Abstract
The optimal therapy for patients with non-metastatic biochemically relapsed prostate cancer (BRPC-M0) after local therapy is elusive. Thus, the evaluation of new non-toxic compounds in BRPC-M0 patients is warranted. PectaSol®-Modified citrus pectin (P-MCP) is a food supplement categorized as GRAS (Generally Recognized As Safe) by the FDA. It is a competitive inhibitor of the galectin-3 protein, which is involved in cancer pathogenesis. In an early report of the present phase 2 study, P-MCP treatment for 6 months led to prostate-specific antigen doubling time (PSADT) improvement in 75% of patients with BRPC-M0. Herein, we report the second long-term treatment phase of an additional 12 months of P-MCP therapy (4.8 g × 3/day orally) in patients without disease progression after the initial 6 months of therapy. Of the 46 patients that entered the second treatment phase, 7 patients withdrew consent and decided to continue therapy out of pocket, and 39 initiated the second treatment phase. After a total of 18 months of P-MCP treatment, 85% (n = 33) had a durable long-term response, with 62% (n = 24) showing decreased/stable PSA, 90% (n = 35) PSADT improvement, and all with negative scans. No patient had grade 3/4 toxicity. In conclusion, P-MCP may have long-term durable efficacy and is safe in BRPC-M0.
1. Introduction
Globally, prostate cancer is the second most common solid tumor [1] and cause of cancer-related death in men from Westernized countries [2]. Although its etiology needs further elucidation, risk factors for prostate cancer development include age, family history, lifestyle, and hereditary syndromes [3]. With an age-standardized incidence rate of 31 per 100,000 [1] and approximately 1.5 million new cases reported in 2020 [1,4], prostate cancer incidence is increasing worldwide [4,5] and presents a significant economic burden [6]. However, with the rapid evolution of treatment options [7], and changes in screening protocols [8], prostate cancer-related mortality patterns have stabilized [5]. Primary treatment options for localized prostate cancer include radical prostatectomy, radiation therapy, and androgen deprivation therapy (ADT) for advanced cases [9]. Within 10 years of treatment, 20–50% of patients will experience a biochemical relapse, with rising PSA and negative scans (BRPC-M0) [9,10,11,12,13]. In BRPC-M0 state, patients may remain asymptomatic and free of clinical evidence of disease for many years [14], and the PSA doubling time (PSADT) is a significant prognostic factor for development of future metastases and mortality [10,13,15,16,17]. The optimal therapy of patients with BRPC-MO is elusive [10,11,17,18,19,20,21,22] Androgen Deprivation Therapy (ADT) in this setting is associated with an uncertain survival benefit, may negatively impact quality of life, and increase comorbidities such as cardiovascular [10,14,23,24,25].
Thus, there is a need to evaluate new non-toxic therapies for BRPC-M0 [2,10,11,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. Pectins comprise carbohydrate-soluble fiber found in plant cell walls and are indigestible to humans in their unmodified form [39]. Modified citrus pectin (MCP), with shorter polysaccharide units, are water soluble [39] and have demonstrated significant anticancer activity [38,39,41,42], potentially by disrupting tumor-promoting signaling by binding galactose-containing side chains to the carbohydrate recognition domains of galectins [38,43,44]. Galectins comprise an evolutionarily conserved family of endogenous glycan-binding proteins and they play multifunctional roles in tumor progression [38] through modulating and recalibrating inter- and intra-cellular signaling [45]. Galectins affect cellular responses, including cell aggregation, growth, differentiation, apoptosis, and proliferation [38,45,46], and may promote immune evasion of cancer cells [47]. Galectins, including Galectin1 (GAL1) and GAL3, have therefore been explored as potentially effective therapeutic targets for cancer patients [38,48,49].
PectaSol®-Modified Citrus Pectin (P-MCP; EcoNugenics Inc., Santa Rosa, CA, USA) is a commercially available polysaccharide and is a Galectin-3 (Gal-3) inhibitor, binding to the Galectin-3 carbohydrate recognition domain [38,43]. Derived from the pith of citrus fruit peels, and classified by the US-FDA as generally regarded as safe (GRAS), data suggest that P-MCP is associated with cytotoxicity and inhibition of cancer cells [39,50], including prostate cancer cells [30,38,41,42].
