Association of Possible Sarcopenia or Sarcopenia with Body Composition, Nutritional Intakes, Serum Vitamin D Levels, and Physical Activity among Patients with Type 2 Diabetes Mellitus in Taiwan
Abstract
:1. Introduction
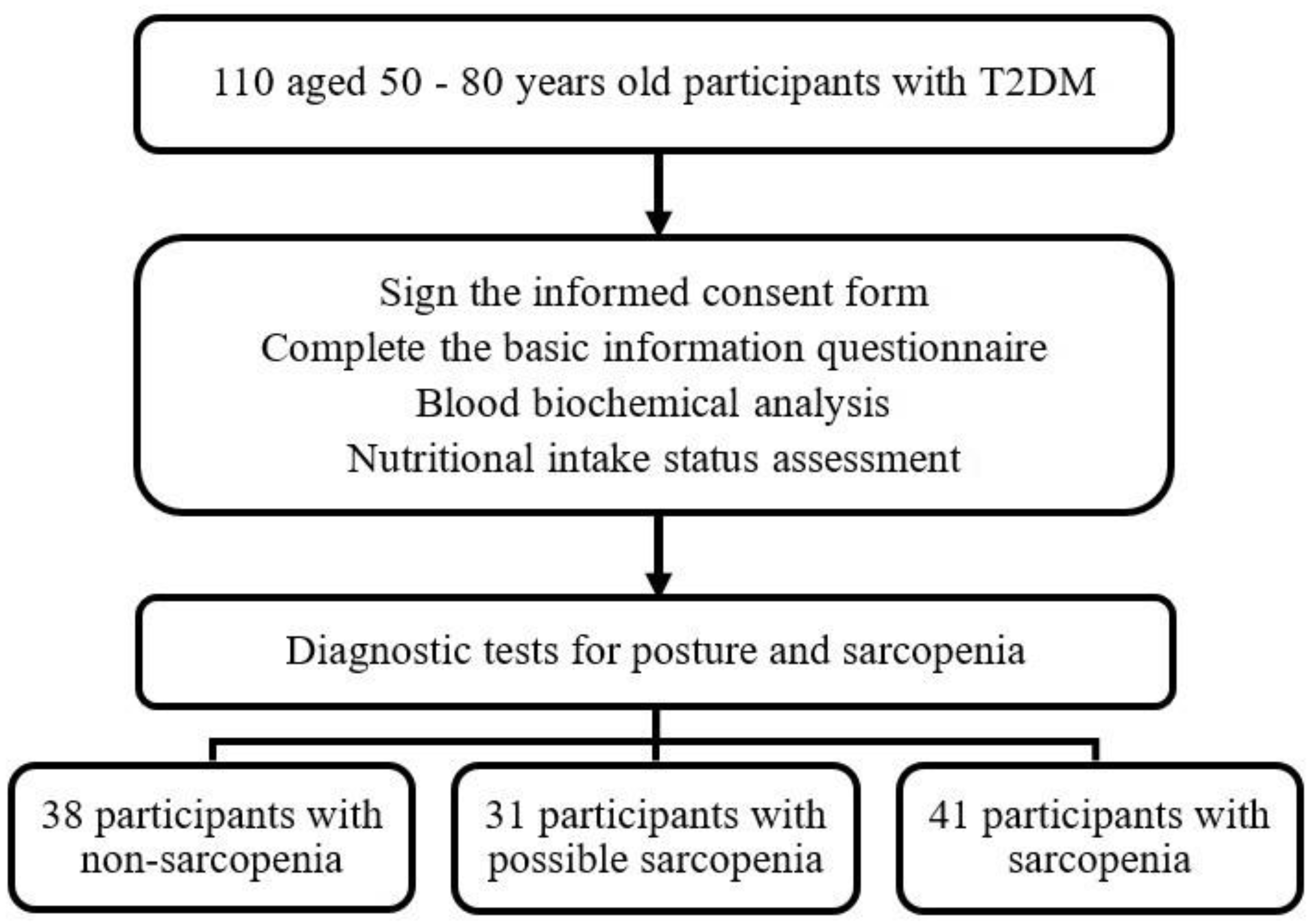
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Measurements
2.3. Screening for Sarcopenia
2.4. Diagnosis of Sarcopenia
2.5. Biochemical Analyses
2.6. Nutritional Intake
2.6.1. 24-h Dietary Recall
2.6.2. Food Frequency Questionnaire
2.7. Leisure-Time Physical Activity Measurement
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Ahmed, N.; Mandel, R.; Fain, M.J. Frailty: An emerging geriatric syndrome. Am. J. Med. 2007, 120, 748–753. [Google Scholar] [CrossRef]
- Casals-Vázquez, C.; Suárez-Cadenas, E.; Estébanez Carvajal, F.M.; Aguilar Trujillo, M.P.; Jiménez Arcos, M.M.; Vázquez Sáchez, M.Á. Relationship between quality of life, physical activity, nutrition, glycemic control and sarcopenia in older adults with type 2 diabetes mellitus. Nutr. Hosp. 2017, 34, 1198–1204. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dietrich, T.; Orav, E.J.; Hu, F.B.; Zhang, Y.; Karlson, E.W.; Dawson-Hughes, B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am. J. Clin. Nutr. 2004, 80, 752–758. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Hwang, A.C.; Liu, L.K.; Lee, W.J.; Peng, L.N.; Chen, L.K. Calf circumference as a screening instrument for appendicular muscle mass measurement. J. Am. Med. Dir. Assoc. 2018, 19, 182–184. [Google Scholar] [CrossRef]
- Ida, S.; Kaneko, R.; Murata, K. SARC-F for screening of sarcopenia among older adults: A meta-analysis of screening test accuracy. J. Am. Med. Dir. Assoc. 2018, 19, 685–689. [Google Scholar] [CrossRef]
- Yang, M.; Hu, X.; Xie, L.; Zhang, L.; Zhou, J.; Lin, J.; Wang, Y.; Li, Y.; Han, Z.; Zhang, D.; et al. Screening sarcopenia in community-dwelling older adults: SARC-F vs SARC-F combined with calf circumference (SARC-CalF). J. Am. Med. Dir. Assoc. 2018, 19, 277.e1–277.e8. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Chow, S.K.; Hung, V.W.; Wong, C.H.; Wong, R.M.; Tsang, C.S.; Kwok, T.; Cheung, W.H. Diagnosis of sarcopenia by evaluating skeletal muscle mass by adjusted bioimpedance analysis validated with dual-energy X-ray absorptiometry. J. Cachexia Sarcopenia Muscle 2021, 12, 2163–2173. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Tang, T.C.; Hwang, A.C.; Liu, L.K.; Lee, W.J.; Chen, L.Y.; Wu, Y.H.; Huang, C.Y.; Hung, C.H.; Wang, C.J.; Lin, M.H.; et al. FNIH-defined sarcopenia predicts adverse outcomes among community-dwelling older People in Taiwan: Results from I-Lan longitudinal aging study. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, T.W.; Arai, H.; Chen, L.K.; Woo, J. Letter to the editor: Normative data of handgrip strength in 26344 older adults-a pooled dataset from eight cohorts in Asia. J. Nutr. Health Aging 2020, 24, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Makiura, D.; Ono, R.; Inoue, J.; Kashiwa, M.; Oshikiri, T.; Nakamura, T.; Kakeji, Y.; Sakai, Y.; Miura, Y. Preoperative sarcopenia is a predictor of postoperative pulmonary complications in esophageal cancer following esophagectomy: A retrospective cohort study. J. Geriatr. Oncol. 2016, 7, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Arima, K.; Okabe, T.; Mizukami, S.; Tomita, Y.; Kanagae, M.; Goto, H.; Horiguchi, I.; Abe, Y.; Aoyagi, K. Usefulness of chair stand time as a surrogate of gait speed in diagnosing sarcopenia. Geriatr. Gerontol. Int. 2017, 17, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Godin, G.; Shephard, R.J. A simple method to assess exercise behavior in the community. Can. J. Appl. Sport Sci. 1985, 10, 141–146. [Google Scholar]
- Kim, T.N.; Park, M.S.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: The Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010, 33, 1497–1499. [Google Scholar] [CrossRef]
- Fung, F.Y.; Koh, Y.L.E.; Malhotra, R.; Ostbye, T.; Lee, P.Y.; Shariff Ghazali, S.; Tan, N.C. Prevalence of and factors associated with sarcopenia among multi-ethnic ambulatory older Asians with type 2 diabetes mellitus in a primary care setting. BMC Geriatr. 2019, 19, 122. [Google Scholar] [CrossRef]
- Pechmann, L.M.; Jonasson, T.H.; Canossa, V.S.; Trierweiler, H.; Kisielewicz, G.; Petterle, R.R.; Moreira, C.A.; Borba, V.Z.C. Sarcopenia in type 2 diabetes mellitus: A cross-sectional observational study. Int. J. Endocrinol. 2020, 2020, 7841390. [Google Scholar] [CrossRef]
- King, P.; Peacock, I.; Donnelly, R. The UK prospective diabetes study (UKPDS): Clinical and therapeutic implications for type 2 diabetes. Br. J. Clin. Pharmacol. 1999, 48, 643–648. [Google Scholar] [CrossRef]
- Chuang, S.C.; Chen, H.L.; Tseng, W.T.; Wu, I.C.; Hsu, C.C.; Chang, H.Y.; Chen, Y.I.; Lee, M.M.; Liu, K.; Hsiung, C.A. Circulating 25-hydroxyvitamin D and physical performance in older adults: A nationwide study in Taiwan. Am. J. Clin. Nutr. 2016, 104, 1334–1344. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Hung, W.C.; Wu, M.Y.; Vhin, W.L.; Chang, Y.F.; Chang, C.S.; Wu, S.J.; Lin, C.W.; Wu, C.H. Prevalence and associated factors of serum vitamin D inadequacy in older men living in Southern Taiwan. Taiwan Assoc. Fam. Med. 2017, 27, 222–231. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Mithal, A.; Bonjour, J.P.; Boonen, S.; Burckhardt, P.; Fuleihan, G.E.; Josse, R.G.; Lips, P.; Morales-Torres, J.; Yoshimura, N. IOF position statement: Vitamin D recommendations for older adults. Osteoporos. Int. 2010, 21, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, Y.; Narita, T.; Fujita, H.; Morii, T.; Sato, T.; Sassa, M.H.; Yamada, Y. Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J. Diabetes Investig. 2019, 10, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.C.; Won, C.W.; Soh, Y. Association of high body mass index, waist circumference, and body fat percentage with sarcopenia in older women. BMC Geriatr. 2022, 22, 937. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cheng, K.Y.; Tong, X.; Cheung, W.H.; Chow, S.K.; Law, S.W.; Wong, R.M.Y. The role of obesity in sarcopenia and the optimal body composition to prevent against sarcopenia and obesity. Front. Endocrinol. 2023, 14, 1077255. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Ji, T.; Li, Y.; Ma, L. Sarcopenic Obesity: An Emerging Public Health Problem. Aging Dis. 2022, 13, 379–388. [Google Scholar] [CrossRef]
- Welch, A.A.; Hayhoe, R.P.G.; Cameron, D. The relationships between sarcopenic skeletal muscle loss during ageing and macronutrient metabolism, obesity and onset of diabetes. Proc. Nutr. Soc. 2020, 79, 158–169. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Yu, M.D.; Zhang, H.Z.; Zhang, Y.; Yang, S.P.; Lin, M.; Zhang, Y.M.; Wu, J.B.; Hong, F.Y.; Chen, W.X. Relationship between chronic kidney disease and sarcopenia. Sci. Rep. 2021, 11, 20523. [Google Scholar] [CrossRef]
| Variables | Non-Sarcopenia (n = 38) | Possible Sarcopenia (n = 31) | Sarcopenia (n = 41) | p |
|---|---|---|---|---|
| Gender | 0.115 | |||
| Female | 17 (44.7%) | 20 (64.5%) | 27 (65.9%) | |
| Male | 21 (55.3%) | 11 (35.5%) | 14 (34.1%) | |
| Age (year) | 64.4 ± 7.3 a | 67.1 ± 7.2 a,b | 70.2 ± 6.0 b | 0.001 |
| Duration of Diabetes (year) | 12.0 (7.0, 15.3) | 14.0 (7.0, 16.0) | 14.0 (11.0, 19.0) | 0.122 |
| Smoking | 10 (26.3%) | 3 (9.7%) | 8 (19.5%) | 0.212 * |
| Alcohol drinking | 9 (23.7%) | 3 (9.7%) | 6 (14.6%) | 0.300 * |
| Betel nut | 5 (13.2%) | 2 (6.5%) | 1 (2.4%) | 0.185 * |
| Oral hypoglycemic medications | 37 (97.4%) | 29 (93.6%) | 39 (95.1%) | 0.744 |
| Insulin injection | 7 (18.4%) | 1 (3.2%) | 9 (22.0%) | 0.064 * |
| Antihypertensive agents | 17 (44.7%) | 11 (35.5%) | 14 (34.2%) | 0.586 |
| Hypolipidemic agents | 33 (86.8%) | 27 (87.1%) | 33 (80.5%) | 0.662 |
| Variables | Non-Sarcopenia (n = 38) | Possible Sarcopenia (n = 31) | Sarcopenia (n = 41) | p |
|---|---|---|---|---|
| Sun exposure (day/week) | 5.4 ± 2.3 | 5.0 ± 2.7 | 4.9 ± 2.7 | 0.709 |
| Sun exposure (time/day) | 0.857 * | |||
| No | 3 (7.9%) | 4 (12.9%) | 7 (17.1%) | |
