Relationship between Oat Consumption, Gut Microbiota Modulation, and Short-Chain Fatty Acid Synthesis: An Integrative Review
Abstract
1. Introduction
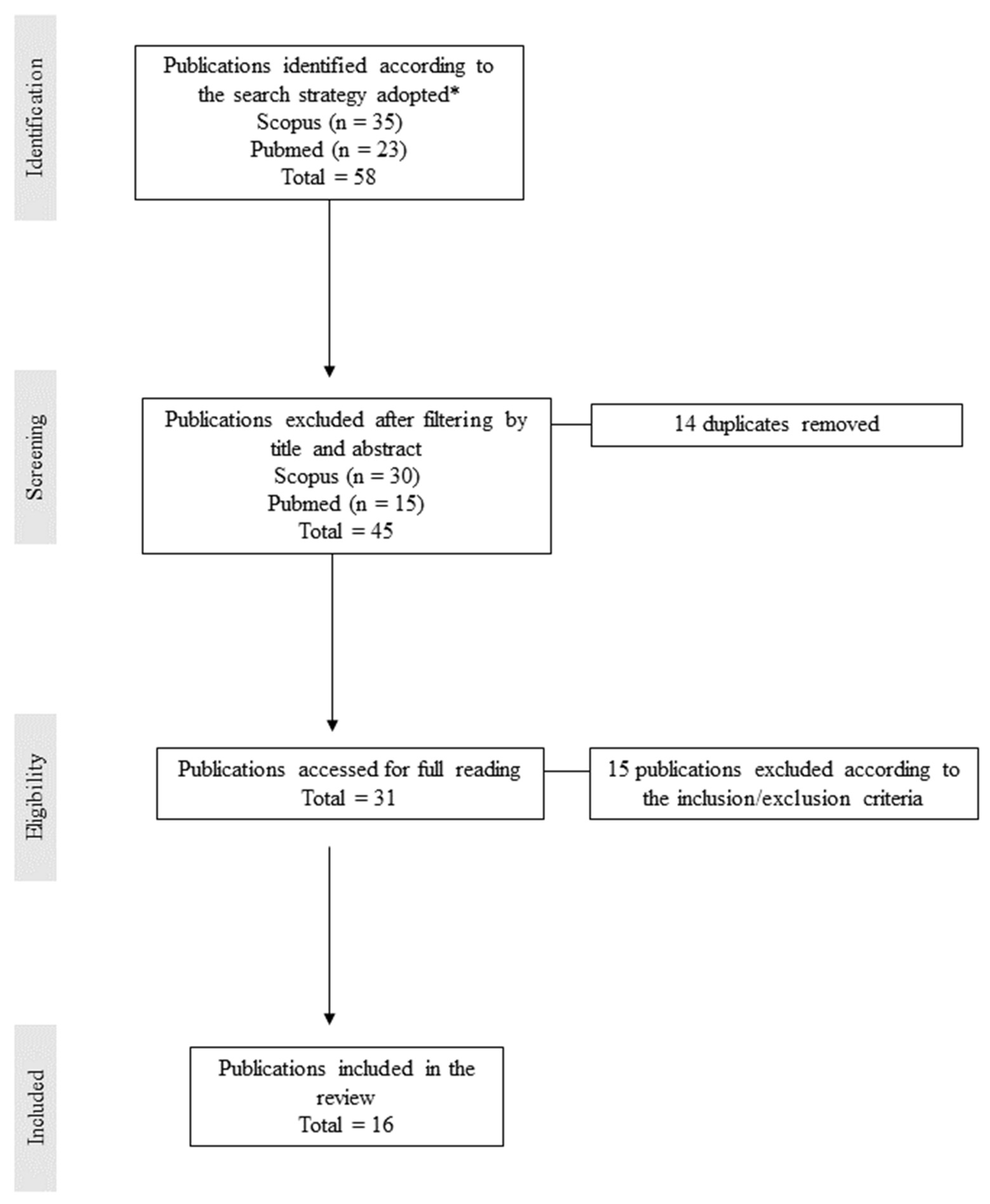
2. Methodology
3. Oat as a Functional Food
3.1. Anticholesterolemic, Hypoglycemic, and Antihypertensive Properties
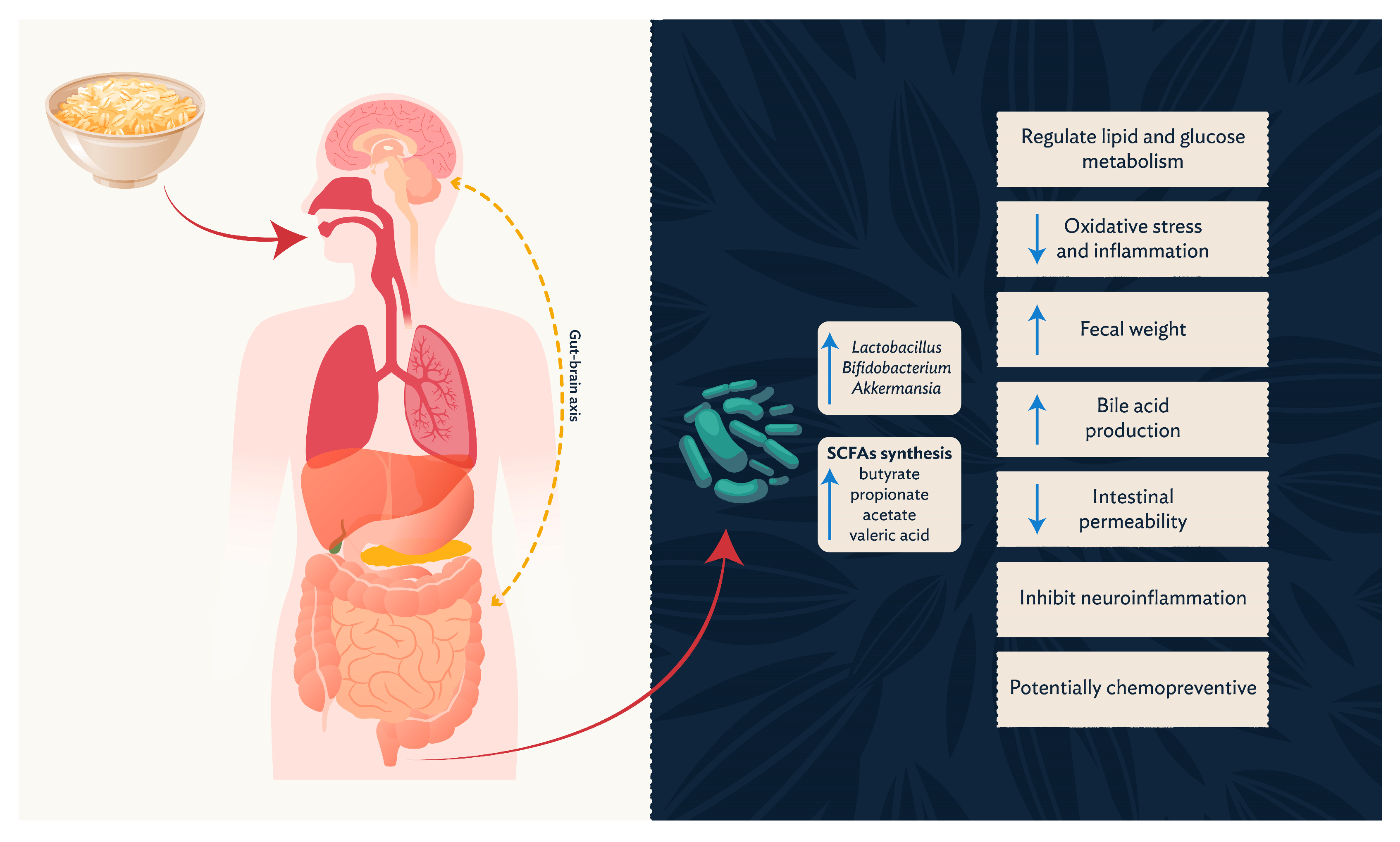
3.2. Prebiotic Potential
3.3. Short-Chain Fatty Acids and Related Metabolites
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Butt, M.S.; Tahir-Nadeem, M.; Khan, M.K.I.; Shabir, R.; Butt, M.S. Oat: Unique among the Cereals. Eur. J. Nutr. 2008, 47, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.J. Impact of Whole Grains on the Gut Microbiota: The next Frontier for Oats? Br. J. Nutr. 2014, 112, S44–S49. [Google Scholar] [CrossRef]
- Chávez de la Vega, M.I.; Alatorre-Santamaría, S.; Gómez-Ruiz, L.; García-Garibay, M.; Guzmán-Rodríguez, F.; González-Olivares, L.G.; Cruz-Guerrero, A.E.; Rodríguez-Serrano, G.M. Influence of Oat β-Glucan on the Survival and Proteolytic Activity of Lactobacillus Rhamnosus GG in Milk Fermentation: Optimization by Response Surface. Fermentation 2021, 7, 210. [Google Scholar] [CrossRef]
- Carlson, J.; Erickson, J.; Hess, J.; Gould, T.; Slavin, J. Prebiotic Dietary Fiber and Gut Health: Comparing the in Vitro Fermentations of Beta-Glucan, Inulin and Xylooligosaccharide. Nutrients 2017, 9, 1361. [Google Scholar] [CrossRef]
- Fehlbaum, S.; Prudence, K.; Kieboom, J.; Heerikhuisen, M.; van den Broek, T.; Schuren, F.; Steinert, R.; Raederstorff, D. In Vitro Fermentation of Selected Prebiotics and Their Effects on the Composition and Activity of the Adult Gut Microbiota. Int. J. Mol. Sci. 2018, 19, 3097. [Google Scholar] [CrossRef]
- EFSA Panel on Dietic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of a Health Claim Related to Oat Beta Glucan and Lowering Blood Cholesterol and Reduced Risk of (Coronary) Heart Disease Pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1885. [Google Scholar] [CrossRef]