In clinical trials evaluating new compounds in BRPC-MO, common endpoints include PSA dynamics as reflected by the PSADT [17,21,51,52] and are used as surrogate endpoints, both as predictive and as stratification factors for clinical disease progression [17,53]. Prolongation of PSADT may be an early signal of efficacy of active compounds in clinical trials [24,26,54].
In previous clinical trials in BRPC-MO, P-MCP therapy was associated with a positive effect on prostate-specific antigen (PSA) dynamics, including a decrease in PSA level and lengthening of PSADT in a significant proportion of patients, [30,41]. A prolongation of PSADT was shown with P-MCP therapy for 6 [30] and 12 [41] months. Herein, we report the efficacy with an extended total treatment period of 18 months, using the cohort reported previously by Keizman et al. (Nutrients 2021) [30], for which an early benefit was reported after 6 months of P-MCP therapy.
2. Methods and Materials
Study design: The eligibility criteria are described in our previous publication [30]. Briefly, patients with BRPC-M0, rising PSA post-primary therapy (surgery and/or radiation), and negative scans were included. All patients had a normal level of serum testosterone > 150 ng/mL and Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2 at study entry. All participating patients signed an Institutional Review Board (IRB)-approved consent form. Study participants were recruited between 2013 and 2019 from 5 medical centers in Israel (Meir, Rabin, Rambam, Soroka, and Tel-Aviv Sourasky). Patients were given 4.8 g of P-MCP to take orally 3 times per day for the duration of the study. The P-MCP was provided by PectaSol-C®, EcoNugenics, Santa Rosa, CA, USA, in packs of 270 capsules. Patients without evidence of disease progression or dose-limiting toxicity after 6 months of therapy (n = 46) entered the second long-term phase of the study and were given an additional 12 months of treatment. A total of 39 patients completed the full 18 months of treatment. The study design is described in Figure 1.
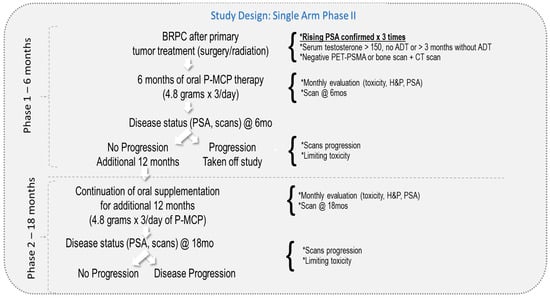
Figure 1.
Study design.
Evaluation of disease status: Patients underwent monthly visits for toxicities, physical examinations, and serum PSA, with baseline measurements taken prior to starting the P-MCP treatment protocol. A positron emission tomography (PET)—prostate-specific membrane antigen (PSMA) scan was conducted after 6 and 18 months in patients without clinical or PSA progression, or earlier upon clinical or PSA progression. The primary efficacy endpoint was the rate of patients without PSA progression (defined as an increase of ≥ 25% from baseline) and/or patients with improvement (lengthening) of PSADT versus baseline. The post-baseline PSADT was calculated using baseline PSA measurements obtained at the start of the study and every month during treatment. Secondary endpoints included the rate of patients without radiologic progression and toxicity, and with treatment benefits according to the PSADT risk grouping (e.g., poor < 3 months, intermediate 3–8.99 months, and good ≥9 months) [30].
Duration of treatment: Treatment as per the protocol continued for 12 additional months or until biochemical or clinical disease progression or dose-limiting toxicity.
Disease progression was defined as biochemical progression without PSADT prolongation and/or new radiological evidence of metastases.
Safety evaluation of toxicity: Toxicity was defined according to the National Cancer Institute (NCI) Common Toxicity Criteria, with treatments terminated at grades 3/4 and patients monitored weekly until ≤grade 1 before restarting therapy. Treatment would be discontinued upon the recurrence of a same grade 3/4 event and for any toxicity requiring longer than 4 weeks to recover to ≤grade 1.
Statistical analysis: Comparisons between pre- and post-treatment endpoint parameters, and within groups were analyzed using the Wilcoxon Signed Rank test for abnormally distributed data, or the two-tailed Student t-test for normally distributed data, with results reported as number, percentage, mean or median, and standard deviation (SD). A p-value ≤ 0.05 was considered statistically significant.