| <20 min | 9 (23.7%) | 5 (16.1%) | 8 (19.5%) | |
| 20 min–1 h | 18 (47.4%) | 14 (45.2%) | 15 (36.6%) | |
| >1 h | 8 (21.1%) | 8 (25.8%) | 11 (26.8%) | |
| Sun protection | 0.657 | |||
| No | 15 (39.5%) | 10 (24.4%) | 17 (41.5%) | |
| 1 | 10 (26.3%) | 12 (38.7%) | 15 (36.6%) | |
| ≥2 | 13 (34.2%) | 9 (29.0%) | 9 (22.0%) |
| Variables | Non-Sarcopenia (n = 38) | Possible Sarcopenia (n = 31) | Sarcopenia (n = 41) | p |
|---|---|---|---|---|
| FBS (mg/dL) | 128.5 (120.0, 143.8) | 134.0 (123.0, 145.0) | 139.0 (117.5, 154.5) | 0.428 |
| HbA1c (%) | 7.1 (6.7, 7.6) | 7.2 (6.7, 7.7) | 7.2 (6.7, 7.8) | 0.762 |
| TG (mg/dL) | 95.5 (71.8, 162.5) | 96.0 (72.0, 127.0) | 86.0 (59.5, 122.0) | 0.293 |
| TC (mg/dL) | 149.7 ± 30.9 a,b | 146.9 ± 33.2 a | 166.2 ± 31.6 b | 0.019 |
| HDL-C (mg/dL) | 58.5 (47.8, 63.5) a | 59.0 (53.0, 74.0) a,b | 64.0 (58.0, 74.0) b | 0.019 |
| LDL-C (mg/dL) | 76.3 ± 24.3 | 72.8 ± 18.0 | 86.2 ± 27.3 | 0.048 |
| eGFR (mL/min/1.73 m2) | 78.8 (69.9, 87.2) | 75.8 (69.7, 85.4) | 75.9 (69.5, 89.5) | 0.822 |
| Serum 25(OH)D (ng/mL) | 38.4 ± 9.3 a | 33.3 ± 5.8 b | 33.2 ± 6.7 b | 0.004 |
| Parameters | Non-Sarcopenia (n = 38) | Possible Sarcopenia (n = 31) | Sarcopenia (n = 41) | p |
|---|---|---|---|---|
| Energy (kcal) | 1820.8 ± 292.4 a | 1620.1 ± 217.0 b | 1411.3 ± 201.1 c | <0.001 |
| Energy (kcal/kg BW) | 27.9 ± 4.6 a | 25.1 ± 4.2 b | 26.4 ± 3.6 a,b | 0.019 |
| Carbohydrate (g) | 213.8 ± 37.2 a | 202.0 ± 41.8 a | 174.9 ± 31.6 b | <0.001 |
| Protein (g) | 67.9 ± 13.9 a | 59.6 ± 11.9 b | 47.2 ± 9.9 c | <0.001 |
| Protein (g/kg BW) | 1.04 ± 0.25 a | 0.92 ± 0.20 a,b | 0.89 ± 0.19 b | 0.004 |
| Fat (g) | 73.4 (63.0, 88.0) a | 64.4 (57.5, 74.0) b | 55.0 (49.7, 64.0) c | <0.001 |
| Vitamin D (μg) | 23.6 (16.7, 36.4) | 21.8 (6.2, 40.6) | 16.9 (7.5, 24.8) | 0.107 |
| Nutritional supplements | ||||
| No | 21 (55.3%) | 14 (45.2%) | 19 (46.3%) | 0.821 |
| 1 | 9 (23.7%) | 7 (22.6%) | 9 (22.0%) | |
| ≥2 | 8 (21.1%) | 10 (32.3%) | 13 (31.7%) | |
| Vitamin D supplements | 3 (7.9%) | 7 (22.6%) | 6 (14.6%) | 0.237 * |
| Anthropometrics | Non-Sarcopenia (n = 38) | Possible Sarcopenia (n = 31) | Sarcopenia (n = 41) | p |
|---|---|---|---|---|
| Body weight (kg) | 65.8 ± 8.6 a | 65.5 ± 8.5 a | 53.7 ± 6.1 b | <0.001 |
| BMI (kg/m2) | 25.4 ± 2.7 a | 26.6 ± 3.1 a | 22.3 ± 2.7 b | <0.001 |
| Waist circumference (cm) | 88.0 ± 7.1 a | 89.4 ± 9.3 a | 81.6 ± 6.7 b | <0.001 |
| Hip circumference (cm) | 95.6 ± 5.0 a | 96.8 ± 5.9 a | 91.2 ± 5.7 b | <0.001 |
| SBP (mmHg) | 131.7 ± 15.5 | 128.3 ± 14.8 | 128.5 ± 16.3 | 0.564 |
| DBP (mmHg) | 76.0 (71.0, 86.0) | 72.0 (66.0, 78.0) | 74.0 (64.5, 82.5) | 0.065 |
| Muscle Mass, Strength and Performance | ||||