- Gangopadhyay, N.; Hossain, M.; Rai, D.; Brunton, N. A Review of Extraction and Analysis of Bioactives in Oat and Barley and Scope for Use of Novel Food Processing Technologies. Molecules 2015, 20, 10884–10909. [Google Scholar] [CrossRef]
- Chen, C.; Huang, X.; Wang, H.; Geng, F.; Nie, S. Effect of β-Glucan on Metabolic Diseases: A Review from the Gut Microbiota Perspective. Curr. Opin. Food Sci. 2022, 47, 100907. [Google Scholar] [CrossRef]
- Boz, H. Phenolic Amides (Avenanthramides) in Oats—A Review. Czech J. Food Sci. 2015, 33, 399–404. [Google Scholar] [CrossRef]
- Meydani, M. Potential Health Benefits of Avenanthramides of Oats. Nutr. Rev. 2009, 67, 731–735. [Google Scholar] [CrossRef]
- Garsed, K.; Scott, B.B. Can Oats Be Taken in a Gluten-Free Diet? A Systematic Review. Scand. J. Gastroenterol. 2007, 42, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Balakireva, A.; Zamyatnin, A. Properties of Gluten Intolerance: Gluten Structure, Evolution, Pathogenicity and Detoxification Capabilities. Nutrients 2016, 8, 644. [Google Scholar] [CrossRef]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac Disease: A Comprehensive Current Review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef]
- Smulders, M.J.M.; van de Wiel, C.C.M.; van den Broeck, H.C.; van der Meer, I.M.; Israel-Hoevelaken, T.P.M.; Timmer, R.D.; van Dinter, B.-J.; Braun, S.; Gilissen, L.J.W.J. Oats in Healthy Gluten-Free and Regular Diets: A Perspective. Food Res. Int. 2018, 110, 3–10. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United States (FAO); WHO. CODEX ALIMENTARIUS: International Food Standards. Standard for Foods for Special Dietary Use for Persons Intolerant to Gluten: CXS 118-1979. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B118-1979%252FCXS_118e_2015.pdf (accessed on 23 February 2023).