Regulatory Considerations: The research was conducted in accordance with the approval by the IRB committee of our institution. The study was registered at ClinicalTrials.gov (accessed on 10 September 2012) (NCT01681823).
3. Results
Patients: Of the 46 patients that displayed a benefit from the initial 6 months of therapy (in terms of stabilization/decrease of PSA, and/or improvement of PSADT, and with negative scans) and that were eligible for the second phase of an additional 12 months of therapy (to create a total of 18 months of treatment), 7 patients withdrew consent during the first month of the additional year of therapy and chose to continue the effective therapy independently, out of pocket, due to the travel distance to monthly medical visits. Thus, 39 patients were treated as per the protocol for a total of 18 months of treatment. Patient pre-treatment characteristics are summarized in Table 1.

Table 1.
Pre-treatment (baseline) patient characteristics.
Long-term outcome as determined by PSA level, PSADT, and disease progression: Out of the 39 patients that entered the second phase of the study (the additional 12 months of therapy, creating a total of 18 months), 85% (n = 33) demonstrated a decreased or stable PSA (Figure 2) and/or improvement of PSADT (54%, n = 21), with negative scans (90%, n = 35). Median PSADT improved significantly versus baseline (p = 0.003), from a median pre-treatment PSADT of 10.3 (median range = 1.4–54.6) months to a median post-treatment PSADT of 43.5 (median range = 3.5–981.0) months (Table 1 and Table 2).
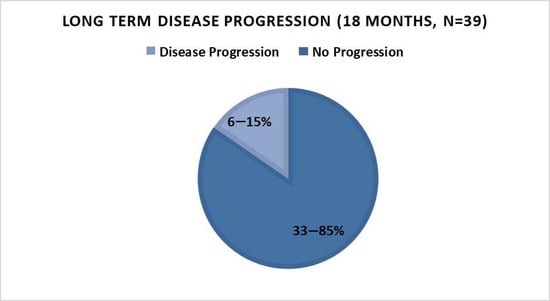
Figure 2.
Disease progression status after 18 months of PectaSol®-Modified Citrus Pectin (P-MCP) therapy.

Table 2.
Treatment characteristics and responses after 18 months of PectaSol®-Modified Citrus Pectin (P-MCP) therapy.
The benefits of 18 months of therapy in terms of PSA stabilization or decrease and/or PSADT lengthening were seen in all PSADT risk groups (Table 2). There was also an improvement in PSADT risk grouping after 18 months of treatment (Table 2 and Figure 3). After 18 months of P-MCP therapy, all patients with a pre-treatment PSADT risk grouping of poor (<3 months) improved their PSADT to an intermediate–good risk, and most patients (77%) with a pre-treatment risk PSADT of intermediate improved their PSADT risk to good (Table 2 and Figure 3). In patients with a good risk pre-treatment PSADT, 91% retained their risk grouping, 87% of whom had an improved PSADT. After 18 months of therapy, no patients remained in the poor risk PSADT group, and the proportion of patients with a PSADT risk of good (≥9.00) increased from 59% (n = 23) at baseline to 87% (n = 34) after 18 months of therapy (Figure 3). The pre-treatment (baseline) distribution of PSADT risk groupings improved after 18 months of P-MCP therapy, with the proportion of patients in the poor risk PSADT group decreasing from 8% to 0%. Similarly, the proportion of patients in the intermediate PSADT risk group decreased from 33% to 13%, and the proportion of patients in the good risk group increased from 59% to 87% (Table 1 and Table 2). There was a significant change in the median PSADT after 18 months of P-MCP therapy (Table 2) as compared to baseline (Table 2) for patients with a pre-treatment PSADT good risk (45.9 versus 14.7 months, p = 0.027) and intermediate risk (22.75 versus 5.1 months, p = 0.0015). Disease progression during the 18 months of therapy was observed in 18% (n = 7) of patients, 8% (n = 3) of which had PSA progression only (without radiological progression) and 10% (n = 4) had both PSA and radiologic progression.
