Underlying Mechanisms of Brain Aging and Neurodegenerative Diseases as Potential Targets for Preventive or Therapeutic Strategies Using Phytochemicals
Highlights
- This review centers on the molecular mechanisms behind senescence and neurodegeneration, specifically in relation to Alzheimer's and Parkinson's diseases (AD and PD), which are age-associated brain conditions.
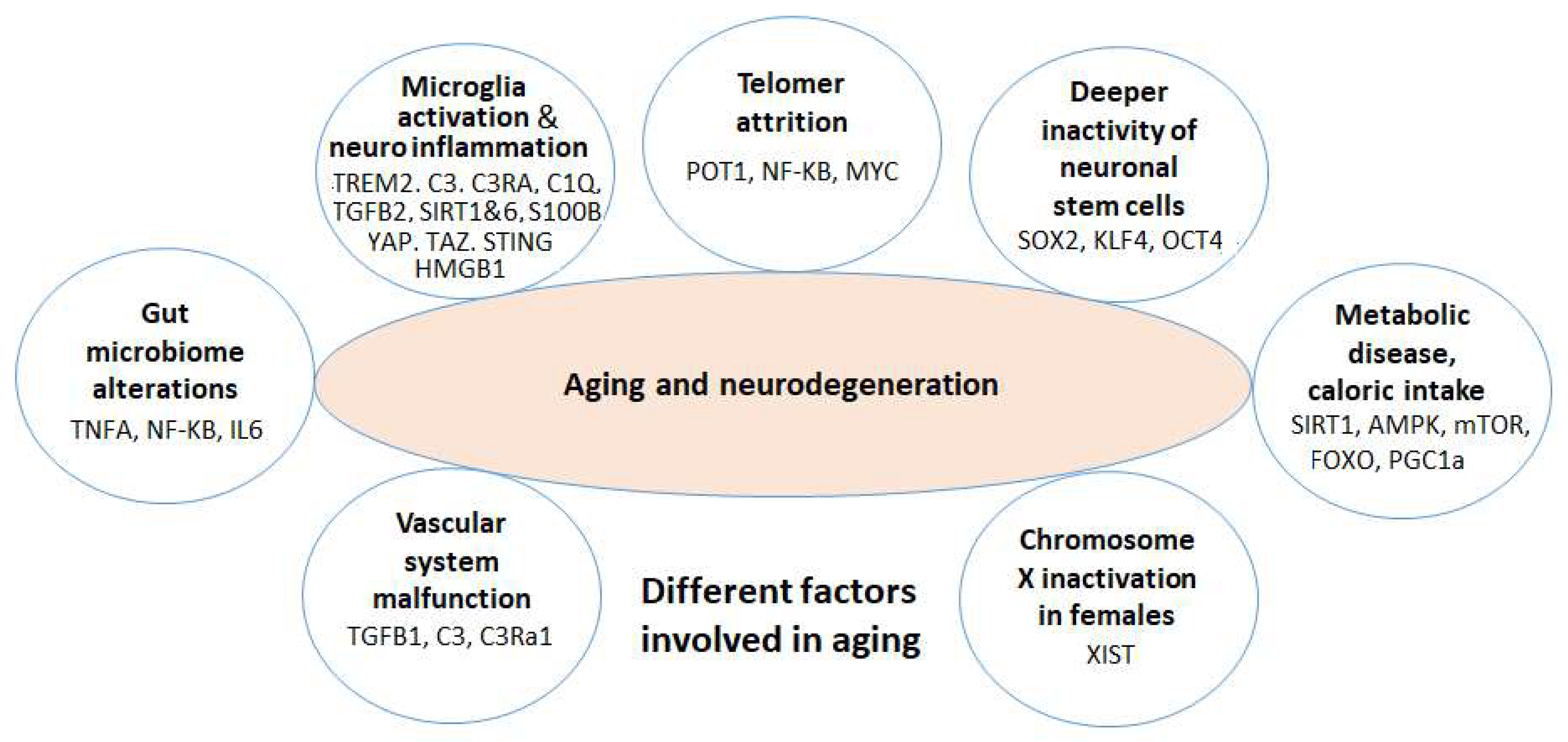
- We introduce several key biological factors contributing to neurodegeneration, including microglia gene dysregulation, neuroinflammation, telomere attrition, neuronal stem cell degradation, vascular dysfunction, and gut microbiome dysbiosis.
- We also highlight various phytochemicals (e.g., curcumin, resveratrol, sulforaphane, and EGCG) that modulate the dysfunction of key genes (e.g., TREM2, SIRT1/6, HMGB1, STING, SOX2, and KLF4) and the gut microbiome, offering possible therapeutic benefits in regard to managing neurodegenerative diseases.
