASH) diet [41], Mediterranean-DASH diet intervention for neurological delay (MIND), with the prevention of CD [35,41]. Among the most-studied DP is the Mediterranean diet and its correlates such as the MIND diet, the DASH diet, and the Healthy diet [19,34,36,42,88,89]. In the 3 C- city cohort, Bordeaux chapter, over 5 years follow up, higher adherence to the Mediterranean diet was associated with slightly slower MMSE decline but not with other measures of cognitive decline. The Mediterranean diet in the presence of other contributing factors is a better protector against decline than Med diet DP alone [36]. A major constituent of the Mediterranean diet is olive oil. Components in the olive oil, such as oleocanthal, have an anti-inflammatory effect, considered to be part of the beneficial effect of the Med diet, and other pro-inflammatory components, such as found in a Westernized-type DP, are considered to negatively affect CS [88,90,91,92,93].
Finally, a major factor associated with CD was frailty in the literate group, with frailty increasing the odds of CD by 4-fold. Whether CD causes increased frailty or frailty increases the risk of CD is not yet clearly understood in the literature. In the context of literacy, our findings suggest an association between frailty and CD, whereas in our analysis on frailty, educational level was not taken into consideration. Older adults with both physical frailty and CI are shown to be at higher risk of adverse health outcomes, including death, disability, hospitalization, and dementia, than those with either condition alone. Such findings could pave the way to the concept of cognitive frailty described in the literature and defined according to specific diagnostic criteria [4,6,94,95]. While the underlying mechanisms of cognitive frailty are still unclear, some factors were previously shown to be associated with cognitive frailty, such as sociodemographic factors, nutritional status, physical and cognitive activities, functional status, comorbidities, medication use, and gut-derived metabolites [10,95,96]. Further studies, taking into consideration the social determinants of health while focusing on the role of DP in the course of cognitive frailty, are required to clarify the mechanisms through which physical frailty and CI would interact particularly in the context of socio-economic insecurity in Lebanon.
Limitations
We noted some limitations to this study. Selection and reporting bias might be limiting factors in reporting diseases. Low intake of some nutrients and vitamins in the total sample, such as vitamin D and W3, hindered the detection of impact of potential nutritional factors in relation to CD. Furthermore, lack of consistency among participants in reporting intakes of vitamins and minerals supplements caused the exclusion of this source of nutrients in the intake analysis, which might have an impact on outcome. Having a small number of participants adopting the WDP-DP could have also been a possible bias. Larger prospective studies are therefore required to further investigate the impact of dietary patterns on risk of CD with a special emphasis on sugar intake and BMI ranges, with equal distribution among DPs. Unifying screening tools in the detection of CI would also allow for more comparable results in relation to CI and help better define the role of education with this regard. A possible way of evaluating cognitive performance would be through clinical dementia rating (CDR) as a more precise tool to evaluate CI. The Washington University Clinical Dementia Rating (CDR) is a global scale frequently used in clinical and research settings to describe stage-dependent features of dementia and in clinical drug trials for measuring clinically meaningful changes. The clinical protocol incorporates interviews to obtain information necessary to rate the subject’s cognitive performance in six domains: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care [60].
5. Conclusions
In summary, our study was the first to explore the association between CD and specific dietary patterns extracted in a posteriori method, in adults over 60 years of age in Lebanon, using specifically validated questionnaires. Despite the difficulties in addressing this specific age group, we succeeded in showing that age, frailty, and adopting a Westernized-type DP, and high sugar intake DP remain the main associated factors related to CD in literate low-socioeconomic settings. Although our results did not evaluate CD through clinical dementia rating (CDR), they support the newly developed concept of cognitive frailty, defined as the presence of frailty concomitantly with cognitive decline (evaluated by the CDR). Most importantly, we demonstrated that in a literate group, adopting a WDP pattern with high sugar consumption and a HI-MEDDP were also linked to CD, and that a more moderate Mediterranean-like pattern was protective. Results also pinpointed a possible mediating effect of education on people’s susceptibility to risk factors, possibly overriding the effect of diet on the progress of CD. Illiteracy favors other factors such as living conditions, but future research would need to explore this. Results directed the attention to individuals living in the capital as being more vulnerable than those living in the rural areas and suburbs. Multi-domain preventive recommendations on a national level would need to tackle social determinants of health, particularly in a low socio-economic context, whether in the urban or rural areas, in addition to tackling prevention of age-related non-communicable diseases, in which diet is a pillar in prevention.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15183911/s1, Table S1: Characteristics of the total sample based on level of cognitive status; Table S2. Comparison between characteristics of literate and illiterate with low CS; Table S3: Health and functional status of participants living in Beirut and participants living in the other regions of Lebanon; Table S4. Nutritional & anthropometric characteristics: comparison between living in Beirut vs. living in other regions; Table S5. Comparison of nutrient intakes between participants living in Beirut vs. participants living in other regions; Table S6: Socio-demographic characteristics of participants living region in Beirut and participants living in the other regions of Lebanon; Table S7. Comparison of food consumption and dietary patterns based on cognitive status and level of literacy.