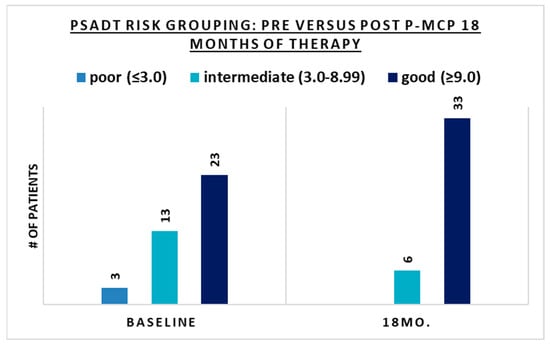
Figure 3.
The number of patients in each PSADT risk group at baseline and after 18 months of PectaSol®-Modified Citrus Pectin (P-MCP) therapy.
Toxicity and compliance: None of the patients had grade 3/4 toxicity during the 18 months of therapy. Grade 1 toxicity was observed in 30% (n = 12) of patients during the first 6 months of therapy, and in 23% (n = 9) during the subsequent 12 months of therapy. This was transient and reversible bloating that did not require treatment discontinuation.
4. Discussion
Prostate cancer is a leading cause of death in men worldwide [1], with non-metastatic biochemical disease progression after primary treatment presenting as a therapeutic challenge [10,20]. In the setting of BRPC-M0. there is no proven standard therapy [2,10,11,14,20]. While ADT is effective in lowering PSA level, its long-term survival benefit is not proven, and it is toxic. Thus, there is a need to investigate less toxic compounds in this setting [26,28,30,31,41].
In an earlier report of the present trial, 6-month therapy with PectaSol®-Modified Citrus Pectin (P-MCP; EcoNugenics Inc., Santa Rosa, CA, USA) [30] led to a benefit in a significant proportion (78%) of patients with BRPC-M0. Herein, we reported continuation of a durable benefit after 12 more months of therapy in this cohort, with 85% of patients having a durable long-term response, 62% a decreased/stable PSA (versus baseline pre-treatment PSA), 90% a PSADT improvement (versus pre-treatment PSADT), and all with negative scans.
PSADT is the most important clinical prognostic factor in patients with BRCP-M0 and is associated with the development of future metastases [10,13,15,16,17,25]. Lengthening of PSADT may be indicative of an effective therapeutic intervention [52]. Thus, the findings of the present study may suggest that therapy with P-MCP is active in managing BRPC-M0 patients over a durable period. Furthermore, most (95%) of the patients in the present study improved or kept to their PSADT risk grouping. Especially, all patients with a baseline pre-treatment poor PSADT risk, improved their risk grouping. Finally, the benefit of long-term P-MCP therapy was shown without significant toxicity, suggesting that P-MCP can be safely administered [16,20]. A major limitation of the present study is the lack of a placebo (control) arm. Additionally, the present study cohort is relatively small. Furthermore, although retrospective studies have shown that PSADT is a strong predictor of metastasis-free survival, overall survival [11,13], or both [26,53], further validation is required to establish whether change in PSADT is an acceptable endpoint for clinical trials in this patient population. Finally, due to the relatively small cohort of the present study, we were unable to correlate our results with the type of local therapy and to analyze predictive factors for the efficacy of P-MCP therapy (e.g., medications, smoking status, level of androgens, and genetic alterations). Moreover, genetic data were not collected during the present study. These should be tested in future studies. Nonetheless, numerically, P-MCP therapy in the present study was associated with a significant improvement of the expected progression rate in these patients, since, as per the historical data on the natural history of BRPC-M0, without active therapy, 80% of patients are expected to progress within 6 months [30]. In conclusion, in the present study we demonstrated that P-MCP therapy may be associated with a sustained benefit and is safe in patients with BRPC-M0. Future randomized larger clinical trials are needed to validate the present study results and to reveal the underlying mechanism of P-MCP therapy as a galectin inhibitor [38].
Author Contributions
D.K., M.F., A.P., I.K., E.R., D.S., I.L., R.M., O.Y., D.M., K.R., A.S., H.D. and I.E. contributed equally to this work. D.K. and I.E. devised the concept and designed the study. D.K., M.F., A.P., I.K., E.R., D.S., I.L., R.M., O.Y., D.M., K.R., I.W., R.G. and H.D. conducted the most experiments. D.K. and I.E. contributed to the manuscript writing. All authors have read and agreed to the published version of the manuscript.