Abstract
:1. Introduction
2. Methods
3. Key Factors in Brain Aging and Neurodegeneration
3.1. Neuroinflammation and Microglia Dysregulated Genes in AD and the Effects of Phytochemicals
3.2. Brain Neuronal Stem Cells (NSCs) in Aging and the Effects of Phytochemicals
| Phytochemicals or Nutrients | In Vitro and/or Animal Models | Effects | Mechanisms of Action | References |
|---|---|---|---|---|
| Alyssum Homolocarpum seed oil | Embryonic NSC (eNSC) |
|
| [86] |
| Daucosterol (walnut meat) | NSC |
|
| [87] |
| Alyssum homolocarpum (Brassicaceae) seed extract | Mice brain |
| Not investigated | [88] |
| Kuwanon V (from mulberry tree (Morus bombycis) root) | Rat NSC |
|
| [89] |
| Silibinin (from Silybum marianum) | Mouse |
|
| [90] |
| Resveratrol | NSC |
|
| [91] |
| Curcumin | Mice hippocampus |
|
| [92] |
| Di-(2-ethylhexyl) phthalate (C. vulgure) | NSC of mice hippocampus |
|
| [96] |
| Sulforaphane (broccoli) | In vitro | Unknown |
| [97] |
| Withaferin A (a medicinal plant) |
| [98] | ||
| Betulinic acid (bark of trees) |
| [99] | ||
| Short-term low-dose ethanol | In vitro | Unknown |
| [100] |
3.3. Telomere Attrition and Aging and the Effects of Phytochemicals
3.4. Gut Microbiome, the Dysfunction of Brain Microglia and Astrocytes in Brain Aging, and Phytochemical Effects
3.5. Metabolic Disease, Caloric Restriction, Physical Exercise, and Aging
3.6. Chromosome X Inactivation and Neurodegeneration
3.7. Vascular System and Neurodegeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Andronie-Cioara, F.L.; Munteanu, M.A.; Brisc, M.C.; et al. Current Trends in Neurodegeneration: Cross Talks between Oxidative Stress, Cell Death, and Inflammation. Int. J. Mol. Sci. 2021, 22, 7432. [Google Scholar] [CrossRef]
- Delbaere, Q.; Chapet, N.; Huet, F.; Delmas, C.; Mewton, N.; Prunier, F.; Angoulvant, D.; Roubille, F. Anti-Inflammatory Drug Candidates for Prevention and Treatment of Cardiovascular Diseases. Pharmaceuticals 2023, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Sadighi Akha, A.A. Aging and the immune system: An overview. J. Immunol. Methods 2018, 463, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Iso-Markku, P.; Kujala, U.M.; Knittle, K.; Polet, J.; Vuoksimaa, E.; Waller, K. Physical activity as a protective factor for dementia and Alzheimer’s disease: Systematic review, meta-analysis and quality assessment of cohort and case-control studies. Br. J. Sports Med. 2022, 56, 701–709. [Google Scholar] [CrossRef]
- Farfel, J.M.; Yu, L.; Boyle, P.A.; Leurgans, S.; Shah, R.C.; Schneider, J.A.; Bennett, D.A. Alzheimer’s disease frequency peaks in the tenth decade and is lower afterwards. Acta Neuropathol. Commun. 2019, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Akram, M.; Daniyal, M.; Zainab, R. Awareness and current knowledge of Parkinson’s disease: A neurodegenerative disorder. Int. J. Neurosci. 2019, 129, 55–93. [Google Scholar] [CrossRef] [PubMed]
- Sehar, U.; Rawat, P.; Reddy, A.P.; Kopel, J.; Reddy, P.H. Amyloid Beta in Aging and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12924. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Rey, N.L.; Tyson, T.; Esquibel, C.; Meyerdirk, L.; Schulz, E.; Pierce, S.; Burmeister, A.R.; Madaj, Z.; Steiner, J.A.; et al. Microglia affect alpha-synuclein cell-to-cell transfer in a mouse model of Parkinson’s disease. Mol. Neurodegener. 2019, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Olah, M.; Menon, V.; Habib, N.; Taga, M.F.; Ma, Y.; Yung, C.J.; Cimpean, M.; Khairallah, A.; Coronas-Samano, G.; Sankowski, R.; et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 2020, 11, 6129. [Google Scholar] [CrossRef]
- Xu, H.; Jia, J. Single-Cell RNA Sequencing of Peripheral Blood Reveals Immune Cell Signatures in Alzheimer’s Disease. Front. Immunol. 2021, 12, 645666. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.L.; Xue, L.L.; Du, R.L.; Niu, R.Z.; Chen, L.; Chen, J.; Hu, Q.; Tan, Y.X.; Shang, H.F.; Liu, J.; et al. Single-cell RNA sequencing reveals B cell-related molecular biomarkers for Alzheimer’s disease. Exp. Mol. Med. 2021, 53, 1888–1901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Silva, T.C.; Young, J.I.; Gomez, L.; Schmidt, M.A.; Hamilton-Nelson, K.L.; Kunkle, B.W.; Chen, X.; Martin, E.R.; Wang, L. Epigenome-wide meta-analysis of DNA methylation differences in prefrontal cortex implicates the immune processes in Alzheimer’s disease. Nat. Commun. 2020, 11, 6114. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Pirazzini, C.; Sala, C.; Sambati, L.; Yusipov, I.; Kalyakulina, A.; Ravaioli, F.; Kwiatkowska, K.M.; Durso, D.F.; Ivanchenko, M.; et al. A Meta-Analysis of Brain DNA Methylation Across Sex, Age, and Alzheimer’s Disease Points for Accelerated Epigenetic Aging in Neurodegeneration. Front. Aging Neurosci. 2021, 13, 639428. [Google Scholar] [CrossRef]