Author Contributions
Conceptualization and methodology, N.Y. and C.B.; validation, M.A., C.B. and C.Y.; formal analysis, C.Y. and N.Y.; data curation, N.Y. and R.E.H.; funding acquisition, M.A.; writing—original draft preparation, N.Y.; review and editing, M.A., C.Y. and R.E.H.; supervision, C.B.; project administration, N.Y. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Council of the Saint Joseph University of Beirut, grant number FPH66.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of SAINT JOSEPH UNIVERSITY (USJ 2016–99).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Informed consent was obtained from all subjects involved in the study.
Acknowledgments
We would like to thank Fernande Abou Haidar, Samar Sleilati, Marie Elias, and all healthcare workers of the Ministry of Social Affairs/Department of Family Affairs, as well as Rafic Baddoura for their valuable efforts and collaboration. A special appreciation to all participants for their contribution.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations. World Population Prospects 2019—Volume II Demog. [Internet]. 2020. Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Volume-II-Demographic-Profiles.pdf (accessed on 23 June 2020).
- Department of Economic and Social Affairs, Population Division. World Population Ageing, 2019 Highlights; United Nations: New York, NY, USA, 2020.
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2017 Highlights. [Internet]. 2017. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf (accessed on 1 November 2021).
- Fabrício, D.d.M.; Chagas, M.H.N.; Diniz, B.S. Frailty and cognitive decline. Transl. Res. 2020, 221, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. Selective review of cognitive aging. J. Int. Neuropsychol. Soc. 2010, 16, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gómez, M.E.; Zapico, S.C. Frailty, Cognitive Decline, Neurodegenerative Diseases and Nutrition Interventions. Int. J. Mol. Sci. 2019, 20, 2842. [Google Scholar] [CrossRef] [PubMed]
- González-Gross, M.; Marcos, A.; Pietrzik, K. Nutrition and cognitive impairment in the elderly. Br. J. Nutr. 2001, 86, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. The New Geriatric Giants. Clin. Geriatr. Med. 2017, 33, xi–xii. [Google Scholar] [CrossRef]
- Robertson, D.A.; Savva, G.M.; Kenny, R.A. Frailty and cognitive impairment—A review of the evidence and causal mechanisms. Ageing Res. Rev. 2013, 12, 840–851. [Google Scholar] [CrossRef]
- Sugimoto, T.; Sakurai, T.; Ono, R.; Kimura, A.; Saji, N.; Niida, S.; Toba, K.; Chen, L.-K.; Arai, H. Epidemiological and clinical significance of cognitive frailty: A mini review. Ageing Res. Rev. 2018, 44, 1–7. [Google Scholar] [CrossRef]
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Wu, B.; Lyu, Y.B.; Cao, Z.J.; Wei, Y.; Shi, W.Y.; Gao, X.; Zhou, J.H.; Kraus, V.B.; Zhao, F.; Chen, X.; et al. Associations of Sarcopenia, Handgrip Strength and Calf Circumference with Cognitive Impairment among Chinese Older Adults. Biomed. Environ. Sci. 2021, 34, 859–870. [Google Scholar]
- Allès, B.; Samieri, C.; Féart, C.; Jutand, M.-A.; Laurin, D.; Barberger-Gateau, P. Dietary patterns: A novel approach to examine the link between nutrition and cognitive function in older individuals. Nutr. Res. Rev. 2012, 25, 207–222. [Google Scholar] [CrossRef]
- Bottiglieri, T. Folate, vitamin B12, and neuropsychiatric disorders. Nutr. Rev. 1996, 54, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Chouet, J.; Ferland, G.; Feart, C.; Rolland, Y.; Presse, N.; Boucher, K.; Barberger-Gateau, P.; Beauchet, O.; Annweiler, C. Dietary Vitamin K Intake Is Associated with Cognition and Behaviour among Geriatric Patients: The CLIP Study. Nutrients 2015, 7, 6739–6750. [Google Scholar] [CrossRef] [PubMed]
- Feart, C.; Letenneur, L.; Helmer, C.; Samieri, C.; Schalch, W.; Etheve, S.; Delcourt, C.; Dartigues, J.-F.; Barberger-Gateau, P. Plasma Carotenoids Are Inversely Associated with Dementia Risk in an Elderly French Cohort. J. Gerontol. Ser. A 2015, 71, 683–688. [Google Scholar] [CrossRef]
- Marseglia, A.; Xu, W.; Fratiglioni, L.; Fabbri, C.; Berendsen, A.A.M.; Bialecka-Debek, A.; Jennings, A.; Gillings, R.; Meunier, N.; Caumon, E.; et al. Effect of the NU-AGE Diet on Cognitive Functioning in Older Adults: A Randomized Controlled Trial. Front. Physiol. 2018, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and Prevention of Cognitive Impairment. The Lancet Neurology. [Internet]. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1474442218303387 (accessed on 13 October 2018).