- Wieser, H.; Segura, V.; Ruiz-Carnicer, Á.; Sousa, C.; Comino, I. Food Safety and Cross-Contamination of Gluten-Free Products: A Narrative Review. Nutrients 2021, 13, 2244. [Google Scholar] [CrossRef] [PubMed]
- Poley, J.R. The Gluten-Free Diet: Can Oats and Wheat Starch Be Part of It? J. Am. Coll. Nutr. 2017, 36, 1–8. [Google Scholar] [CrossRef]
- Pinto-Sánchez, M.I.; Causada-Calo, N.; Bercik, P.; Ford, A.C.; Murray, J.A.; Armstrong, D.; Semrad, C.; Kupfer, S.S.; Alaedini, A.; Moayyedi, P.; et al. Safety of Adding Oats to a Gluten-Free Diet for Patients With Celiac Disease: Systematic Review and Meta-Analysis of Clinical and Observational Studies. Gastroenterology 2017, 153, 395–409.e3. [Google Scholar] [CrossRef]
- Kosová, K.; Leišová-Svobodová, L.; Dvořáček, V. Oats as a Safe Alternative to Triticeae Cereals for People Suffering from Celiac Disease? A Review. Plant Foods Hum. Nutr. 2020, 75, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Hoffmanová, I.; Sánchez, D.; Szczepanková, A.; Tlaskalová-Hogenová, H. The Pros and Cons of Using Oat in a Gluten-Free Diet for Celiac Patients. Nutrients 2019, 11, 2345. [Google Scholar] [CrossRef]
- Gilissen, L.; van der Meer, I.; Smulders, M. Why Oats Are Safe and Healthy for Celiac Disease Patients. Med. Sci. 2016, 4, 21. [Google Scholar] [CrossRef]
- La Vieille, S.; Pulido, O.M.; Abbott, M.; Koerner, T.B.; Godefroy, S. Celiac Disease and Gluten-Free Oats: A Canadian Position Based on a Literature Review. Can. J. Gastroenterol. Hepatol. 2016, 2016, 1870305. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health Benefits of Dietary Fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- McBurney, M.I.; Davis, C.; Fraser, C.M.; Schneeman, B.O.; Huttenhower, C.; Verbeke, K.; Walter, J.; Latulippe, M.E. Establishing What Constitutes a Healthy Human Gut Microbiome: State of the Science, Regulatory Considerations, and Future Directions. J. Nutr. 2019, 149, 1882–1895. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Manos, J. The Human Microbiome in Disease and Pathology. APMIS 2022, 130, 690–705. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Garcia-Gil, L.J. Escherichia Coli in Chronic Inflammatory Bowel Diseases: An Update on Adherent Invasive Escherichia coli Pathogenicity. World J. Gastrointest. Pathophysiol. 2014, 5, 213. [Google Scholar] [CrossRef]
- Lane, E.R.; Zisman, T.; Suskind, D. The Microbiota in Inflammatory Bowel Disease: Current and Therapeutic Insights. J. Inflamm. Res. 2017, 10, 63–73. [Google Scholar] [CrossRef]
- Zhu, W.; Winter, M.G.; Byndloss, M.X.; Spiga, L.; Duerkop, B.A.; Hughes, E.R.; Büttner, L.; de Lima Romão, E.; Behrendt, C.L.; Lopez, C.A.; et al. Precision Editing of the Gut Microbiota Ameliorates Colitis. Nature 2018, 553, 208–211. [Google Scholar] [CrossRef]
- Hollander, D. Intestinal Permeability, Leaky Gut, and Intestinal Disorders. Curr. Gastroenterol. Rep. 1999, 1, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Baliou, S.; Adamaki, M.; Spandidos, A.D.; Kyriakopoulos, M.A.; Christodoulou, L.; Zoumpourlis, V. The Microbiome, Its Molecular Mechanisms and Its Potential as a Therapeutic Strategy against Colorectal Carcinogenesis (Review). World Acad. Sci. J. 2019, 1, 3–19. [Google Scholar]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic Analysis Identifies Association of Fusobacterium with Colorectal Carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef]
- Lv, G.; Cheng, N.; Wang, H. The Gut Microbiota, Tumorigenesis, and Liver Diseases. Engineering 2017, 3, 110–114. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1–Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Korem, T.; Zeevi, D.; Suez, J.; Weinberger, A.; Avnit-Sagi, T.; Pompan-Lotan, M.; Matot, E.; Jona, G.; Harmelin, A.; Cohen, N.; et al. Growth Dynamics of Gut Microbiota in Health and Disease Inferred from Single Metagenomic Samples. Science 2015, 349, 1101–1106. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Biel, W.; Kazimierska, K.; Bashutska, U. Nutritional Value of Wheat, Triticale, Barley and Oat Grains. Acta Sci. Pol. Zootech. 2020, 19, 19–28. [Google Scholar] [CrossRef]
- Guo, H.; Wu, H.; Sajid, A.; Li, Z. Whole Grain Cereals: The Potential Roles of Functional Components in Human Health. Crit. Rev. Food Sci. Nutr. 2022, 62, 8388–8402. [Google Scholar] [CrossRef]
- Simpson, H.L.; Campbell, B.J. Review Article: Dietary Fibre-Microbiota Interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef]
- Chudan, S.; Ishibashi, R.; Nishikawa, M.; Tabuchi, Y.; Nagai, Y.; Ikushiro, S.; Furusawa, Y. Effect of Soluble Oat Fiber on Intestinal Microenvironment and TNBS-Induced Colitis. Food Funct. 2023, 14, 2188–2199. [Google Scholar] [CrossRef]
- Kutcher, A.M.; LeBaron, V.T. A Simple Guide for Completing an Integrative Review Using an Example Article. J. Prof. Nurs. 2022, 40, 13–19. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, W.; Guo, X.; Song, G.; Pang, S.; Fang, W.; Peng, Z. Effects of Oats, Tartary Buckwheat, and Foxtail Millet Supplementation on Lipid Metabolism, Oxido-Inflammatory Responses, Gut Microbiota, and Colonic SCFA Composition in High-Fat Diet Fed Rats. Nutrients 2022, 14, 2760. [Google Scholar] [CrossRef]
