Vitamin D Supplementation Influences Ultramarathon-Induced Changes in Serum Amino Acid Levels, Tryptophan/Branched-Chain Amino Acid Ratio, and Arginine/Asymmetric Dimethylarginine Ratio
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Overview
2.2. Participants
2.3. Ultramarathon Run
2.4. Vitamin D Supplementation
2.5. Sample Collection
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Limitations
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mieszkowski, J.; Niespodzinski, B.; Kochanowicz, A.; Gmiat, A.; Prusik, K.; Prusik, K.; Kortas, J.; Ziemann, E.; Antosiewicz, J. The Effect of Nordic Walking Training Combined with Vitamin D Supplementation on Postural Control and Muscle Strength in Elderly People—A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2018, 15, 1951. [Google Scholar] [CrossRef] [Green Version]
- Grimaldi, A.S.; Parker, B.A.; Capizzi, J.A.; Clarkson, P.M.; Pescatello, L.S.; White, M.C.; Thompson, P.D. 25(OH) vitamin D is associated with greater muscle strength in healthy men and women. Med. Sci. Sports Exerc. 2013, 45, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, R.J.; Close, G.L.; Farooq, A.; Riding, N.R.; Salah, O.; Hamilton, B.; Wilson, M.G. Severely vitamin D-deficient athletes present smaller hearts than sufficient athletes. Eur. J. Prev. Cardiol. 2015, 22, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Angeline, M.E.; Gee, A.O.; Shindle, M.; Warren, R.F.; Rodeo, S.A. The effects of vitamin D deficiency in athletes. Am. J. Sports Med. 2013, 41, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Mieszkowski, J.; Kochanowicz, A.; Piskorska, E.; Niespodzinski, B.; Siodmiak, J.; Busko, K.; Stankiewicz, B.; Olszewska-Slonina, D.; Antosiewicz, J. Serum levels of bone formation and resorption markers in relation to vitamin D status in professional gymnastics and physically active men during upper and lower body high-intensity exercise. J. Int. Soc. Sports Nutr. 2021, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin D status and nutrition in Europe and Asia. J. Steroid Biochem. Mol. Biol. 2007, 103, 620–625. [Google Scholar] [CrossRef]
- Farrokhyar, F.; Tabasinejad, R.; Dao, D.; Peterson, D.; Ayeni, O.R.; Hadioonzadeh, R.; Bhandari, M. Prevalence of vitamin D inadequacy in athletes: A systematic-review and meta-analysis. Sports Med. 2015, 45, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Valle, M.S.; Casabona, A.; Spicuzza, L.; Sambataro, G.; Malaguarnera, L. Vitamin D Impacts on Skeletal Muscle Dysfunction in Patients with COPD Promoting Mitochondrial Health. Biomedicines 2022, 10, 898. [Google Scholar] [CrossRef] [PubMed]
- Capiati, D.; Benassati, S.; Boland, R.L. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J. Cell. Biochem. 2002, 86, 128–135. [Google Scholar] [CrossRef]