- Selhub, J.; Troen, A.; Rosenberg, I.H. B vitamins and the aging brain. Nutr. Rev. 2010, 68, S112–S118. [Google Scholar]
- Cooper, J.K. Nutrition and the brain: What advice should we give? Neurobiol. Aging 2014, 35, S79–S83. [Google Scholar] [CrossRef]
- Allès, B.; Samieri, C.; Jutand, M.-A.; Carmichael, P.-H.; Shatenstein, B.; Gaudreau, P.; Ferland, G.; Barberger-Gateau, P.; Laurin, D. Nutrient Patterns, Cognitive Function, and Decline in Older Persons: Results from the Three-City and NuAge Studies. Nutrients 2019, 11, 1808. [Google Scholar] [CrossRef]
- Barberger-Gateau, P.; Lambert, J.-C.; Féart, C.; Pérès, K.; Ritchie, K.; Dartigues, J.-F.; Alpérovitch, A. From Genetics to Dietetics: The Contribution of Epidemiology to Understanding Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 33, S457–S463. [Google Scholar] [CrossRef]
- Barberger-Gateau, P.; Samieri, C.; Feart, C.; Plourde, M. Dietary Omega 3 Polyunsaturated Fatty Acids and Alzheimers Disease: Interaction with Apolipoprotein E Genotype. Curr. Alzheimer Res. 2011, 8, 479–491. [Google Scholar] [CrossRef]
- Barnard, N.D.; Bush, A.I.; Ceccarelli, A.; Cooper, J.; de Jager, C.A.; Erickson, K.I.; Fraser, G.; Kesler, S.; Levin, S.M.; Lucey, B.; et al. Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiol. Aging 2014, 35, S74–S78. [Google Scholar] [CrossRef] [PubMed]
- Rathod, R.; Kale, A.; Joshi, S. Novel insights into the effect of vitamin B12 and omega-3 fatty acids on brain function. J. Biomed. Sci. 2016, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Féart, C.; Letenneur, L.; Dartigues, J.-F.; Pérès, K.; Auriacombe, S.; Peuchant, E.; Delcourt, C.; Barberger-Gateau, P. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am. J. Clin. Nutr. 2008, 88, 714–721. [Google Scholar] [CrossRef]
- Barnard, N.D.; Bunner, A.E.; Agarwal, U. Saturated and trans fats and dementia: A systematic review. Neurobiol. Aging 2014, 35, S65–S73. [Google Scholar] [CrossRef] [PubMed]
- Ancelin, M.-L.; Ripoche, E.; Dupuy, A.-M.; Samieri, C.; Rouaud, O.; Berr, C.; Carrière, I.; Ritchie, K. Gender-specific associations between lipids and cognitive decline in the elderly. Eur. Neuropsychopharmacol. 2014, 24, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Plourde, M.; Pifferi, F.; Bégin, M.; Féart, C.; Barberger-Gateau, P. Fish, docosahexaenoic acid and Alzheimer’s disease. Prog. Lipid Res. 2009, 48, 239–256. [Google Scholar] [CrossRef]
- Parkinson, L.; Keast, R. Oleocanthal, a Phenolic Derived from Virgin Olive Oil: A Review of the Beneficial Effects on Inflammatory Disease. Int. J. Mol. Sci. 2014, 15, 12323–12334. [Google Scholar] [CrossRef]
- Parkinson, L.; Cicerale, S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef]
- Samieri, C.; Jutand, M.-A.; Féart, C.; Capuron, L.; Letenneur, L.; Barberger-Gateau, P. Dietary Patterns Derived by Hybrid Clustering Method in Older People: Association with Cognition, Mood, and Self-Rated Health. J. Am. Diet. Assoc. 2008, 108, 1461–1471. [Google Scholar] [CrossRef]