- Han, S.; Gao, H.; Song, R.; Zhang, W.; Li, Y.; Zhang, J. Oat Fiber Modulates Hepatic Circadian Clock via Promoting Gut Microbiota-Derived Short Chain Fatty Acids. J. Agric. Food Chem. 2021, 69, 15624–15635. [Google Scholar] [CrossRef]
- Ji, Y.; Ma, N.; Zhang, J.; Wang, H.; Tao, T.; Pei, F.; Hu, Q. Dietary Intake of Mixture Coarse Cereals Prevents Obesity by Altering the Gut Microbiota in High-Fat Diet Fed Mice. Food Chem. Toxicol. 2021, 147, 111901. [Google Scholar] [CrossRef] [PubMed]
- Kundi, Z.M.; Lee, J.C.; Pihlajamäki, J.; Chan, C.B.; Leung, K.S.; So, S.S.Y.; Nordlund, E.; Kolehmainen, M.; El-Nezami, H. Dietary Fiber from Oat and Rye Brans Ameliorate Western Diet–Induced Body Weight Gain and Hepatic Inflammation by the Modulation of Short-Chain Fatty Acids, Bile Acids, and Tryptophan Metabolism. Mol. Nutr. Food Res. 2021, 65, 1900580. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Song, R.; Li, Y.; Zhang, W.; Wan, Z.; Wang, Y.; Zhang, H.; Han, S. Effects of Oat Fiber Intervention on Cognitive Behavior in LDLR–/– Mice Modeling Atherosclerosis by Targeting the Microbiome–Gut–Brain Axis. J. Agric. Food Chem. 2020, 68, 14480–14491. [Google Scholar] [CrossRef]
- Huang, K.; Yu, W.; Li, S.; Guan, X.; Liu, J.; Song, H.; Liu, D.; Duan, R. Effect of Embryo-Remaining Oat Rice on the Lipid Profile and Intestinal Microbiota in High-Fat Diet Fed Rats. Food Res. Int. 2020, 129, 108816. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.-X.; Tong, L.-T.; Liang, T.-T.; Wang, L.-L.; Liu, L.-Y.; Zhou, X.-R.; Zhou, S.-M. Effect of Oat and Tartary Buckwheat—Based Food on Cholesterol—Lowering and Gut Microbiota in Hypercholesterolemic Hamsters. J. Oleo Sci. 2019, 68, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhu, Y.; Ma, Y.; Xiang, Q.; Shen, R.; Liu, Y. Oat Products Modulate the Gut Microbiota and Produce Anti-Obesity Effects in Obese Rats. J. Funct. Foods 2016, 25, 408–420. [Google Scholar] [CrossRef]
- Wilczak, J.; Błaszczyk, K.; Kamola, D.; Gajewska, M.; Harasym, J.P.; Jałosińska, M.; Gudej, S.; Suchecka, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. The Effect of Low or High Molecular Weight Oat Beta-Glucans on the Inflammatory and Oxidative Stress Status in the Colon of Rats with LPS-Induced Enteritis. Food Funct. 2015, 6, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, M.; Zhang, Y.; Zhang, Y.; Huo, R.; Guo, X. In Vitro Fermentation of Pretreated Oat Bran by Human Fecal Inoculum and Impact on Microbiota. J. Funct. Foods 2022, 98, 105278. [Google Scholar] [CrossRef]
- Liang, S.; Xie, Q.; Evivie, S.E.; Zhao, L.; Chen, Q.; Xu, B.; Liu, F.; Li, B.; Huo, G. Study on Supplementary Food with Beneficial Effects on the Gut Microbiota of Infants. Food Biosci. 2021, 43, 101291. [Google Scholar] [CrossRef]
- Glei, M.; Zetzmann, S.; Lorkowski, S.; Dawczynski, C.; Schlörmann, W. Chemopreventive effects of raw and roasted oat flakes after in vitro fermentation with human faecal microbiota. Int. J. of Food Sci. Nutr. 2021, 72, 57–69. [Google Scholar] [CrossRef]
- Kristek, A.; Wiese, M.; Heuer, P.; Kosik, O.; Schär, M.Y.; Soycan, G.; Alsharif, S.; Kuhnle, G.G.C.; Walton, G.; Spencer, J.P.E. Oat Bran, but Not Its Isolated Bioactive β-Glucans or Polyphenols, Have a Bifidogenic Effect in an in Vitro Fermentation Model of the Gut Microbiota. Br. J. Nutr. 2019, 121, 549–559. [Google Scholar] [CrossRef]
- Xu, D.; Feng, M.; Chu, Y.; Wang, S.; Shete, V.; Tuohy, K.M.; Liu, F.; Zhou, X.; Kamil, A.; Pan, D.; et al. The Prebiotic Effects of Oats on Blood Lipids, Gut Microbiota, and Short-Chain Fatty Acids in Mildly Hypercholesterolemic Subjects Compared with Rice: A Randomized, Controlled Trial. Front. Immunol. 2021, 12, 787797. [Google Scholar] [CrossRef]
- Valeur, J.; Puaschitz, N.G.; Midtvedt, T.; Berstad, A. Oatmeal Porridge: Impact on Microflora-Associated Characteristics in Healthy Subjects. Br. J. Nutr. 2016, 115, 62–67. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J. Acad. Nutr. Diet. 2015, 115, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.J. Functional Foods. Eur. J. Clin. Nutr. 2010, 64, 657–659. [Google Scholar] [CrossRef]
- Joyce, S.A.; Kamil, A.; Fleige, L.; Gahan, C.G.M. The Cholesterol-Lowering Effect of Oats and Oat Beta Glucan: Modes of Action and Potential Role of Bile Acids and the Microbiome. Front. Nutr. 2019, 6, 171. [Google Scholar] [CrossRef]
- Ross, S. Functional Foods: The Food and Drug Administration Perspective. Am. J. Clin. Nutr. 2000, 71, 1735S–1738S. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services; Food and Drug Administration. Food Labeling: Health Claims; Oats and Coronaty Heart Disease. Final Rule; Federal Register: Washington, DC, USA, 1997; 62, pp. 3584–3601. Available online: https://www.govinfo.gov/content/pkg/FR-1997-01-23/pdf/97-1598.pdf (accessed on 7 April 2023).