Funding
This study received funding from EcoNugenics Inc., Santa Rosa, CA, USA. EcoNugenics was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Meir Medical Center (Protocol 019-2-12 MMC. Current ongoing approval date 23 August 2019). The study was registered at ClinicalTrials.gov (accessed on 10 September 2012) (NCT01681823).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in the study are available with the investigator Daniel Keizman.
Conflicts of Interest
I.E. discloses being the developer of the sponsoring dietary supplement company but holds no ownership in the company. The other authors declare no potential conflict of interest.
References
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and Prevention of Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, A.N.; Usman, A.; Morgans, A.; VanderWeele, D.J.; Sosman, J.; Wu, J.D. Past, Current, and Future of Immunotherapies for Prostate Cancer. Front. Oncol. 2019, 9, 884. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Zheng, Y.; Li, N.; Deng, Y.; Zhou, L.; Tian, T.; Yang, S.; Hao, Q.; Song, D.; Wu, Y.; et al. Incidence and Disease Burden of Prostate Cancer from 1990 to 2017: Results from the Global Burden of Disease Study 2017. Cancer 2020, 126, 1969–1978. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Black, L.K. The Economic Burden of Prostate Cancer. BJU Int. 2011, 108, 806–813. [Google Scholar] [CrossRef]
- Swami, U.; McFarland, T.R.; Nussenzveig, R.; Agarwal, N. Advanced Prostate Cancer: Treatment Advances and Future Directions. Trends Cancer 2020, 6, 702–715. [Google Scholar] [CrossRef]
- Catalona, W.J. Prostate Cancer Screening. Med. Clin. N. Am. 2018, 102, 199–214. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU Guidelines on Prostate Cancer. Part II: Treatment of Advanced, Relapsing, and Castration-Resistant Prostate Cancer. Eur. Urol. 2014, 65, 467–479. [Google Scholar] [CrossRef]
- Paller, C.J.; Antonarakis, E.S. Management of Biochemically Recurrent Prostate Cancer after Local Therapy: Evolving Standards of Care and New Directions. Clin. Adv. Hematol. Oncol. 2013, 11, 14–23. [Google Scholar]
- Simon, N.I.; Parker, C.; Hope, T.A.; Paller, C.J. Best Approaches and Updates for Prostate Cancer Biochemical Recurrence. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 352–359. [Google Scholar] [CrossRef]
- Heijnsdijk, E.A.M.; Bangma, C.H.; Borràs, J.M.; de Carvalho, T.M.; Castells, X.; Eklund, M.; Espinàs, J.A.; Graefen, M.; Grönberg, H.; Lansdorp-Vogelaar, I.; et al. Summary Statement on Screening for Prostate Cancer in Europe: Prostate Cancer Screening in Europe. Int. J. Cancer 2018, 142, 741–746. [Google Scholar] [CrossRef]
- Arlen, P.M.; Bianco, F.; Dahut, W.L.; D’Amico, A.; Figg, W.D.; Freedland, S.J.; Gulley, J.L.; Kantoff, P.W.; Kattan, M.W.; Lee, A.; et al. Prostate Specific Antigen Working Group Guidelines on Prostate Specific Antigen Doubling Time. J. Urol. 2008, 179, 2181–2186. [Google Scholar] [CrossRef]
- Pound, C.R. Natural History of Progression After PSA Elevation Following Radical Prostatectomy. JAMA 1999, 281, 1591. [Google Scholar] [CrossRef]
- Aggarwal, R.; Heller, G.; Hillman, D.; Xiao, H.; Picus, J.; Wang, J.; Taplin, M.E.; Dorff, T.; Appleman, L.J.; Weckstein, D.; et al. LBA63 PRESTO: A Phase III, Open-Label Study of Androgen Annihilation in Patients (Pts) with High-Risk Biochemically Relapsed Prostate Cancer (AFT-19). Ann. Oncol. 2022, 33, S1428. [Google Scholar] [CrossRef]