- Marcason, W. What Are the Components to the MIND Diet? J. Acad. Nutr. Diet. 2015, 115, 1744. [Google Scholar] [CrossRef] [PubMed]
- Féart, C.; Samieri, C.; Rondeau, V.; Amieva, H.; Portet, F.; Dartigues, J.F.; Scarmeas, N.; Barberger-Gateau, P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009, 302, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, C.A.; Yannakoulia, M.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Arampatzi, X.; Bougea, A.; Labropoulos, I.; Scarmeas, N. Mediterranean diet and cognitive health: Initial results from the Hellenic Longitudinal Investigation of Ageing and Diet. PLoS ONE 2017, 12, e0182048. [Google Scholar] [CrossRef]
- Capurso, C.; Bellanti, F.; Buglio, A.L.; Vendemiale, G. The Mediterranean Diet Slows Down the Progression of Aging and Helps to Prevent the Onset of Frailty: A Narrative Review. Nutrients 2019, 12, 35. [Google Scholar] [CrossRef]
- Pelletier, A.; Barul, C.; Féart, C.; Helmer, C.; Bernard, C.; Periot, O.; Dilharreguy, B.; Dartigues, J.; Allard, M.; Barberger-Gateau, P.; et al. Mediterranean diet and preserved brain structural connectivity in older subjects. Alzheimer’s Dement. 2015, 11, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A. Epigenetics of Mediterranean Diet: Altering Disease Risk. In Mediterranean Diet. Nutrition and Health; Humana Press: Cham, Switzerland, 2016; pp. 203–216. [Google Scholar] [CrossRef]
- Talegawkar, S.A.; Jin, Y.; Simonsick, E.M.; Tucker, K.L.; Ferrucci, L.; Tanaka, T. The Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet is associated with physical function and grip strength in older men and women. Am. J. Clin. Nutr. 2022, 115, 625–632. [Google Scholar] [CrossRef]
- Talegawkar, S.A.; Bandinelli, S.; Bandeen-Roche, K.; Chen, P.; Milaneschi, Y.; Tanaka, T.; Semba, R.D.; Guralnik, J.M.; Ferrucci, L. A Higher Adherence to a Mediterranean-Style Diet Is Inversely Associated with the Development of Frailty in Community-Dwelling Elderly Men and Women. J. Nutr. 2012, 142, 2161–2166. [Google Scholar] [CrossRef]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef]
- Abdulrahim, S.; Ajrouch, K.J.; Antonucci, T.C. Aging in Lebanon: Challenges and Opportunities. Gerontol. 2015, 55, 511–518. [Google Scholar] [CrossRef]
- Economic and Social Commission for Western Asia (ESCWA). Ageing in ESCWA Member States: Third Review and Appraisal of the Madrid International Plan of Action on Ageing; ESCWA: Beirut, Lebanon; p. 83.
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2020 Highlights: Living Arrangements of Older Persons; United Nations: New York, NY, USA, 2020.
- Faour, M.A. Religion, demography, and politics in Lebanon. Middle East. Studies 2007, 43, 909–921. [Google Scholar] [CrossRef]
- ESCWA. Launch of the National Strategy for Older Persons in Lebanon 2020–2030. Available online: http://www.unescwa.org/news/launch-national-strategy-older-persons-lebanon-2020-2030 (accessed on 25 October 2021).