- Rafique, H.; Dong, R.; Wang, X.; Alim, A.; Aadil, R.M.; Li, L.; Zou, L.; Hu, X. Dietary-Nutraceutical Properties of Oat Protein and Peptides. Front. Nutr. 2022, 9, 950400. [Google Scholar] [CrossRef] [PubMed]
- Raguindin, P.F.; Adam Itodo, O.; Stoyanov, J.; Dejanovic, G.M.; Gamba, M.; Asllanaj, E.; Minder, B.; Bussler, W.; Metzger, B.; Muka, T.; et al. A Systematic Review of Phytochemicals in Oat and Buckwheat. Food. Chem. 2021, 338, 127982. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S.; Hwang, C.-W.; Yang, W.-S.; Kim, C.-H. Multiple Antioxidative and Bioactive Molecules of Oats (Avena sativa L.) in Human Health. Antioxidants 2021, 10, 1454. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the Vitamin E of the 21st Century: Its Potential against Cancer and Other Chronic Diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef]
- Paudel, D.; Dhungana, B.; Caffe, M.; Krishnan, P. A Review of Health-Beneficial Properties of Oats. Foods 2021, 10, 2591. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, O.; Pereira-Caro, G.; Borges, G.; Crozier, A.; Olsson, O. Characterization and Antioxidant Activity of Avenanthramides from Selected Oat Lines Developed by Mutagenesis Technique. Food Chem. 2021, 343, 128408. [Google Scholar] [CrossRef]
- Wolever, T.M.; Tosh, S.M.; Gibbs, A.L.; Brand-Miller, J.; Duncan, A.M.; Hart, V.; Lamarche, B.; Thomson, B.A.; Duss, R.; Wood, P.J. Physicochemical Properties of Oat β-Glucan Influence Its Ability to Reduce Serum LDL Cholesterol in Humans: A Randomized Clinical Trial. Am. J. Clin. Nutr. 2010, 92, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M. Cholesterol-Lowering Effects of Oat β-Glucan: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The Effect of Oat β -Glucan on LDL-Cholesterol, Non-HDL-Cholesterol and ApoB for CVD Risk Reduction: A Systematic Review and Meta-Analysis of Randomised-Controlled Trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef]
- Tiwari, U.; Cummins, E. Meta-Analysis of the Effect of β-Glucan Intake on Blood Cholesterol and Glucose Levels. Nutrition 2011, 27, 1008–1016. [Google Scholar] [CrossRef]
- McRorie, J.W.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Andersson, M.; Ellegård, L.; Andersson, H. Oat Bran Stimulates Bile Acid Synthesis within 8 h as Measured by 7α-Hydroxy-4-Cholesten-3-One. Am. J. Clin. Nutr. 2002, 76, 1111–1116. [Google Scholar] [CrossRef]
- Tong, L.-T.; Guo, L.; Zhou, X.; Qiu, J.; Liu, L.; Zhong, K.; Zhou, S. Effects of Dietary Oat Proteins on Cholesterol Metabolism of Hypercholesterolaemic Hamsters. J. Sci. Food Agric. 2016, 96, 1396–1401. [Google Scholar] [CrossRef]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.M.; Marchesi, J.R. Functional and Comparative Metagenomic Analysis of Bile Salt Hydrolase Activity in the Human Gut Microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 13580–13585. [Google Scholar] [CrossRef]
- Guo, L.; Tong, L.-T.; Liu, L.; Zhong, K.; Qiu, J.; Zhou, S. The Cholesterol-Lowering Effects of Oat Varieties Based on Their Difference in the Composition of Proteins and Lipids. Lipids Health Dis. 2014, 13, 182. [Google Scholar] [CrossRef]
- Ryan, D.; Kendall, M.; Robards, K. Bioactivity of Oats as It Relates to Cardiovascular Disease. Nutr. Res. Rev. 2007, 20, 147–162. [Google Scholar] [CrossRef]
- Panahi, S.; Ezatagha, A.; Temelli, F.; Vasanthan, T.; Vuksan, V. β-Glucan from Two Sources of Oat Concentrates Affect Postprandial Glycemia in Relation to the Level of Viscosity. J. Am. Coll. Nutr. 2007, 26, 639–644. [Google Scholar] [CrossRef]
- Xue, Y.; Cui, L.; Qi, J.; Ojo, O.; Du, X.; Liu, Y.; Wang, X. The Effect of Dietary Fiber (Oat Bran) Supplement on Blood Pressure in Patients with Essential Hypertension: A Randomized Controlled Trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2458–2470. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Kasubuchi, M.; Nakajima, A.; Irie, J.; Itoh, H.; Kimura, I. The Role of Short-Chain Fatty Acid on Blood Pressure Regulation. Curr. Opin. Nephrol. Hypertens. 2016, 25, 379–383. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, L.; Qian, H.; Qi, X.; Ding, X.; Hu, B.; Li, J. The Effect of Oat β -Glucan on in Vitro Glucose Diffusion and Glucose Transport in Rat Small Intestine. J. Sci. Food Agric. 2016, 96, 484–491. [Google Scholar] [CrossRef]
- Quraishi, M.N.; Sergeant, M.; Kay, G.; Iqbal, T.; Chan, J.; Constantinidou, C.; Trivedi, P.; Ferguson, J.; Adams, D.H.; Pallen, M.; et al. The Gut-Adherent Microbiota of PSC–IBD Is Distinct to That of IBD. Gut 2017, 66, 386–388. [Google Scholar] [CrossRef]
- Kataoka, K. The Intestinal Microbiota and Its Role in Human Health and Disease. J. Med. Investig. 2016, 63, 27–37. [Google Scholar] [CrossRef]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why Definitions Matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pineiro, M.; Asp, N.-G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO Technical Meeting on Prebiotics. J. Clin. Gastroenterol. 2008, 42 (Suppl. 3), S156–S159. [Google Scholar] [CrossRef]