- Duan, R.; Fu, Q.; Sun, Y.; Li, Q. Epigenetic clock: A promising biomarker and practical tool in aging. Ageing Res. Rev. 2022, 81, 101743. [Google Scholar] [CrossRef]
- Chiavellini, P.; Canatelli-Mallat, M.; Lehmann, M.; Gallardo, M.D.; Herenu, C.B.; Cordeiro, J.L.; Clement, J.; Goya, R.G. Aging and rejuvenation—A modular epigenome model. Aging 2021, 13, 4734–4746. [Google Scholar] [CrossRef]
- Yang, J.H.; Hayano, M.; Griffin, P.T.; Amorim, J.A.; Bonkowski, M.S.; Apostolides, J.K.; Salfati, E.L.; Blanchette, M.; Munding, E.M.; Bhakta, M.; et al. Loss of epigenetic information as a cause of mammalian aging. Cell 2023, 186, 305–326.e27. [Google Scholar] [CrossRef]
- Smith, A.R.; Smith, R.G.; Condliffe, D.; Hannon, E.; Schalkwyk, L.; Mill, J.; Lunnon, K. Increased DNA methylation near TREM2 is consistently seen in the superior temporal gyrus in Alzheimer’s disease brain. Neurobiol. Aging 2016, 47, 35–40. [Google Scholar] [CrossRef]
- Celarain, N.; Sanchez-Ruiz de Gordoa, J.; Zelaya, M.V.; Roldan, M.; Larumbe, R.; Pulido, L.; Echavarri, C.; Mendioroz, M. TREM2 upregulation correlates with 5-hydroxymethycytosine enrichment in Alzheimer’s disease hippocampus. Clin. Epigenet. 2016, 8, 37. [Google Scholar] [CrossRef]
- Ozaki, Y.; Yoshino, Y.; Yamazaki, K.; Sao, T.; Mori, Y.; Ochi, S.; Yoshida, T.; Mori, T.; Iga, J.I.; Ueno, S.I. DNA methylation changes at TREM2 intron 1 and TREM2 mRNA expression in patients with Alzheimer’s disease. J. Psychiatr. Res. 2017, 92, 74–80. [Google Scholar] [CrossRef]
- Wu, M.; Liao, M.; Huang, R.; Chen, C.; Tian, T.; Wang, H.; Li, J.; Li, J.; Sun, Y.; Wu, C.; et al. Hippocampal overexpression of TREM2 ameliorates high fat diet induced cognitive impairment and modulates phenotypic polarization of the microglia. Genes Dis. 2022, 9, 401–414. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.; Jiang, Y.; Xu, Y.; Zhao, H.; Lyu, X.; Wu, T. Bilberry anthocyanins improve neuroinflammation and cognitive dysfunction in APP/PSEN1 mice via the CD33/TREM2/TYROBP signaling pathway in microglia. Food Funct. 2020, 11, 1572–1584. [Google Scholar] [CrossRef] [PubMed]
- Sanjay; Shin, J.H.; Park, M.; Lee, H.J. Cyanidin-3-O-Glucoside Regulates the M1/M2 Polarization of Microglia via PPARgamma and Abeta42 Phagocytosis Through TREM2 in an Alzheimer’s Disease Model. Mol. Neurobiol. 2022, 59, 5135–5148. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Daborg, J.; Andreasson, U.; Pekna, M.; Lautner, R.; Hanse, E.; Minthon, L.; Blennow, K.; Hansson, O.; Zetterberg, H. Cerebrospinal fluid levels of complement proteins C3, C4 and CR1 in Alzheimer’s disease. J. Neural Transm. 2012, 119, 789–797. [Google Scholar] [CrossRef]
- Noguchi, A.; Nawa, M.; Aiso, S.; Okamoto, K.; Matsuoka, M. Transforming growth factor beta2 level is elevated in neurons of Alzheimer’s disease brains. Int. J. Neurosci. 2010, 120, 168–175. [Google Scholar] [CrossRef]
- Presumey, J.; Bialas, A.R.; Carroll, M.C. Complement System in Neural Synapse Elimination in Development and Disease. Adv. Immunol. 2017, 135, 53–79. [Google Scholar] [CrossRef]
- Lian, H.; Yang, L.; Cole, A.; Sun, L.; Chiang, A.C.; Fowler, S.W.; Shim, D.J.; Rodriguez-Rivera, J.; Taglialatela, G.; Jankowsky, J.L.; et al. NFkappaB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron 2015, 85, 101–115. [Google Scholar] [CrossRef]
- Bhatia, K.; Ahmad, S.; Kindelin, A.; Ducruet, A.F. Complement C3a receptor-mediated vascular dysfunction: A complex interplay between aging and neurodegeneration. J. Clin. Investig. 2021, 131, e144348. [Google Scholar] [CrossRef]
- Litvinchuk, A.; Wan, Y.W.; Swartzlander, D.B.; Chen, F.; Cole, A.; Propson, N.E.; Wang, Q.; Zhang, B.; Liu, Z.; Zheng, H. Complement C3aR Inactivation Attenuates Tau Pathology and Reverses an Immune Network Deregulated in Tauopathy Models and Alzheimer’s Disease. Neuron 2018, 100, 1337–1353.e5. [Google Scholar] [CrossRef]
- Lardenoije, R.; Roubroeks, J.A.Y.; Pishva, E.; Leber, M.; Wagner, H.; Iatrou, A.; Smith, A.R.; Smith, R.G.; Eijssen, L.M.T.; Kleineidam, L.; et al. Alzheimer’s disease-associated (hydroxy)methylomic changes in the brain and blood. Clin. Epigenet. 2019, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Li, N.; Wei, W.; Zhang, L. Detection of molecular signatures and pathways shared by Alzheimer’s disease and type 2 diabetes. Gene 2022, 810, 146070. [Google Scholar] [CrossRef] [PubMed]