- Pruchno, R. International Aging: Spotlighting the Spotlights. Gerontologist 2017, 57, 392–395. [Google Scholar] [CrossRef]
- Darwish, H.; Farran, N.; Assaad, S.; Chaaya, M. Cognitive Reserve Factors in a Developing Country: Education and Occupational Attainment Lower the Risk of Dementia in a Sample of Lebanese Older Adults. Front. Aging Neurosci. 2018, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Phung, K.T.T.; Chaaya, M.; Prince, M.; Atweh, S.; El Asmar, K.; Karam, G.; Khoury, R.M.; Ghandour, L.; Ghusn, H.; Nielsen, T.R.; et al. Dementia prevalence, care arrangement and access to care in lebnon: A pilot study. Alzheimers Dement. 2017, 13, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Assaf, G.; Tanielian, M. Mild cognitive impairment in primary care: A clinical review. Postgrad. Med. J. 2018, 94, 647–652. [Google Scholar] [CrossRef]
- Hayek, M.; Tarabey, L.; Abou-Mrad, T.; Fadel, P.; Abou-Mrad, F. Normative Data for the Montreal Cognitive Assessment in a Lebanese Older Adult Population. J. Clin. Neurosci. 2020, 74, 81–86. [Google Scholar] [CrossRef]
- Boulos, C.; Salameh, P.; Barberger-Gateau, P. Factors associated with poor nutritional status among community dwelling Lebanese elderly subjects living in rural areas: Results of the AMEL study. J. Nutr. Health Aging 2014, 18, 487–494. [Google Scholar] [CrossRef]
- Jomaa, L.; Hwalla, N.; Itani, L.; Chamieh, M.C.; Mehio-Sibai, A.; Naja, F. A Lebanese dietary pattern promotes better diet quality among older adults: Findings from a national cross-sectional study. BMC Geriatr. 2016, 16, 85. [Google Scholar] [CrossRef]
- Yaghi, N.; Yaghi, C.; Abifadel, M.; Boulos, C.; Feart, C. Dietary Patterns and Risk Factors of Frailty in Lebanese Older Adults. Nutrients 2021, 13, 2188. [Google Scholar] [CrossRef]
- El-Hayeck, R.; Baddoura, R.; Wehbé, A.; Bassil, N.; Koussa, S.; Khaled, K.A.; Richa, S.; Khoury, R.; Alameddine, A.; Sellal, F. An Arabic Version of the Mini-Mental State Examination for the Lebanese Population: Reliability, Validity, and Normative Data. J. Alzheimer’s Dis. 2019, 71, 525–540. [Google Scholar] [CrossRef]
- El-Hayeck, R.; Baddoura, R.; Wehbé, A.; Bassil, N.; Koussa, S.; Khaled, K.A.; Richa, S.; Khoury, R.; Alameddine, A.; Sellal, F. An adapted Arabic version of the Test of Nine Images for the illiterate Lebanese population: Validation and preliminary normative data. J. Int. Neuropsychol. Soc. 2023, 29, 316–323. [Google Scholar] [CrossRef]
- Maillet, D.; Matharan, F.; Le Clésiau, H.; Bailon, O.; Pérès, K.; Amieva, H.; Belin, C. TNI-93: A New Memory Test for Dementia Detection in Illiterate and Low-Educated Patients. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2016, 31, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Júnior, J.W.L.; de Souza, A.C.C.; Alves, G.S.; Bonfadini, J.d.C.; Siqueira-Neto, J.I.; Braga-Neto, P. Cognitive Assessment Tools for Screening Older Adults with Low Levels of Education: A Critical Review. Front. Psychiatry 2019, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- Hallit, S.; Hallit, R.; Boulos, C.; Hachem, D.; Nasra, M.C.; Kheir, N.; Salameh, P. Validation of the Arabic Geriatric Depression Scale (GDS-5) among the Lebanese Geriatric Population. J. Psychopathol. 2017, 23, 87–90. [Google Scholar]
- Hoyl, M.T.; Alessi, C.A.; Harker, J.O.; Josephson, K.R.; Pietruszka, F.M.; Koelfgen, M.; Mervis, J.R.; Fitten, L.J.; Rubenstein, L.Z. Development and Testing of a Five-Item Version of the Geriatric Depression Scale. J. Am. Geriatr. Soc. 1999, 47, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y. The mini nutritional assessment (MNA®) review of the literature-what does it tell us? J. Nutr. Health Aging 2006, 10, 466. [Google Scholar]
- Yaghi, N.; Boulos, C.; Baddoura, R.; Abifadel, M.; Yaghi, C. Validity and reliability of a food frequency questionnaire for community dwelling older adults in a Mediterranean country: Lebanon. Nutr. J. 2022, 21, 40. [Google Scholar] [CrossRef]
- Dietary Reference Intakes: The Essential Guide to Nutrient Requirements|The National Academies Press [Internet]. Available online: https://www.nap.edu/download/11537 (accessed on 5 February 2021).