| BF% | 31.1 ± 7.1 a | 35.6 ± 6.5 b | 32.3 ± 8.0 a,b | 0.041 |
| SMI (ASM/Height2) (kg/m2) | 7.0 ± 0.8 a | 6.7 ± 0.8 a | 5.6 ± 0.7 b | <0.001 |
| SMI (ASM/BMI) (m2) | 0.73 ± 0.16 a | 0.63 ± 0.12 b | 0.62 ± 0.15 b | 0.001 |
| HGS (kg) | 30.5 (22.5, 36.0) a | 21.9 (18.9, 25.7) b | 21.2 (17.3, 27.0) b | <0.001 |
| SPPB (score) | 11.4 ± 0.8 a | 9.8 ± 1.5 b | 9.3 ± 2.1 b | <0.001 |
| 5-time chair stand test (s) | 9.3 (8.2, 10.5) a | 12.3 (9.9, 13.6) b | 12.7 (11.5, 13.4) b | <0.001 |
| CC (right) (cm) | 35.7 ± 2.3 a | 34.8 ± 2.5 a | 31.6 ± 2.0 b | <0.001 |
| CC (left) (cm) | 35.7 ± 2.3 a | 34.8 ± 2.7 a | 31.6 ± 2.0 b | <0.001 |
| SARC-F (score) | 0.13 ± 0.41 a | 0.58 ± 1.41 a,b | 0.95 ± 1.84 b | 0.033 |
| PA (score) | 21.0 (9.8, 35.0) | 12.0 (0.0, 21.0) | 15.0 (0.0, 21.0) | 0.062 |
| Parameters | Nagelkerke R-Square | Odds Ratio (95% CI) | p |
|---|---|---|---|
| Possible sarcopenia | 0.210 | ||
| Serum 25(OH)D (ng/mL) | 0.927 (0.863–0.997) | 0.040 | |
| Energy (per kcal/kg BW) | 0.877 (0.774–0.994) | 0.040 | |
| Sarcopenia | 0.890 | ||
| Age (per year) | 1.231 (1.071–1.415) | 0.004 | |
| BMI (kg/m2) | 0.438 (0.283–0.680) | 0.000 | |
| Serum 25(OH)D (ng/mL) | 0.901 (0.812–0.999) | 0.048 | |
| Protein intake (per g/kg BW) | 0.001 (0.000–0.166) | 0.009 | |
| Vitamin D intake (per μg) | 0.915 (0.849–0.986) | 0.020 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-T.; Lin, J.-Y.; Lin, C.-J.; Lee, Y.-J.; Chang, W.-H. Association of Possible Sarcopenia or Sarcopenia with Body Composition, Nutritional Intakes, Serum Vitamin D Levels, and Physical Activity among Patients with Type 2 Diabetes Mellitus in Taiwan. Nutrients 2023, 15, 3892. https://doi.org/10.3390/nu15183892
Hsu Y-T, Lin J-Y, Lin C-J, Lee Y-J, Chang W-H. Association of Possible Sarcopenia or Sarcopenia with Body Composition, Nutritional Intakes, Serum Vitamin D Levels, and Physical Activity among Patients with Type 2 Diabetes Mellitus in Taiwan. Nutrients. 2023; 15(18):3892. https://doi.org/10.3390/nu15183892
Chicago/Turabian StyleHsu, Yu-Ting, Jian-Yu Lin, Chien-Ju Lin, Yau-Jiunn Lee, and Wen-Hsin Chang. 2023. "Association of Possible Sarcopenia or Sarcopenia with Body Composition, Nutritional Intakes, Serum Vitamin D Levels, and Physical Activity among Patients with Type 2 Diabetes Mellitus in Taiwan" Nutrients 15, no. 18: 3892. https://doi.org/10.3390/nu15183892
APA StyleHsu, Y.-T., Lin, J.-Y., Lin, C.-J., Lee, Y.-J., & Chang, W.-H. (2023). Association of Possible Sarcopenia or Sarcopenia with Body Composition, Nutritional Intakes, Serum Vitamin D Levels, and Physical Activity among Patients with Type 2 Diabetes Mellitus in Taiwan. Nutrients, 15(18), 3892. https://doi.org/10.3390/nu15183892