- Nicolia, V.; Cavallaro, R.A.; Lopez-Gonzalez, I.; Maccarrone, M.; Scarpa, S.; Ferrer, I.; Fuso, A. DNA Methylation Profiles of Selected Pro-Inflammatory Cytokines in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2017, 76, 27–31. [Google Scholar] [CrossRef]
- Gasparoni, G.; Bultmann, S.; Lutsik, P.; Kraus, T.F.J.; Sordon, S.; Vlcek, J.; Dietinger, V.; Steinmaurer, M.; Haider, M.; Mulholland, C.B.; et al. DNA methylation analysis on purified neurons and glia dissects age and Alzheimer’s disease-specific changes in the human cortex. Epigenet. Chromatin 2018, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.H. The neurology of folic acid deficiency. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 120, pp. 927–943. [Google Scholar] [CrossRef]
- Sahu, P.; Thippeswamy, H.; Chaturvedi, S.K. Neuropsychiatric manifestations in vitamin B12 deficiency. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2022; Volume 119, pp. 457–470. [Google Scholar] [CrossRef]
- Taylor, J.R.; Wood, J.G.; Mizerak, E.; Hinthorn, S.; Liu, J.; Finn, M.; Gordon, S.; Zingas, L.; Chang, C.; Klein, M.A.; et al. Sirt6 regulates lifespan in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2022, 119, e2111176119. [Google Scholar] [CrossRef]
- Simon, M.; Yang, J.; Gigas, J.; Earley, E.J.; Hillpot, E.; Zhang, L.; Zagorulya, M.; Tombline, G.; Gilbert, M.; Yuen, S.L.; et al. A rare human centenarian variant of SIRT6 enhances genome stability and interaction with Lamin A. EMBO J. 2022, 41, e110393. [Google Scholar] [CrossRef]
- Rahnasto-Rilla, M.K.; McLoughlin, P.; Kulikowicz, T.; Doyle, M.; Bohr, V.A.; Lahtela-Kakkonen, M.; Ferrucci, L.; Hayes, M.; Moaddel, R. The Identification of a SIRT6 Activator from Brown Algae Fucus distichus. Mar. Drugs 2017, 15, 190. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Hu, C.; Liu, A.; Chen, J.; Gu, C.; Zhang, X.; You, C.; Tong, H.; Wu, M.; et al. Sargassum fusiforme Fucoidan SP2 Extends the Lifespan of Drosophila melanogaster by Upregulating the Nrf2-Mediated Antioxidant Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 8918914. [Google Scholar] [CrossRef]
- Subaraja, M.; Anantha Krishnan, D.; Edwin Hillary, V.; William Raja, T.R.; Mathew, P.; Ravikumar, S.; Gabriel Paulraj, M.; Ignacimuthu, S. Fucoidan serves a neuroprotective effect in an Alzheimer’s disease model. Front. Biosci. 2020, 12, 1–34. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Duan, L.; Li, X.; Yang, W.; Huang, T.; Kong, M.; Guan, F.; Ma, S. Fucoidan ameliorates LPS-induced neuronal cell damage and cognitive impairment in mice. Int. J. Biol. Macromol. 2022, 222, 759–771. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Hou, Y.; Huang, S.; Pei, G. Alzheimer’s Amyloid-beta Accelerates Human Neuronal Cell Senescence Which Could Be Rescued by Sirtuin-1 and Aspirin. Front. Cell. Neurosci. 2022, 16, 906270. [Google Scholar] [CrossRef] [PubMed]
- Anekonda, T.S.; Wadsworth, T.L.; Sabin, R.; Frahler, K.; Harris, C.; Petriko, B.; Ralle, M.; Woltjer, R.; Quinn, J.F. Phytic acid as a potential treatment for alzheimer’s pathology: Evidence from animal and in vitro models. J. Alzheimer’s Dis. 2011, 23, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, K.P.; Beak, S.; Kang, H.R.; Kim, Y.K.; Lim, K. Effect of black chokeberry on skeletal muscle damage and neuronal cell death. J. Exerc. Nutr. Biochem. 2019, 23, 26–31. [Google Scholar] [CrossRef]
- Mizwicki, M.T.; Liu, G.; Fiala, M.; Magpantay, L.; Sayre, J.; Siani, A.; Mahanian, M.; Weitzman, R.; Hayden, E.Y.; Rosenthal, M.J.; et al. 1alpha,25-dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-beta phagocytosis and inflammation in Alzheimer’s disease patients. J. Alzheimer’s Dis. 2013, 34, 155–170. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Chiba, T.; Yamada, M.; Nawa, M.; Kanekura, K.; Suzuki, H.; Terashita, K.; Aiso, S.; Nishimoto, I.; Matsuoka, M. Transforming growth factor beta2 is a neuronal death-inducing ligand for amyloid-beta precursor protein. Mol. Cell. Biol. 2005, 25, 9304–9317. [Google Scholar] [CrossRef]
- Peddakkulappagari, C.S.; Saifi, M.A.; Khurana, A.; Anchi, P.; Singh, M.; Godugu, C. Withaferin A ameliorates renal injury due to its potent effect on inflammatory signaling. Biofactors 2019, 45, 750–762. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, X.; Li, M.; Ji, R.; Li, Z.; Wang, Y.; Guo, Y.; Liu, D.; Huang, B.; Du, H. Active fractions of golden-flowered tea (Camellia nitidissima Chi) inhibit epidermal growth factor receptor mutated non-small cell lung cancer via multiple pathways and targets in vitro and in vivo. Front. Nutr. 2022, 9, 1014414. [Google Scholar] [CrossRef]