- Harada, C.N.; Natelson Love, M.C.N.; Triebel, K.L. Normal Cognitive Aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef]
- Au, B.; Dale-McGrath, S.; Tierney, M.C. Sex differences in the prevalence and incidence of mild cognitive impairment: A meta-analysis. Ageing Res Rev. 2017, 35, 176–199. [Google Scholar] [CrossRef]
- Miquel, S.; Champ, C.; Day, J.; Aarts, E.; Bahr, B.A.; Bakker, M.; Bánáti, D.; Calabrese, V.; Cederholm, T.; Cryan, J. Poor cognitive ageing: Vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res. Rev. 2018, 42, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. Cognitive Reserve: Implications for Assessment and Intervention. Folia Phoniatr. et Logop. 2013, 65, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-R.; Xu, W.; Zhang, W.; Wang, H.-F.; Ou, Y.-N.; Qu, Y.; Shen, X.-N.; Chen, S.-D.; Wu, K.-M.; Zhao, Q.-H.; et al. Modifiable risk factors for incident dementia and cognitive impairment: An umbrella review of evidence. J. Affect. Disord. 2022, 314, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.S.; Zuidersma, M.; Zuidema, S.U.; Burgerhof, J.G.; Stolk, R.P.; Oude Voshaar, R.C.; Smidt, N. Social relationships and cognitive decline: A systematic review and meta-analysis of longitudinal cohort studies. Int. J. Epidemiol. 2016, 45, 1169–1206. [Google Scholar] [CrossRef]
- Marseglia, A.; Darin-Mattsson, A.; Skoog, J.; Rydén, L.; Hadarsson-Bodin, T.; Kern, S.; Sterner, T.R.; Shang, Y.; Zettergren, A.; Westman, E.; et al. Metabolic Syndrome Is Associated With Poor Cognition: A Population-Based Study of 70-Year-Old Adults Without Dementia. J. Gerontol. Ser. A 2021, 76, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Chye, L.; Wei, K.; Nyunt, M.S.Z.; Gao, Q.; Wee, S.L.; Ng, T.-P. Strong Relationship Between Malnutrition And Cognitive Frailty In The Singapore Longitudinal Ageing Studies (SLAS-1 and SLAS-2). J. Prev. Alzheimer’s Dis. 2018, 5, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chu, Z.; Quan, X.; Zhang, Y.; Yuan, W.; Yao, Y.; Zhao, Y.; Fu, S. Malnutrition is positively associated with cognitive decline in centenarians and oldest-old adults: A cross-sectional study. EClinicalMedicine 2022, 47, 101336. [Google Scholar] [CrossRef]
- Flicker, L. Cardiovascular Risk Factors, Cerebrovascular Disease Burden, and Healthy Brain Aging. Clin. Geriatr. Med. 2010, 26, 17–27. [Google Scholar] [CrossRef]
- Hajjar, I.; Quach, L.; Yang, F.; Chaves, P.H.; Newman, A.B.; Mukamal, K.; Longstreth, W., Jr.; Inzitari, M.; Lipsitz, L.A. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: The Cardiovascular Health Study. Circulation 2011, 123, 858–865. [Google Scholar] [CrossRef]
- Wei, M.Y.; Levine, D.A.; Zahodne, L.B.; Kabeto, M.U.; Langa, K.M. Multimorbidity and Cognitive Decline Over 14 Years in Older Americans. J. Gerontol. Ser. A 2019, 75, 1206–1213. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, S.M.; de Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-Lowering by B Vitamins Slows the Rate of Accelerated Brain Atrophy in Mild Cognitive Impairment: A Randomized Controlled Trial. PLoS ONE 2010, 8, e12244. [Google Scholar] [CrossRef]
- Robbins, R.N.; Scott, T.; Joska, J.A.; Gouse, H. Impact of urbanization on cognitive disorders. Curr. Opin. Psychiatry 2019, 32, 210–217. [Google Scholar] [CrossRef]