- Dhesi, J.K.; Bearne, L.M.; Moniz, C.; Hurley, M.V.; Jackson, S.H.; Swift, C.G.; Allain, T.J. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J. Bone Miner. Res. 2002, 17, 891–897. [Google Scholar] [CrossRef]
- Costa, E.M.; Blau, H.M.; Feldman, D. 1,25-dihydroxyvitamin D3 receptors and hormonal responses in cloned human skeletal muscle cells. Endocrinology 1986, 119, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.; Saini, A.; Stromberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for Vitamin D Receptor Expression and Direct Effects of 1alpha,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology 2016, 157, 98–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlinson, P.B.; Joseph, C.; Angioi, M. Effects of vitamin D supplementation on upper and lower body muscle strength levels in healthy individuals. A systematic review with meta-analysis. J. Sci. Med. Sport 2015, 18, 575–580. [Google Scholar] [CrossRef]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.D.; Wang, X.; Lanza, I.R.; Schaible, N.S.; Salisbury, J.L.; Nair, K.S.; Terzic, A.; Sieck, G.C.; et al. 1alpha,25-Dihydroxyvitamin D3 Regulates Mitochondrial Oxygen Consumption and Dynamics in Human Skeletal Muscle Cells. J. Biol. Chem. 2016, 291, 1514–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyere, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [Green Version]
- Rowland, S.N.; Da Boit, M.; Tan, R.; Robinson, G.P.; O’Donnell, E.; James, L.J.; Bailey, S.J. Dietary Nitrate Supplementation Enhances Performance and Speeds Muscle Deoxyhaemoglobin Kinetics during an End-Sprint after Prolonged Moderate-Intensity Exercise. Antioxidants 2022, 12, 25. [Google Scholar] [CrossRef]
- Olek, R.A.; Ziemann, E.; Grzywacz, T.; Kujach, S.; Luszczyk, M.; Antosiewicz, J.; Laskowski, R. A single oral intake of arginine does not affect performance during repeated Wingate anaerobic test. J. Sports Med. Phys. Fit. 2010, 50, 52–56. [Google Scholar]
- Tain, Y.L.; Hsu, C.N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92. [Google Scholar] [CrossRef] [Green Version]
- Palm, F.; Onozato, M.L.; Luo, Z.; Wilcox, C.S. Dimethylarginine dimethylaminohydrolase (DDAH): Expression, regulation, and function in the cardiovascular and renal systems. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3227–H3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzik, K.; Skrobot, W.; Flis, D.J.; Karnia, M.; Libionka, W.; Kloc, W.; Kaczor, J.J. Vitamin D supplementation attenuates oxidative stress in paraspinal skeletal muscles in patients with low back pain. Eur. J. Appl. Physiol. 2018, 118, 143–151. [Google Scholar] [CrossRef]