- Tsuji, Y.; Denda, S.; Soma, T.; Raftery, L.; Momoi, T.; Hibino, T. A potential suppressor of TGF-beta delays catagen progression in hair follicles. J. Investig. Dermatol. Symp. Proc. 2003, 8, 65–68. [Google Scholar] [CrossRef]
- Yuan, J.; Zou, M.; Xiang, X.; Zhu, H.; Chu, W.; Liu, W.; Chen, F.; Lin, J. Curcumin improves neural function after spinal cord injury by the joint inhibition of the intracellular and extracellular components of glial scar. J. Surg. Res. 2015, 195, 235–245. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Aamir, K.; Shaikh, M.F. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s Disease (AD): From Risk Factors to Therapeutic Targeting. Cells 2020, 9, 383. [Google Scholar] [CrossRef]
- Gulmammadli, N.; Konukoglu, D.; Merve Kurtulus, E.; Tezen, D.; Ibrahim Erbay, M.; Bozluolcay, M. Serum Sirtuin-1, HMGB1-TLR4, NF-KB and IL-6 levels in Alzheimer’s: The Relation Between Neuroinflammatory Pathway and Severity of Dementia. Curr. Alzheimer Res. 2022, 19, 841–848. [Google Scholar] [CrossRef]
- Nishibori, M.; Wang, D.; Ousaka, D.; Wake, H. High Mobility Group Box-1 and Blood-Brain Barrier Disruption. Cells 2020, 9, 2650. [Google Scholar] [CrossRef]
- Gaikwad, S.; Puangmalai, N.; Bittar, A.; Montalbano, M.; Garcia, S.; McAllen, S.; Bhatt, N.; Sonawane, M.; Sengupta, U.; Kayed, R. Tau oligomer induced HMGB1 release contributes to cellular senescence and neuropathology linked to Alzheimer’s disease and frontotemporal dementia. Cell Rep. 2021, 36, 109419. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Liang, J.; Jiang, X.; Fang, X.; Wu, M.; Wei, X.; Yang, W.; Hou, W.; Zhang, Q. Quercetin Attenuates d-GaLN-Induced L02 Cell Damage by Suppressing Oxidative Stress and Mitochondrial Apoptosis via Inhibition of HMGB1. Front. Pharmacol. 2020, 11, 608. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zheng, W.; Cheng, L.; Li, M.; Huang, J.; Bao, S.; Xu, Q.; Ma, Z. Citrus fruits are rich in flavonoids for immunoregulation and potential targeting ACE2. Nat. Prod. Bioprospect. 2022, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aal, S.A.; AbdElrahman, M.; Reda, A.M.; Afify, H.; Ragab, G.M.; El-Gazar, A.A.; Ibrahim, S.S.A. Galangin mitigates DOX-induced cognitive impairment in rats: Implication of NOX-1/Nrf-2/HMGB1/TLR4 and TNF-alpha/MAPKs/RIPK/MLKL/BDNF. Neurotoxicology 2022, 92, 77–90. [Google Scholar] [CrossRef]
- Rohde, K.; Ronningen, T.; la Cour Poulsen, L.; Keller, M.; Bluher, M.; Bottcher, Y. Role of the DNA repair genes H2AX and HMGB1 in human fat distribution and lipid profiles. BMJ Open Diabetes Res. Care 2020, 8, e000831. [Google Scholar] [CrossRef]
- Su, J.; Fang, M.; Tian, B.; Luo, J.; Jin, C.; Wang, X.; Ning, Z.; Li, X. Hypoxia induces hypomethylation of the HMGB1 promoter via the MAPK/DNMT1/HMGB1 pathway in cardiac progenitor cells. Acta Biochim. Biophys. Sin. 2018, 50, 1121–1130. [Google Scholar] [CrossRef]
- He, M.; Zhang, B.; Wei, X.; Wang, Z.; Fan, B.; Du, P.; Zhang, Y.; Jian, W.; Chen, L.; Wang, L.; et al. HDAC4/5-HMGB1 signalling mediated by NADPH oxidase activity contributes to cerebral ischaemia/reperfusion injury. J. Cell. Mol. Med. 2013, 17, 531–542. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, J.Q.; Zhang, X.; Shen, L.F. MiR-129-2 functions as a tumor suppressor in glioma cells by targeting HMGB1 and is down-regulated by DNA methylation. Mol. Cell. Biochem. 2015, 404, 229–239. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Chen, P.; Ruan, X.; Liu, W.; Li, Y.; Sun, C.; Hou, L.; Yin, B.; Qiang, B.; et al. MicroRNA-129 modulates neuronal migration by targeting Fmr1 in the developing mouse cortex. Cell Death Dis. 2019, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Gonzalez, I.; Wang, Y.; Friday, W.B.; Vickers, K.C.; Toth, C.L.; Molina-Torres, L.; Surzenko, N.; Zeisel, S.H. MicroRNA-129-5p is regulated by choline availability and controls EGF receptor synthesis and neurogenesis in the cerebral cortex. FASEB J. 2019, 33, 3601–3612. [Google Scholar] [CrossRef] [PubMed]