- van Loveren, H.; Sanz, Y.; Salminen, S. Health Claims in Europe: Probiotics and Prebiotics as Case Examples. Annu. Rev. Food Sci. Technol. 2012, 3, 247–261. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Various Food(s)/Food Constituents(s) and Increasing Numbers of Gastro-Intestinal Microorganisms (ID 760, 761, 779, 780, 779, 1905), and Decreasing Potentially Pathogenic Gastro-Intestina. EFSA J. 2010, 8, 1809. [Google Scholar] [CrossRef]
- Saito, M. Role of FOSHU (Food for Specified Health Uses) for Healthier Life. YAKUGAKU ZASSHI 2007, 127, 407–416. [Google Scholar] [CrossRef][Green Version]
- Canadian Food Inspection Agency. Use of the Term “Prebiotic(s)”. In Health Claims on Food Labels; Canadian Food Inspection Agency: Ottawa, ON, Canada, 2019. [Google Scholar]
- Lin, B.; Gong, J.; Wang, Q.; Cui, S.; Yu, H.; Huang, B. In-Vitro Assessment of the Effects of Dietary Fibers on Microbial Fermentation and Communities from Large Intestinal Digesta of Pigs. Food Hydrocoll. 2011, 25, 180–188. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Belzer, C.; Chia, L.W.; Aalvink, S.; Chamlagain, B.; Piironen, V.; Knol, J.; de Vos, W.M. Microbial Metabolic Networks at the Mucus Layer Lead to Diet-Independent Butyrate and Vitamin B12 Production by Intestinal Symbionts. mBio 2017, 8, e00770-17. [Google Scholar] [CrossRef]
- Flint, H.J. Gut Microbial Metabolites in Health and Disease. Gut Microbes 2016, 7, 187–188. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The Gut Microbiota, Bacterial Metabolites and Colorectal Cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic Availability and Metabolism of Colonic-Derived Short-Chain Fatty Acids in Healthy Subjects: A Stable Isotope Study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Tazoe, H.; Otomo, Y.; Karaki, S.; Kato, I.; Fukami, Y.; Terasaki, M.; Kuwahara, A. Expression of Short-Chain Fatty Acid Receptor GPR41 in the Human Colon. Biomed. Res. 2009, 30, 149–156. [Google Scholar] [CrossRef]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs Strongly Stimulate PYY Production in Human Enteroendocrine Cells. Sci. Rep. 2018, 8, 74. [Google Scholar] [CrossRef]
- Arora, T.; Tremaroli, V. Therapeutic Potential of Butyrate for Treatment of Type 2 Diabetes. Front. Endocrinol. 2021, 12, 761834. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of Inflammatory Responses by Gut Microbiota and Chemoattractant Receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and Anti-Inflammatory Effects of Short Chain Fatty Acids on Immune and Endothelial Cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Shen, T.-C.D.; Albenberg, L.; Bittinger, K.; Chehoud, C.; Chen, Y.-Y.; Judge, C.A.; Chau, L.; Ni, J.; Sheng, M.; Lin, A.; et al. Engineering the Gut Microbiota to Treat Hyperammonemia. J. Clin. Investig. 2015, 125, 2841–2850. [Google Scholar] [CrossRef]
| Authors (Year) | Country | Title | Journal | Food Matrix or Supplement | Aim | Methodology | Outcomes of Interest |
|---|---|---|---|---|---|---|---|
| Wang, Qi, Guo, Song, Pang, Fang, and Peng (2022) [46] | China | Effects of Oats, Tartary Buckwheat, and Foxtail Millet Supplementation on Lipid Metabolism, Oxide-Inflammatory Responses, Gut Microbiota, and Colonic SCFA Composition in High-Fat-Diet-Fed Rats | Nutrients | Cooked oats, tartaric buckwheat, and foxtail millet | Investigate the effect of cooked oats, tartaric buckwheat, and foxtail millet action on lipid levels, oxide-inflammatory responses, intestinal microbiota, and SCFA in rats. | Sixty male Sprague Dawley rats (n = 12 per group) were fed a basal diet, a high-fat diet (HFD), HFD with 22% cooked oats, HFD with 22% buckwheat, and HFD with 22% millet for 12 weeks. |
|
| Han, Gao, Song, Zhang, Li, and Zhang (2021) [47] | China | Oat Fiber Modulates Hepatic Circadian Clock via Promoting Gut Microbiota-Derived Short-Chain Fatty Acids | Journal of Agricultural and Food Chemistry | Oat Fiber | Evaluate the action of SCFAs produced by gut microbiota on circadian rhythm. | Seventy-two male C57BL/6 mice (24 per group) were fed a control diet, HFD, or HFD with 0.8% oat for 21 weeks. |
|
| Ji, Ma, Zhang, Wang, Tao, Pei, and Hu (2021) [48] | China | Dietary Intake of Mixture Coarse Cereals Prevents Obesity by Altering the Gut Microbiota in High-Fat-Diet-Fed Mice | Food and Chemical Toxicology | Mix with millet, maize, oat, soybean, and purple potato | Evaluate the consumption of mixture coarse cereals on obesity prevention and gut microbiota in HFD-fed mice. | Forty-eight male C57BL/6 mice (n = 8 per group) were fed a chow diet (10% calories from fat) containing either 0%, 20%, or 40% mixture coarse cereals; or a HFD (45% calories from fat) containing either 0%, 20%, or 40% mixture coarse cereals for 8 weeks. |
|