- Tsuda, Y.; Yamaguchi, M.; Noma, T.; Okaya, E.; Itoh, H. Combined Effect of Arginine, Valine, and Serine on Exercise-Induced Fatigue in Healthy Volunteers: A Randomized, Double-Blinded, Placebo-Controlled Crossover Study. Nutrients 2019, 11, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohm, G.L. Protein as a fuel for endurance exercise. Exerc. Sport Sci. Rev. 1986, 14, 143–173. [Google Scholar] [CrossRef] [PubMed]
- Nyborg, C.; Bonnevie-Svendsen, M.; Melsom, H.S.; Melau, J.; Seljeflot, I.; Hisdal, J. Reduced L-Arginine and L-Arginine-ADMA-Ratio, and Increased SDMA after Norseman Xtreme Triathlon. Sports 2021, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Dzik, K.P.; Skrobot, W.; Kaczor, K.B.; Flis, D.J.; Karnia, M.J.; Libionka, W.; Antosiewicz, J.; Kloc, W.; Kaczor, J.J. Vitamin D Deficiency Is Associated with Muscle Atrophy and Reduced Mitochondrial Function in Patients with Chronic Low Back Pain. Oxid. Med. Cell. Longev. 2019, 2019, 6835341. [Google Scholar] [CrossRef]
- Bode-Boger, S.M.; Scalera, F.; Ignarro, L.J. The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef]
- Tsikas, D. Urinary Dimethylamine (DMA) and Its Precursor Asymmetric Dimethylarginine (ADMA) in Clinical Medicine, in the Context of Nitric Oxide (NO) and Beyond. J. Clin. Med. 2020, 9, 1843. [Google Scholar] [CrossRef]
- Areces, F.; Gonzalez-Millan, C.; Salinero, J.J.; Abian-Vicen, J.; Lara, B.; Gallo-Salazar, C.; Ruiz-Vicente, D.; Del Coso, J. Changes in Serum Free Amino Acids and Muscle Fatigue Experienced during a Half-Ironman Triathlon. PLoS ONE 2015, 10, e0138376. [Google Scholar] [CrossRef]
- Mieszkowski, J.; Brzezinska, P.; Stankiewicz, B.; Kochanowicz, A.; Niespodzinski, B.; Reczkowicz, J.; Waldzinski, T.; Kacprzak, B.; Siuba-Jarosz, N.; Petr, M.; et al. Direct Effects of Vitamin D Supplementation on Ultramarathon-Induced Changes in Kynurenine Metabolism. Nutrients 2022, 14, 4485. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Newsholme, E.A. The effect of tryptophan deficiency in the brain on rat fatigue levels: A rat model of fatigue reduction. Adv. Exp. Med. Biol. 2003, 527, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Chaouloff, F. Effects of acute physical exercise on central serotonergic systems. Med. Sci. Sports Exerc. 1997, 29, 58–62. [Google Scholar] [CrossRef]
- Blomstrand, E.; Hassmen, P.; Ekblom, B.; Newsholme, E.A. Administration of branched-chain amino acids during sustained exercise--effects on performance and on plasma concentration of some amino acids. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 63, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Spoto, B.; Parlongo, R.M.; Parlongo, G.; Sgro, E.; Zoccali, C. The enzymatic machinery for ADMA synthesis and degradation is fully expressed in human adipocytes. J. Nephrol. 2007, 20, 554–559. [Google Scholar] [PubMed]