- Sladitschek-Martens, H.L.; Guarnieri, A.; Brumana, G.; Zanconato, F.; Battilana, G.; Xiccato, R.L.; Panciera, T.; Forcato, M.; Bicciato, S.; Guzzardo, V.; et al. YAP/TAZ activity in stromal cells prevents ageing by controlling cGAS-STING. Nature 2022, 607, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Shiwaku, H.; Tanaka, H.; Obita, T.; Ohuchi, S.; Yoshioka, Y.; Jin, X.; Kondo, K.; Fujita, K.; Homma, H.; et al. Tau activates microglia via the PQBP1-cGAS-STING pathway to promote brain inflammation. Nat. Commun. 2021, 12, 6565. [Google Scholar] [CrossRef]
- Hou, Y.; Wei, Y.; Lautrup, S.; Yang, B.; Wang, Y.; Cordonnier, S.; Mattson, M.P.; Croteau, D.L.; Bohr, V.A. NAD+ supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS-STING. Proc. Natl. Acad. Sci. USA 2021, 118, e2011226118. [Google Scholar] [CrossRef]
- Li, S.; Hong, Z.; Wang, Z.; Li, F.; Mei, J.; Huang, L.; Lou, X.; Zhao, S.; Song, L.; Chen, W.; et al. The Cyclopeptide Astin C Specifically Inhibits the Innate Immune CDN Sensor STING. Cell Rep. 2018, 25, 3405–3421.E7. [Google Scholar] [CrossRef]
- Liu, D.; Wu, H.; Wang, C.; Li, Y.; Tian, H.; Siraj, S.; Sehgal, S.A.; Wang, X.; Wang, J.; Shang, Y.; et al. STING directly activates autophagy to tune the innate immune response. Cell Death Differ. 2019, 26, 1735–1749. [Google Scholar] [CrossRef]
- Li, Y.; Xia, X.; Wang, Y.; Zheng, J.C. Mitochondrial dysfunction in microglia: A novel perspective for pathogenesis of Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 248. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Zhao, Q.; Zhang, J.; Wang, J.W.; Qian, Y.; Fan, Y.C.; Wang, K. Methylation status of the stimulator of interferon genes promoter in patients with chronic hepatitis B. Medicine 2018, 97, e13904. [Google Scholar] [CrossRef]
- Suebsaard, P.; Charerntantanakul, W. Rutin, alpha-tocopherol, and l-ascorbic acid up-regulate type I interferon-regulated gene and type I and II interferon expressions and reduce inflammatory cytokine expressions in monocyte-derived macrophages infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 2021, 235, 110231. [Google Scholar] [CrossRef]
- Balez, R.; Steiner, N.; Engel, M.; Munoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M.; et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 6, 31450. [Google Scholar] [CrossRef] [PubMed]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef]
- Liu, M.; Chen, F.; Sha, L.; Wang, S.; Tao, L.; Yao, L.; He, M.; Yao, Z.; Liu, H.; Zhu, Z.; et al. (−)-Epigallocatechin-3-gallate ameliorates learning and memory deficits by adjusting the balance of TrkA/p75NTR signaling in APP/PS1 transgenic mice. Mol. Neurobiol. 2014, 49, 1350–1363. [Google Scholar] [CrossRef]
- Tang, M.; Taghibiglou, C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1003–1016. [Google Scholar] [CrossRef]
- Kim, H.G.; Ju, M.S.; Ha, S.K.; Lee, H.; Lee, H.; Kim, S.Y.; Oh, M.S. Acacetin protects dopaminergic cells against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neuroinflammation in vitro and in vivo. Biol. Pharm. Bull. 2012, 35, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.K.; Moon, E.; Lee, P.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. Acacetin attenuates neuroinflammation via regulation the response to LPS stimuli in vitro and in vivo. Neurochem. Res. 2012, 37, 1560–1567. [Google Scholar] [CrossRef]
- Hassan, S.S.U.; Samanta, S.; Dash, R.; Karpinski, T.M.; Habibi, E.; Sadiq, A.; Ahmadi, A.; Bunagu, S. The neuroprotective effects of fisetin, a natural flavonoid in neurodegenerative diseases: Focus on the role of oxidative stress. Front. Pharmacol. 2022, 13, 1015835. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Jha, N.K.; Jha, S.K.; Verma, P.R.P.; Ashraf, G.M.; Singh, S.K. Neuroprotective Role of Quercetin against Alpha-Synuclein-Associated Hallmarks in Parkinson’s Disease. Curr. Neuropharmacol. 2023, 21, 1464–1466. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef]
- Md, S.; Gan, S.Y.; Haw, Y.H.; Ho, C.L.; Wong, S.; Choudhury, H. In vitro neuroprotective effects of naringenin nanoemulsion against beta-amyloid toxicity through the regulation of amyloidogenesis and tau phosphorylation. Int. J. Biol. Macromol. 2018, 118, 1211–1219. [Google Scholar] [CrossRef]
- Goyal, A.; Verma, A.; Dubey, N.; Raghav, J.; Agrawal, A. Naringenin: A prospective therapeutic agent for Alzheimer’s and Parkinson’s disease. J. Food Biochem. 2022, 46, e14415. [Google Scholar] [CrossRef] [PubMed]
- Fatima, U.; Roy, S.; Ahmad, S.; Al-Keridis, L.A.; Alshammari, N.; Adnan, M.; Islam, A.; Hassan, M.I. Investigating neuroprotective roles of Bacopa monnieri extracts: Mechanistic insights and therapeutic implications. Biomed. Pharmacother. 2022, 153, 113469. [Google Scholar] [CrossRef] [PubMed]