| Kundi, Lee, Pihlajamaki, Chan, Leung, Yu So, Nordlund, Kolehmainen, and El-Nezami (2020) [49] | China | Dietary Fiber from Oat and Rye Brans Ameliorate Western Diet–Induced Body Weight Gain and Hepatic Inflammation by the Modulation of Short-Chain Fatty Acids, Bile Acids, and Tryptophan Metabolism | Molecular Nutrition and Food Research | Oat bran | Elucidate the protective mechanisms conferred by oat and rye fibers in a Western diet. | Forty-eight male C57BL/6N mice (n = 12 per group) were fed a chow diet, a Western diet (WD), or a WD with 10% oat or rye bran, for 17 weeks. |
|
| Gao, Song, Li, Zhang, Wan, Wang, Zhang, and Han (2020) [50] | China | Effects of Oat Fiber Intervention on Cognitive Behavior in LDLR−/− Mice Modeling Atherosclerosis by Targeting the Microbiome-Gut-Brain Axis | Journal of Agricultural and Food Chemistry | Oat fiber | Elucidate the oat fiber action on cognitive behavior through neuroinflammatory signals and the gut–brain axis. | Twenty male LDLR−/− mice (n = 10 per group) were fed a high-fat-and-cholesterol (HFC) diet (46% kcal from fat) with or without 0.8% oat fiber for 14 weeks. Ten male wild-type mice who were fed chow were used as controls. |
|
| Huang, Yu, Li, Guan, Liu, Song, Liu, and Duan (2020) [51] * | China | Effect of Embryo-Remaining Oat Rice on the Lipid Profile and Intestinal Microbiota in High-Fat-Diet-Fed Rats | Food Research International | Embryo-remaining oat rice (EROR) | Investigate the effects of Embryo-remaining oat rice (EROR) on lipid profile, cecal SCFAs, and intestinal microbiota in HFD-fed rats. | Twenty-four male SD rats (n = 6 per group) were fed a normal diet, HFD, HFD with 10% EROR, HFD with 50% EROR for 4 weeks. |
|
| Sun, Tong, Liang, Wang, Liu, Zhou, and Zhou (2019) [52] | China | Effect of Oat and Tartary Buckwheat-based Food on Cholesterol-Lowering and Gut Microbiota in Hypercholesterolemic Hamsters | Journal of Oleo science | Oat (65%) and tartary buckwheat (25%) blend | Investigate the effects of oat-based food on cholesterol and the composition of the gut microbiota. | Thirty male golden hamsters (n = 10 per group) were fed a control diet, HFD, and HFD with 10% oat/buckwheat blend for 30 days. |
|
| Dong, Zhu, Ma, Xiang, Shen, and Liu (2016) [53] | China | Oat Products Modulate the Gut Microbiota and Produce Anti-Obesity Effects in Obese Rats | Journal of Functional Foods | Oat meal: OM; oat flour: OF; and oat bran: OB | Compare the actions of oat meal, oat flour, and high-fiber oat bran on lipid metabolism, as well as gut microbiota of HFD-fed rats. | Eighty male SD (n = 10 per group) rats were fed either a chow-control diet, an HFD, an HFD with oat meal, an HFD with oat flour, or an HFD with high-fiber oat bran for 8 weeks. |
|
| Wilczak, Blaszczyk, Kamola, Gajewska, Harasym, Jatosinska, Gudej, Suchecka, Oczkowski, Gromadzka–Ostrowska (2015) [54] | Poland | The Effect of Low- or High-Molecular-Weight Oat β-glucans on the Inflammatory and Oxidative Stress Status in the Colon of Rats with LPS-Indices Enteritis | Food and Function | Low- and high-molecular-weight oat β-glucans | Investigate the protective effect of low- and high-molecular-weight β-glucans in gut immune markers, microbiota changes and SCFAs. | Seventy-two male SD rats (n = 12 per group) were fed a control diet, low-molecular-weight β-glucan (1%) diet, or high-molecular-weight β-glucan (1%) diet for 6 weeks; half were administered LPS to induce enteritis. |
|
| Authors (Year) | Country | Title | Journal | Food Matrix or Supplement | The Aim | Methodology | Outcomes of Interest |
|---|---|---|---|---|---|---|---|
| Bai, Zhang, Zhang, Zhang, Huo, Guo (2022) [55] | China | In Vitro Fermentation of Pretreated Oat Bran by Human Fecal Inoculum and Impact on Microbiota | Journal of Functional Foods | Oat bran pretreated | Determine the prebiotic effects of different pretreatments of oat bran. | Oat bran was steamed, microwaved, or dryed with hot air. The samples were exposed to in vitro simulated digestion and added to human fecal inoculum to investigate fermentation metabolites and impacts on microbiota. |
|
| Liang, xie, Evivie, Zhao, Chen, Xu, Liu, Li, Huo (2021) [56] | China | Study on Supplementary Food with Beneficial Effects on the Gut Microbiota of Infants | Food Bioscience | Ready-to-use supplementary food with oat, corn, and millet | Investigate the impact of oat-, corn-, and millet-supplementary foods on gut microbiota and SCFA production. | Oat-, corn-, and millet-supplementary foods and an oligosaccharide control were added to human fecal inoculum from 6 infant donors (age 6–12 months) to investigate fermentation metabolites and impacts on microbiota. |
|