- Freedland, S.J.; Humphreys, E.B.; Mangold, L.A.; Eisenberger, M.; Dorey, F.J.; Walsh, P.C.; Partin, A.W. Risk of Prostate Cancer–Specific Mortality Following Biochemical Recurrence After Radical Prostatectomy. JAMA 2005, 294, 433. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, M.-H.; McLeod, D.; Carroll, P.R.; Moul, J.W.; D’Amico, A.V. Predictors of Prostate Cancer–Specific Mortality After Radical Prostatectomy or Radiation Therapy. JCO 2005, 23, 6992–6998. [Google Scholar] [CrossRef]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J.; et al. Variation in the Definition of Biochemical Recurrence in Patients Treated for Localized Prostate Cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel Report and Recommendations for a Standard in the Reporting of Surgical Outcomes. J. Urol. 2007, 177, 540–545. [Google Scholar] [CrossRef]
- Van den Broeck, T.; van den Bergh, R.C.N.; Arfi, N.; Gross, T.; Moris, L.; Briers, E.; Cumberbatch, M.; De Santis, M.; Tilki, D.; Fanti, S.; et al. Prognostic Value of Biochemical Recurrence Following Treatment with Curative Intent for Prostate Cancer: A Systematic Review. Eur. Urol. 2019, 75, 967–987. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Feng, Z.; Trock, B.J.; Humphreys, E.B.; Carducci, M.A.; Partin, A.W.; Walsh, P.C.; Eisenberger, M.A. The Natural History of Metastatic Progression in Men with Prostate-Specific Antigen Recurrence after Radical Prostatectomy: Long-Term Follow-Up: Metastatic Progression in PSA-Recurrent Prostate Cancer. BJU Int. 2012, 109, 32–39. [Google Scholar] [CrossRef]
- Paller, C.J.; Antonarakis, E.S.; Eisenberger, M.A.; Carducci, M.A. Management of Patients with Biochemical Recurrence After Local Therapy for Prostate Cancer. Hematol./Oncol. Clin. N. Am. 2013, 27, 1205–1219. [Google Scholar] [CrossRef]
- Roach, M.; Hanks, G.; Thames, H.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining Biochemical Failure Following Radiotherapy with or without Hormonal Therapy in Men with Clinically Localized Prostate Cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Keating, N.L.; O’Malley, A.J.; Smith, M.R. Diabetes and Cardiovascular Disease During Androgen Deprivation Therapy for Prostate Cancer. JCO 2006, 24, 4448–4456. [Google Scholar] [CrossRef] [PubMed]
- Keizman, D.; Zahurak, M.; Sinibaldi, V.; Carducci, M.; Denmeade, S.; Drake, C.; Pili, R.; Antonarakis, E.S.; Hudock, S.; Eisenberger, M. Lenalidomide in Nonmetastatic Biochemically Relapsed Prostate Cancer: Results of a Phase I/II Double-Blinded, Randomized Study. Clin. Cancer Res. 2010, 16, 5269–5276. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Ben-Porat, L.; Scher, H.I.; Chan, H.M.; Fearn, P.A.; Fuks, Z.Y.; Leibel, S.A.; Venkatraman, E.S. Outcome Predictors for the Increasing PSA State After Definitive External-Beam Radiotherapy for Prostate Cancer. JCO 2005, 23, 826–831. [Google Scholar] [CrossRef]
- Paller, C.J.; Ye, X.; Wozniak, P.J.; Gillespie, B.K.; Sieber, P.R.; Greengold, R.H.; Stockton, B.R.; Hertzman, B.L.; Efros, M.D.; Roper, R.P.; et al. A Randomized Phase II Study of Pomegranate Extract for Men with Rising PSA Following Initial Therapy for Localized Prostate Cancer. Prostate Cancer Prostatic Dis. 2013, 16, 50–55. [Google Scholar] [CrossRef]