- Wassner, S.J.; Li, J.B.; Sperduto, A.; Norman, M.E. Vitamin D Deficiency, hypocalcemia, and increased skeletal muscle degradation in rats. J. Clin. Investig. 1983, 72, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Deeg, D.J.; Lips, P.; Longitudinal Aging Study, A. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef] [Green Version]
- Cannell, J.J.; Hollis, B.W.; Sorenson, M.B.; Taft, T.N.; Anderson, J.J. Athletic performance and vitamin D. Med. Sci. Sports Exerc. 2009, 41, 1102–1110. [Google Scholar] [CrossRef]
- Ceglia, L.; Harris, S.S. Vitamin D and its role in skeletal muscle. Calcif. Tissue Int. 2013, 92, 151–162. [Google Scholar] [CrossRef]

| Variable | Effect | F | Df | p | Effect Size (η2) | Post Hoc Outcome |
|---|---|---|---|---|---|---|
| Alanine (Ala) | GR | 1.46 | 1, 33 | 0.24 | 0.10 | |
| UM | 2.30 | 2, 66 | 0.12 | 0.16 | ||
| GR × UM | 8.34 | 2, 66 | 0.01 ** | 0.41 | SI > SII, SIII; CII < SII | |
| Arginine (Arg) | GR | 4.07 | 1, 33 | 0.66 | 0.25 | |
| UM | 79.84 | 2, 66 | 0.01 ** | 0.86 | II < I, III; I > III | |
| GR × UM | 57.15 | 2, 66 | 0.01 ** | 0.82 | SI > SII; CII < SII | |
| Asparagine (Asn) | GR | 0.02 | 1, 33 | 0.86 | 0.01 | |
| UM | 17.98 | 2, 66 | 0.01 ** | 0.59 | II < I, III | |
| GR × UM | 8.04 | 2, 66 | 0.01 ** | 0.40 | SII < SI, SIII | |
| Aspartic acid (Asp) | GR | 0.40 | 1, 33 | 0.53 | 0.03 | |
| UM | 50.57 | 2, 66 | 0.01 ** | 0.80 | II < I, III; I > III | |
| GR × UM | 15.50 | 2, 66 | 0.01 ** | 0.56 | SII < SI, SIII; CII < SII | |
| Cysteine (Cys) | GR | 11.05 | 1, 33 | 0.01 ** | 0.47 | S > C |
| UM | 3.84 | 2, 66 | 0.03 * | 0.24 | II < I | |
| GR × UM | 1.10 | 2, 66 | 0.34 | 0.08 | ||
| Glutamic acid (Glu) | GR | 2.49 | 1, 33 | 0.14 | 0.17 | I > III |
| UM | 3.65 | 2, 66 | 0.04 * | 0.23 | ||
| GR × UM | 0.68 | 2, 66 | 0.51 | 0.05 | ||
| Glutamine (Gln) | GR | 1.29 | 1, 33 | 0.27 | 0.09 | |
| UM | 7.53 | 2, 66 | 0.01 ** | 0.38 | II < I, III | |
| GR × UM | 9.05 | 2, 66 | 0.01 ** | 0.43 | SII < SI, SIII | |
| Glycine (Gly) | GR | 0.21 | 1, 33 | 0.65 | 0.01 | |
| UM | 14.04 | 2, 66 | 0.01 ** | 0.53 | II < I, III | |
| GR × UM | 14.03 | 2, 66 | 0.01 ** | 0.53 | SII < SI, SIII | |
| Histidine (His) | GR | 1.54 | 1, 33 | 0.11 | 0.11 | |
| UM | 9.80 | 2, 66 | 0.44 | 0.44 | II < I, III | |
| GR × UM | 11.34 | 2, 66 | 0.48 | 0.48 | SII < SI, SIII; CII < SII | |
| Isoleucine (Ile) | GR | 0.12 | 1, 33 | 0.72 | 0.01 | II < I, III |
| UM | 10.31 | 2, 66 | 0.01 ** | 0.46 | ||
| GR × UM | 1.73 | 2, 66 | 0.19 | 0.12 | ||
| Leucine (Leu) | GR | 0.75 | 1, 33 | 0.78 | 0.01 | II < I, III |
| UM | 12.99 | 2, 66 | 0.01 ** | 0.51 | ||
| GR × UM | 2.18 | 2, 66 | 0.13 | 0.15 | ||
| Lysine (Lys) | GR | 0.89 | 1, 33 | 0.36 | 0.06 | II < I, III |