| Glei, Zetzmann, Lorkowschi, Dawczynski, and Scholormann (2020) [57] | Germany | Chemo-Preventive Effects of Raw and Roasted Oat Flakes After In Vitro Fermentation With Human Fecal Microbiota | International Journal of Food Sciences and Nutrition | Oat flakes | Analyzed the chemo-preventive effects of raw and roasted oat flakes, evaluating the processing in colon adenoma cells. | The oat flakes were roasted at 140 °C–160 °C for 20 min. The raw and roasted oat flakes were exposed to an in vitro simulated digestion and fermentation with human microbiota. Then, the fermentation supernatants (FS) obtained were characterized, and chemo-preventive effects were analyzed in LT97 colon adenoma cells. |
|
| Huang, Yu, Li, Guan, Liu, Song, Liu, and Duan (2020) [51] * | China | Effect of Embryo-Remaining Oat Rice on the Lipid Profile and Intestinal Microbiota in High-Fat-Diet-Fed Rats | Food Research International | Embryo-remaining oat rice (EROR) | Investigate the effects of an extract of EROR on lipid accumulation. | Water and lipid extracts of EROR were incubated with HepG2 cells to evaluate lipid accumulation in vitro. |
|
| Kristek, Wiese, Heuer, Kosik, Schar, Soycan, Alsharif, Kuhnle, Walton, and Spencer (2019) [58] | United Kingdom and Denmark | Oat Bran, But Not Its Isolated Bioactive β-glucans or Polyphenols, Has a Bifidogenic Effect in an In Vitro Fermentation Model of the Gut Microbiota | British Journal of Nutrition | Oat bran, β-glucan extract, oat polyphenols | Evaluate and compare the effects of an oatpolyphenol mix (avenanthramide, hydroxycinnamic acids, and benzoic acid derivatives), β-glucan extract (BG), and oat bran on gut microbiota. | The oat bran was digested in vitro, and the polyphenols were extracted from undigested (raw) and digested (after in vitro digestion) oat bran. Two different doses (1 and 3% (w/v)) of oat bran and matched concentrations of β-glucan extract or polyphenol mix were added to anaerobic fecal batch cultures. |
|
| Carlson, Erickson, Hess, Gould and Slavin (2017) [4] | United States | Prebiotic Dietary Fiber and Gut Health: Comparing the In Vitro Fermentations of Beta-Glucan, Inulin, and Xylooligosaccharide | Nutrients | β-glucan and Oatwell (oat bran with 22% β-glucan) | Compare the fermentation effects of prebiotics (inulin, xylooligosaccharides and β-glucan based products) in the production of SCFA. | Five common prebiotic dietary fibers, OatWell, WholeFiber (dried chicory root blend with inulin, pectin, hemi and cellulose), xylooligosaccharide, pure inulin, and pure β-glucan, were incubated in fecal inoculum from healthy adults to measure microbiota, SCFA, and gas production. |
|
| Authors (Year) | Country | Title | Journal | Food Matrix or Supplement | The Aim | Methodology | Outcomes of Interest |
|---|---|---|---|---|---|---|---|
| Xu, Feng, Chu, Wang, Shete, Tuohy, Liu, Zhou, Kamil, Pan, Liu, Yang, Yang, Zhu, Lv, Xiong, Wang, Sun, Sun, and Yang (2021) [59] | China | The Prebiotic Effects of Oats on Blood Lipids, Gut Microbiota, and Short-Chain Fatty Acids in Mildly Hypercholesterolemic Subjects Compared with Rice: A Randomized, Controlled Trial | Frontiers in Immunology | Oat | Evaluate the relationship of blood lipids, intestinal microbiota, and SCFAs in a Chinese population with mild hypercholesterolemia. | In a randomized parallel design, 210 mildly hypercholesterolemic males and females from Beijing, Nanjing, and Shanghai were assigned to a diet containing 80 g/d of oats or rice (control) for 45 days, along with their habitual diet. |
|
| Valeur, Puaschitz, Midtvedt, and Berstad (2016) [60] | Norway | Oatmeal Porridge: Impact on Microflora-Associated Characteristics in Healthy Subjects | British Journal of Nutrition | Oatmeal porridge | Evaluate the effect of oatmeal porridge consumption every day for one week on fecal SCFAs, gas production, and inflammatory markers. | Ten healthy males and females consumed a daily portion of 60 g oatmeal porridge for 7 days. Lactulose-induced intestinal gas production, fecal excretion of SCFA, fecal levels of β-galactosidase and urease, and PGE2 levels were analyzed. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabiano, G.A.; Shinn, L.M.; Antunes, A.E.C. Relationship between Oat Consumption, Gut Microbiota Modulation, and Short-Chain Fatty Acid Synthesis: An Integrative Review. Nutrients 2023, 15, 3534. https://doi.org/10.3390/nu15163534
Fabiano GA, Shinn LM, Antunes AEC. Relationship between Oat Consumption, Gut Microbiota Modulation, and Short-Chain Fatty Acid Synthesis: An Integrative Review. Nutrients. 2023; 15(16):3534. https://doi.org/10.3390/nu15163534
Chicago/Turabian StyleFabiano, Giovanna Alexandre, Leila Marie Shinn, and Adriane Elisabete Costa Antunes. 2023. "Relationship between Oat Consumption, Gut Microbiota Modulation, and Short-Chain Fatty Acid Synthesis: An Integrative Review" Nutrients 15, no. 16: 3534. https://doi.org/10.3390/nu15163534
APA StyleFabiano, G. A., Shinn, L. M., & Antunes, A. E. C. (2023). Relationship between Oat Consumption, Gut Microbiota Modulation, and Short-Chain Fatty Acid Synthesis: An Integrative Review. Nutrients, 15(16), 3534. https://doi.org/10.3390/nu15163534