- Ibrayeva, A.; Bay, M.; Pu, E.; Jorg, D.J.; Peng, L.; Jun, H.; Zhang, N.; Aaron, D.; Lin, C.; Resler, G.; et al. Early stem cell aging in the mature brain. Cell Stem Cell 2021, 28, 955–966.e7. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, R.; Ghareghani, M.; Zibara, K.; Tajali Ardakani, M.; Jand, Y.; Azari, H.; Nikbakht, J.; Ghanbari, A. Alyssum homolocarpum seed oil (AHSO), containing natural alpha linolenic acid, stearic acid, myristic acid and beta-sitosterol, increases proliferation and differentiation of neural stem cells in vitro. BMC Complement. Altern. Med. 2019, 19, 113. [Google Scholar] [CrossRef]
- Jiang, L.H.; Yang, N.Y.; Yuan, X.L.; Zou, Y.J.; Zhao, F.M.; Chen, J.P.; Wang, M.Y.; Lu, D.X. Daucosterol promotes the proliferation of neural stem cells. J. Steroid Biochem. Mol. Biol. 2014, 140, 90–99. [Google Scholar] [CrossRef]
- Hamedi, A.; Ghanbari, A.; Razavipour, R.; Saeidi, V.; Zarshenas, M.M.; Sohrabpour, M.; Azari, H. Alyssum homolocarpum seeds: Phytochemical analysis and effects of the seed oil on neural stem cell proliferation and differentiation. J. Nat. Med. 2015, 69, 387–396. [Google Scholar] [CrossRef]
- Kong, S.Y.; Park, M.H.; Lee, M.; Kim, J.O.; Lee, H.R.; Han, B.W.; Svendsen, C.N.; Sung, S.H.; Kim, H.J. Kuwanon V inhibits proliferation, promotes cell survival and increases neurogenesis of neural stem cells. PLoS ONE 2015, 10, e0118188. [Google Scholar] [CrossRef]
- Li, Y.J.; Li, Y.J.; Yang, L.D.; Zhang, K.; Zheng, K.Y.; Wei, X.M.; Yang, Q.; Niu, W.M.; Zhao, M.G.; Wu, Y.M. Silibinin exerts antidepressant effects by improving neurogenesis through BDNF/TrkB pathway. Behav. Brain Res. 2018, 348, 184–191. [Google Scholar] [CrossRef]
- Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Zhou, L.; Zeng, L.; Yang, Q. Resveratrol pretreatment attenuates injury and promotes proliferation of neural stem cells following oxygen-glucose deprivation/reoxygenation by upregulating the expression of Nrf2, HO-1 and NQO1 in vitro. Mol. Med. Rep. 2016, 14, 3646–3654. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Agarwal, S.; Tripathi, A.; Chaturvedi, R.K. Bisphenol-A Mediated Inhibition of Hippocampal Neurogenesis Attenuated by Curcumin via Canonical Wnt Pathway. Mol. Neurobiol. 2016, 53, 3010–3029. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, J.J.; Cai, Z.N.; Xu, C.J. The effect of curcumin on the differentiation, apoptosis and cell cycle of neural stem cells is mediated through inhibiting autophagy by the modulation of Atg7 and p62. Int. J. Mol. Med. 2018, 42, 2481–2488. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Yon, J.M.; Lin, C.; Jung, A.Y.; Jung, K.Y.; Nam, S.Y. Combined treatment with capsaicin and resveratrol enhances neuroprotection against glutamate-induced toxicity in mouse cerebral cortical neurons. Food Chem. Toxicol. 2012, 50, 3877–3885. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Brommer, B.; Tian, X.; Krishnan, A.; Meer, M.; Wang, C.; Vera, D.L.; Zeng, Q.; Yu, D.; Bonkowski, M.S.; et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 2020, 588, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Abdanipour, A.; Noori-Zadeh, A.; Mesbah-Namin, S.A.; Bakhtiyari, S.; Nejatbakhsh, R.; Anarkooli, I.J. Di-(2-ethylhexyl) Phthalate-Induced Hippocampus-Derived Neural Stem Cells Proliferation. Cell J. 2017, 19, 166–172. [Google Scholar] [CrossRef]
- Traka, M.H.; Chambers, K.F.; Lund, E.K.; Goodlad, R.A.; Johnson, I.T.; Mithen, R.F. Involvement of KLF4 in sulforaphane- and iberin-mediated induction of p21(waf1/cip1). Nutr. Cancer 2009, 61, 137–145. [Google Scholar] [CrossRef]
- Kim, S.H.; Singh, S.V. Mammary cancer chemoprevention by withaferin A is accompanied by in vivo suppression of self-renewal of cancer stem cells. Cancer Prev. Res. 2014, 7, 738–747. [Google Scholar] [CrossRef]
- Chintharlapalli, S.; Papineni, S.; Liu, S.; Jutooru, I.; Chadalapaka, G.; Cho, S.D.; Murthy, R.S.; You, Y.; Safe, S. 2-cyano-lup-1-en-3-oxo-20-oic acid, a cyano derivative of betulinic acid, activates peroxisome proliferator-activated receptor gamma in colon and pancreatic cancer cells. Carcinogenesis 2007, 28, 2337–2346. [Google Scholar] [CrossRef]
- Gelfand, R.; Vernet, D.; Bruhn, K.W.; Sarkissyan, S.; Heber, D.; Vadgama, J.V.; Gonzalez-Cadavid, N.F. Long-term exposure of MCF-7 breast cancer cells to ethanol stimulates oncogenic features. Int. J. Oncol. 2017, 50, 49–65. [Google Scholar] [CrossRef]
- Effros, R.B. Telomere/telomerase dynamics within the human immune system: Effect of chronic infection and stress. Exp. Gerontol. 2011, 46, 135–140. [Google Scholar] [CrossRef]