| UM | 9.90 | 2, 66 | 0.01 ** | 0.45 | ||
| GR × UM | 1.70 | 2, 66 | 0.20 | 0.12 | ||
| Methionine (Met) | GR | 0.89 | 1, 33 | 0.36 | 0.06 | |
| UM | 4.22 | 2, 66 | 0.02 * | 0.26 | II < III | |
| GR × UM | 2.86 | 2, 66 | 0.01 * | 0.28 | SII < SI, SIII; CII < SII | |
| Phenylalanine Tyrosine (Phe + Tyr) | GR | 2.85 | 1, 33 | 0.11 | 0.19 | |
| UM | 0.54 | 2, 66 | 0.58 | 0.04 | ||
| GR × UM | 27.26 | 2, 66 | 0.01 ** | 0.69 | SII < SI, SIII; CII > CI, CIII; CII > SII | |
| Proline (Pro) | GR | 1.17 | 1, 33 | 0.30 | 0.08 | |
| UM | 13.21 | 2, 66 | 0.01 ** | 0.52 | I > II, III | |
| GR × UM | 0.74 | 2, 66 | 0.48 | 0.05 | ||
| Serine (Ser) | GR | 0.88 | 1, 33 | 0.36 | 0.68 | |
| UM | 24.40 | 2, 66 | 0.01 ** | 0.67 | I > II, III; II < III | |
| GR × UM | 23.15 | 2, 66 | 0.01 ** | 0.65 | SI > SII, SIII; SII < SIII; SII < CII | |
| Taurine (Tau) | GR | 2.13 | 1, 33 | 0.16 | 0.15 | |
| UM | 7.80 | 2, 66 | 0.01 ** | 0.39 | I > III | |
| GR × UM | 16.18 | 2, 66 | 0.01 ** | 0.57 | SII < SI; CII > SII | |
| Threonine (Thr) | GR | 0.68 | 1, 33 | 0.51 | 0.03 | |
| UM | 14.41 | 2, 66 | 0.01 ** | 0.55 | II < I, III | |
| GR × UM | 6.72 | 2, 66 | 0.01 ** | 0.30 | SII < SI, SIII | |
| Tryptophan (Trp) | GR | 0.53 | 1, 33 | 0.45 | 0.04 | |
| UM | 13.45 | 2, 66 | 0.01 ** | 0.52 | II < I, III | |
| GR × UM | 5.04 | 2, 66 | 0.01 * | 0.29 | SII < SI, SIII; CII > SII | |
| Valine (Val) | GR | 0.18 | 1, 33 | 0.67 | 0.01 | II < I, III |
| UM | 13.9 | 2, 66 | 0.01 ** | 0.53 | ||
| GR × UM | 0.32 | 2, 66 | 0.32 | 0.03 |
| Variable | Effect | F | Df | p | Effect Size (η2) | Post Hoc Outcome |
|---|---|---|---|---|---|---|
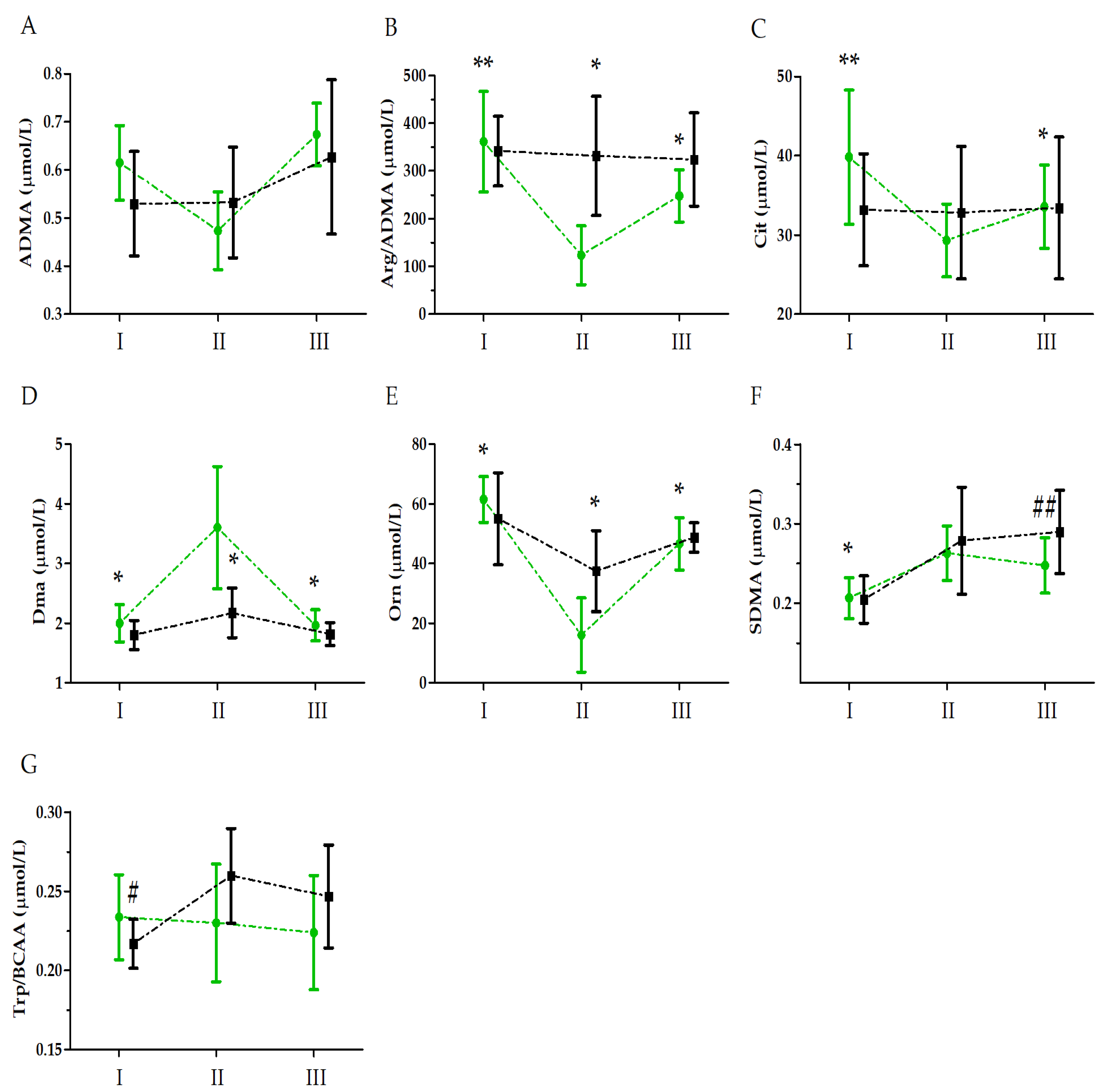
| Asymmetric dimethylarginine (ADMA) | GR | 0.32 | 1, 33 | 0.57 | 0.02 | |
| UM | 12.10 | 2, 66 | 0.01 ** | 0.50 | II < I, III; I < III | |
| GR × UM | 3.12 | 2, 66 | 0.06 | 0.20 | ||
| Arginine to asymmetric dimethylarginine ratio (Arg/ADMA) | GR | 4.11 | 1, 33 | 0.06 | 0.25 | |
| UM | 28.11 | 2, 66 | 0.01 ** | 0.70 | II < I, III; I > III | |
| GR × UM | 23.96 | 2, 66 | 0.01 ** | 0.66 | SII < SI, SIII; SI > SIII; CII > SII | |
| Beta-Alanine (bAla) | GR | 0.44 | 1, 33 | 0.51 | 0.03 | |
| UM | 2.64 | 2, 66 | 0.09 | 0.18 | ||
| GR × UM | 2.65 | 2, 66 | 0.09 | 0.18 | ||
| Citrulline (Cit) | GR | 0.35 | 1, 33 | 0.55 | 0.01 | |
| UM | 6.01 | 2, 66 | 0.01 ** | 0.16 | II < I | |
| GR × UM | 8.02 | 2, 66 | 0.01 ** | 0.20 | SII < SI, SIII; SI > SIII | |
| Dimethylamine (DMA) | GR | 8.18 | 1, 33 | 0.01 * | 0.42 | S > C |
| UM | 23.49 | 2, 66 | 0.01 ** | 0.68 | II > I, III | |
| GR × UM | 3.63 | 2, 66 | 0.04 * | 0.24 | SII > SI, SIII; SII > CII | |
| Gamma-aminobutyric acid (GABA) | GR | 0.08 | 1, 33 | 0.77 | 0.01 | |
| UM | 0.54 | 2, 66 | 0.58 | 0.04 | ||