- Gillessen, S.; Bossi, A.; Davis, I.D.; de Bono, J.; Fizazi, K.; James, N.D.; Mottet, N.; Shore, N.; Small, E.; Smith, M.; et al. Management of Patients with Advanced Prostate Cancer. Part I: Intermediate-/High-Risk and Locally Advanced Disease, Biochemical Relapse, and Side Effects of Hormonal Treatment: Report of the Advanced Prostate Cancer Consensus Conference 2022. Eur. Urol. 2023, 83, 267–293. [Google Scholar] [CrossRef]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Di Domizio, A.; Limonta, P. Natural Compounds in Prostate Cancer Prevention and Treatment: Mechanisms of Action and Molecular Targets. Cells 2020, 9, 460. [Google Scholar] [CrossRef]
- Bilgin, S.; Erden Tayhan, S.; Yıldırım, A.; Koç, E. Investigation of the Effects of Isoeugenol-Based Phenolic Compounds on Migration and Proliferation of HT29 Colon Cancer Cells at Cellular and Molecular Level. Bioorganic Chem. 2023, 130, 106230. [Google Scholar] [CrossRef]
- Keizman, D.; Frenkel, M.; Peer, A.; Kushnir, I.; Rosenbaum, E.; Sarid, D.; Leibovitch, I.; Mano, R.; Yossepowitch, O.; Margel, D.; et al. Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Results of a Prospective Phase II Study. Nutrients 2021, 13, 4295. [Google Scholar] [CrossRef]
- Pantuck, A.J.; Leppert, J.T.; Zomorodian, N.; Aronson, W.; Hong, J.; Barnard, R.J.; Seeram, N.; Liker, H.; Wang, H.; Elashoff, R.; et al. Phase II Study of Pomegranate Juice for Men with Rising Prostate-Specific Antigen Following Surgery or Radiation for Prostate Cancer. Clin. Cancer Res. 2006, 12, 4018–4026. [Google Scholar] [CrossRef]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen Signaling in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030452. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Yamthe, L.R.T.; Tali, B.T.; Adetunji, C.O.; Rahavian, A.; Mudau, F.N.; Martorell, M.; Setzer, W.N.; Rodrigues, C.F.; et al. Phytochemicals in Prostate Cancer: From Bioactive Molecules to Upcoming Therapeutic Agents. Nutrients 2019, 11, 1483. [Google Scholar] [CrossRef]
- Li, F.; Li, S.; Li, H.-B.; Deng, G.-F.; Ling, W.-H.; Wu, S.; Xu, X.-R.; Chen, F. Antiproliferative Activity of Peels, Pulps and Seeds of 61 Fruits. J. Funct. Foods 2013, 5, 1298–1309. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Tang, L.; Chen, H.; Wu, C.; Zhao, M.; Yang, Y.; Chen, X.; Liu, G. Resveratrol Inhibits TGF-Β1-Induced Epithelial-to-Mesenchymal Transition and Suppresses Lung Cancer Invasion and Metastasis. Toxicology 2013, 303, 139–146. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry Anthocyanidins Synergistically Suppress Growth and Invasive Potential of Human Non-Small-Cell Lung Cancer Cells. Cancer Lett. 2012, 325, 54–62. [Google Scholar] [CrossRef]
- Mariño, K.V.; Cagnoni, A.J.; Croci, D.O.; Rabinovich, G.A. Targeting Galectin-Driven Regulatory Circuits in Cancer and Fibrosis. Nat. Rev. Drug Discov. 2023, 22, 295–316. [Google Scholar] [CrossRef]
- Yan, J.; Katz, A. PectaSol-C Modified Citrus Pectin Induces Apoptosis and Inhibition of Proliferation in Human and Mouse Androgen-Dependent and- Independent Prostate Cancer Cells. Integr. Cancer Ther. 2010, 9, 197–203. [Google Scholar] [CrossRef]
- Demotte, N.; Wieërs, G.; Van Der Smissen, P.; Moser, M.; Schmidt, C.; Thielemans, K.; Squifflet, J.-L.; Weynand, B.; Carrasco, J.; Lurquin, C.; et al. A Galectin-3 Ligand Corrects the Impaired Function of Human CD4 and CD8 Tumor-Infiltrating Lymphocytes and Favors Tumor Rejection in Mice. Cancer Res. 2010, 70, 7476–7488. [Google Scholar] [CrossRef]