- Lehmann, M.; Regland, B.; Blennow, K.; Gottfries, C. Vitamin B12-B6-Folate Treatment Improves Blood-Brain Barrier Function in Patients with Hyperhomocysteinaemia and Mild Cognitive Impairment. Dement. Geriatr. Cogn. Disord. 2003, 16, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Delyfer, M.-N.; Korobelnik, J.-F.; Rougier, M.-B.; Malet, F.; Féart, C.; Le Goff, M.; Peuchant, E.; Letenneur, L.; Dartigues, J.-F.; et al. High Concentrations of Plasma n3 Fatty Acids Are Associated with Decreased Risk for Late Age-Related Macular Degeneration. J. Nutr. 2013, 143, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, S.; Gao, F.; Li, C. Vitamin B6, B9, and B12 Intakes and Cognitive Performance in Elders: National Health and Nutrition Examination Survey, 2011–2014. Neuropsychiatr. Dis. Treat. 2022, 18, 537–553. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, S.; Gu, Y.; Wang, P.; Zhang, Q.; Shan, C. Interaction of High-Sugar Diet and History of Stroke with Risk of Cognitive Decline in Older Adults. Experiment 2022, 28, e937572. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Gao, X.; Scott, T.; Tucker, K.L. Habitual sugar intake and cognitive function among middle-aged and older Puerto Ricans without diabetes. Br. J. Nutr. 2011, 106, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-Q.; Yu, L.-L.; Qi, G.-Y.; Mi, Y.-S.; Wu, W.-Q.; Lee, Y.-K.; Zhai, Q.-X.; Tian, F.-W.; Chen, W. Can dietary patterns prevent cognitive impairment and reduce Alzheimer’s disease risk: Exploring the underlying mechanisms of effects. Neurosci. Biobehav. Rev. 2022, 135, 104556. [Google Scholar] [CrossRef]
- Feart, C.; Samieri, C.; Barberger-Gateau, P. Mediterranean diet and cognitive health: An update of available knowledge. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 51–62. [Google Scholar] [CrossRef]
- Gu, Y.; Brickman, A.M.; Stern, Y.; Habeck, C.G.; Razlighi, Q.R.; Luchsinger, J.A.; Manly, J.J.; Schupf, N.; Mayeux, R.; Scarmeas, N. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology 2015, 85, 1744–1751. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Vernuccio, L.; Catanese, G.; Inzerillo, F.; Salemi, G.; Barbagallo, M. Nutrition, Physical Activity, and Other Lifestyle Factors in the Prevention of Cognitive Decline and Dementia. Nutrients 2021, 13, 4080. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef]
- Gu, Y.; Manly, J.J.; Mayeux, R.P.; Brickman, A.M. An Inflammation-related Nutrient Pattern is Associated with Both Brain and Cognitive Measures in a Multiethnic Elderly Population. Curr. Alzheimer Res. 2018, 15, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Misirli, G.; Benetou, V.; Lagiou, P.; Bamia, C.; Trichopoulos, D.; Trichopoulou, A. Relation of the Traditional Mediterranean Diet to Cerebrovascular Disease in a Mediterranean Population. Am. J. Epidemiol. 2012, 176, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Naja, F.; Shivappa, N.; Nasreddine, L.; Kharroubi, S.; Itani, L.; Hwalla, N.; Sibai, A.M.; Hebert, J.R. Role of inflammation in the association between the western dietary pattern and metabolic syndrome among Lebanese adults. Int. J. Food Sci. Nutr. 2017, 68, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Shakersain, B.; Santoni, G.; Larsson, S.C.; Faxén-Irving, G.; Fastbom, J.; Fratiglioni, L.; Xu, W. Prudent diet may attenuate the adverse effects of Western diet on cognitive decline. Alzheimer’s Dement. 2016, 12, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; van Kan, G.A.; Ousset, P.-J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) International Consensus Group. J. Nutr. Heal. Aging 2013, 17, 726–734. [Google Scholar] [CrossRef]
- Mantovani, E.; Zucchella, C.; Schena, F.; Romanelli, M.G.; Venturelli, M.; Tamburin, S. Towards a Redefinition of Cognitive Frailty. J. Alzheimer’s Dis. 2020, 76, 831–843. [Google Scholar] [CrossRef]
- Sugimoto, T.; Arai, H.; Sakurai, T. An update on cognitive frailty: Its definition, impact, associated factors and underlying mechanisms, and interventions. Geriatr. Gerontol. Int. 2022, 22, 99–109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).