| GR × UM | 2.50 | 2, 66 | 0.10 | 0.17 | ||
| Homoarginine (hArg) | GR | 0.34 | 1, 33 | 0.56 | 0.03 | |
| UM | 7.43 | 2, 66 | 0.01 ** | 0.42 | II < I, III; I > III | |
| GR × UM | 0.29 | 2, 66 | 0.74 | 0.02 | ||
| Ornithine (Orn) | GR | 2.58 | 1, 33 | 0.13 | 0.19 | |
| UM | 27.29 | 2, 66 | 0.01 ** | 0.71 | II < I, III; I > III | |
| GR × UM | 5.41 | 2, 66 | 0.01 * | 0.33 | SII < SI, SIII; CII > SII | |
| Sarcosine | GR | 0.82 | 1, 33 | 0.38 | 0.06 | |
| UM | 0.47 | 2, 66 | 0.62 | 0.03 | ||
| GR × UM | 3.13 | 2, 66 | 0.06 | 0.20 | ||
| Symmetric dimethylarginine (SDMA) | GR | 2.59 | 1, 33 | 0.11 | 0.07 | |
| UM | 44.56 | 2, 66 | 0.01 ** | 0.57 | I < II, III | |
| GR × UM | 3.96 | 2, 66 | 0.02 * | 0.11 | SI < SII, SIII; SIII < CIII | |
| Tryptophan to branched-chain amino acid ratio (Trp/BCAA) | GR | 0.79 | 1, 33 | 0.39 | 0.06 | |
| UM | 2.52 | 2, 66 | 0.10 | 0.17 | ||
| GR × UM | 4.61 | 2, 66 | 0.02 * | 0.27 | CII > CI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieszkowski, J.; Brzezińska, P.; Stankiewicz, B.; Kochanowicz, A.; Zolodkiewicz, K.; Niespodziński, B.; Reczkowicz, J.; Kowalik, T.; Waldziński, T.; Antosiewicz, J. Vitamin D Supplementation Influences Ultramarathon-Induced Changes in Serum Amino Acid Levels, Tryptophan/Branched-Chain Amino Acid Ratio, and Arginine/Asymmetric Dimethylarginine Ratio. Nutrients 2023, 15, 3536. https://doi.org/10.3390/nu15163536
Mieszkowski J, Brzezińska P, Stankiewicz B, Kochanowicz A, Zolodkiewicz K, Niespodziński B, Reczkowicz J, Kowalik T, Waldziński T, Antosiewicz J. Vitamin D Supplementation Influences Ultramarathon-Induced Changes in Serum Amino Acid Levels, Tryptophan/Branched-Chain Amino Acid Ratio, and Arginine/Asymmetric Dimethylarginine Ratio. Nutrients. 2023; 15(16):3536. https://doi.org/10.3390/nu15163536
Chicago/Turabian StyleMieszkowski, Jan, Paulina Brzezińska, Błażej Stankiewicz, Andrzej Kochanowicz, Katarzyna Zolodkiewicz, Bartłomiej Niespodziński, Joanna Reczkowicz, Tomasz Kowalik, Tomasz Waldziński, and Jędrzej Antosiewicz. 2023. "Vitamin D Supplementation Influences Ultramarathon-Induced Changes in Serum Amino Acid Levels, Tryptophan/Branched-Chain Amino Acid Ratio, and Arginine/Asymmetric Dimethylarginine Ratio" Nutrients 15, no. 16: 3536. https://doi.org/10.3390/nu15163536
APA StyleMieszkowski, J., Brzezińska, P., Stankiewicz, B., Kochanowicz, A., Zolodkiewicz, K., Niespodziński, B., Reczkowicz, J., Kowalik, T., Waldziński, T., & Antosiewicz, J. (2023). Vitamin D Supplementation Influences Ultramarathon-Induced Changes in Serum Amino Acid Levels, Tryptophan/Branched-Chain Amino Acid Ratio, and Arginine/Asymmetric Dimethylarginine Ratio. Nutrients, 15(16), 3536. https://doi.org/10.3390/nu15163536