- Guess, B.W.; Scholz, M.C.; Strum, S.B.; Lam, R.Y.; Johnson, H.J.; Jennrich, R.I. Modified Citrus Pectin (MCP) Increases the Prostate-Specific Antigen Doubling Time in Men with Prostate Cancer: A Phase II Pilot Study. Prostate Cancer Prostatic Dis. 2003, 6, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J.; Nailk, H.; Akhtar, A.; Yamazaki, K.; Replogle, T.S.; Lehr, J.; Donat, T.L.; Tait, L.; Hogan, V.; Raz, A. Inhibition of Spontaneous Metastasis in a Rat Prostate Cancer Model by Oral Administration of Modified Citrus Pectin. JNCI J. Natl. Cancer Inst. 1995, 87, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Glinskii, O.V.; Sud, S.; Mossine, V.V.; Mawhinney, T.P.; Anthony, D.C.; Glinsky, G.V.; Pienta, K.J.; Glinsky, V.V. Inhibition of Prostate Cancer Bone Metastasis by Synthetic TF Antigen Mimic/Galectin-3 Inhibitor Lactulose-l-Leucine. Neoplasia 2012, 14, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Stegmayr, J.; Lepur, A.; Kahl-Knutson, B.; Aguilar-Moncayo, M.; Klyosov, A.A.; Field, R.A.; Oredsson, S.; Nilsson, U.J.; Leffler, H. Low or No Inhibitory Potency of the Canonical Galectin Carbohydrate-Binding Site by Pectins and Galactomannans. J. Biol. Chem. 2016, 291, 13318–13334. [Google Scholar] [CrossRef] [PubMed]
- Girotti, M.R.; Salatino, M.; Dalotto-Moreno, T.; Rabinovich, G.A. Sweetening the Hallmarks of Cancer: Galectins as Multifunctional Mediators of Tumor Progression. J. Exp. Med. 2020, 217, e20182041. [Google Scholar] [CrossRef]
- Yang, R.-Y.; Rabinovich, G.A.; Liu, F.-T. Galectins: Structure, Function and Therapeutic Potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef]
- Méndez-Huergo, S.P.; Blidner, A.G.; Rabinovich, G.A. Galectins: Emerging Regulatory Checkpoints Linking Tumor Immunity and Angiogenesis. Curr. Opin. Immunol. 2017, 45, 8–15. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a Novel Biomarker for Disease Diagnosis and a Target for Therapy. Int. J. Mol. Med. 2017, 41, 599–614. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Hogan, V.; Honjo, Y.; Baccarini, S.; Tait, L.; Bresalier, R.; Raz, A. Inhibition of Human Cancer Cell Growth and Metastasis in Nude Mice by Oral Intake of Modified Citrus Pectin. JNCI J. Natl. Cancer Inst. 2002, 94, 1854–1862. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Wu, J.M. Changes in Cell Growth, Cyclin/Kinase, Endogenous Phosphoproteins and Nm23 Gene Expression in Human Prostatic JCA-1 Cells Treated with Modified Citrus Pectin. Biochem. Mol. Biol. Int. 1995, 37, 833–841. [Google Scholar]
- Suzman, D.L.; Zhou, X.C.; Zahurak, M.L.; Lin, J.; Antonarakis, E.S. Change in PSA Velocity Is a Predictor of Overall Survival in Men with Biochemically-Recurrent Prostate Cancer Treated with Nonhormonal Agents: Combined Analysis of Four Phase-2 Trials. Prostate Cancer Prostatic Dis. 2015, 18, 49–55. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Moul, J.W.; Carroll, P.R.; Sun, L.; Lubeck, D.; Chen, M.-H. Surrogate End Point for Prostate Cancer-Specific Mortality After Radical Prostatectomy or Radiation Therapy. JNCI J. Natl. Cancer Inst. 2003, 95, 1376–1383. [Google Scholar] [CrossRef]
- Gleason, D.F.; Mellinger, G.T.; The Veterans Administration Cooperative Urological Research Group. Prediction of Prognosis for Prostatic Adenocarcinoma by Combined Histological Grading and Clinical Staging. J. Urol. 1974, 111, 58–64. [Google Scholar] [CrossRef]
- Rosenbaum, E.; Zahurak, M.; Sinibaldi, V.; Carducci, M.A.; Pili, R.; Laufer, M.; DeWeese, T.L.; Eisenberger, M.A. Marimastat in the Treatment of Patients with Biochemically Relapsed Prostate Cancer: A Prospective Randomized, Double-Blind, Phase I/II Trial. Clin. Cancer Res. 2005, 11, 4437–4443. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).