- Lin, J.; Epel, E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res. Rev. 2022, 73, 101507. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Schreiner, M.; Schlotz, N.; Lamy, E. Short-Term Dietary Intervention with Cooked but Not Raw Brassica Leafy Vegetables Increases Telomerase Activity in CD8+ Lymphocytes in a Randomized Human Trial. Nutrients 2019, 11, 786. [Google Scholar] [CrossRef] [PubMed]
- Berezutsky, M.A.; Durnova, N.A.; Vlasova, I.A. Experimental and clinical studies of mechanisms of the anti-aging effects of chemical compounds in Astragalus membranaceus (review). Adv. Gerontol. 2019, 32, 702–710. [Google Scholar] [PubMed]
- Radwan, R.A.; El-Sherif, Y.A.; Salama, M.M. A Novel Biochemical Study of Anti-Ageing Potential of Eucalyptus Camaldulensis Bark Waste Standardized Extract and Silver Nanoparticles. Colloids Surfaces B Biointerfaces 2020, 191, 111004. [Google Scholar] [CrossRef]
- Refaey, M.S.; Abdelhamid, R.A.; Elimam, H.; Elshaier, Y.; Ali, A.A.; Orabi, M.A.A. Bioactive constituents from Thunbergia erecta as potential anticholinesterase and anti-ageing agents: Experimental and in silico studies. Bioorg. Chem. 2021, 108, 104643. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Lin, S.; Chen, K.; Yin, S.; Peng, H.; Cai, N.; Ma, W.; Songyang, Z.; Huang, Y. Natural Product Library Screens Identify Sanguinarine Chloride as a Potent Inhibitor of Telomerase Expression and Activity. Cells 2022, 11, 1485. [Google Scholar] [CrossRef]
- Saretzki, G. The Telomerase Connection of the Brain and Its Implications for Neurodegenerative Diseases. Stem Cells 2023, 41, 233–241. [Google Scholar] [CrossRef]
- Shibu, M.A.; Lin, Y.J.; Chiang, C.Y.; Lu, C.Y.; Goswami, D.; Sundhar, N.; Agarwal, S.; Islam, M.N.; Lin, P.Y.; Lin, S.Z.; et al. Novel anti-aging herbal formulation Jing Si displays pleiotropic effects against aging associated disorders. Biomed. Pharmacother. 2022, 146, 112427. [Google Scholar] [CrossRef]
- Perez, R.F.; Alba-Linares, J.J.; Tejedor, J.R.; Fernandez, A.F.; Calero, M.; Roman-Dominguez, A.; Borras, C.; Vina, J.; Avila, J.; Medina, M.; et al. Blood DNA Methylation Patterns in Older Adults with Evolving Dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1743–1749. [Google Scholar] [CrossRef]
- Mandal, S.; Denham, M.M.; Spencer, S.J.; Denham, J. Exercise regulates shelterin genes and microRNAs implicated in ageing in Thoroughbred horses. Pflug. Arch. Eur. J. Physiol. 2022, 474, 1159–1169. [Google Scholar] [CrossRef]
- Davinelli, S.; Trichopoulou, A.; Corbi, G.; De Vivo, I.; Scapagnini, G. The potential nutrigeroprotective role of Mediterranean diet and its functional components on telomere length dynamics. Ageing Res. Rev. 2019, 49, 1–10. [Google Scholar] [CrossRef]
- Progatzky, F.; Shapiro, M.; Chng, S.H.; Garcia-Cassani, B.; Classon, C.H.; Sevgi, S.; Laddach, A.; Bon-Frauches, A.C.; Lasrado, R.; Rahim, M.; et al. Regulation of intestinal immunity and tissue repair by enteric glia. Nature 2021, 599, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio 2019, 10, e00632-19. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, C.C.; Chiang, H.L.; Liou, J.M.; Chang, C.M.; Lu, T.P.; Chuang, E.Y.; Tai, Y.C.; Cheng, C.; Lin, H.Y.; et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflamm. 2019, 16, 129. [Google Scholar] [CrossRef]
- Kaur, G.; Behl, T.; Bungau, S.; Kumar, A.; Uddin, M.S.; Mehta, V.; Zengin, G.; Mathew, B.; Shah, M.A.; Arora, S. Dysregulation of the Gut-Brain Axis, Dysbiosis and Influence of Numerous Factors on Gut Microbiota Associated Parkinson’s Disease. Curr. Neuropharmacol. 2021, 19, 233–247. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.; Di Cesare Mannelli, L.; Lucarini, E.; Man, A.L.; Le Gall, G.; Branca, J.J.V.; Ghelardini, C.; Amedei, A.; Bertelli, E.; Regoli, M.; et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 2020, 8, 140. [Google Scholar] [CrossRef]
- Scheperjans, F.; Aho, V.; Pereira, P.A.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Qin, N.; Chen, S.D.; Xiao, Q. Detection of Microbial 16S rRNA Gene in the Blood of Patients with Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 156. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Quan, M.; Zhao, H.; Jia, J. Gut Microbiota Changes and Their Correlation with Cognitive and Neuropsychiatric Symptoms in Alzheimer’s Disease. J. Alzheime’rs Dis. 2021, 81, 583–595. [Google Scholar] [CrossRef]
- Yildirim, S.; Nalbantoglu, O.U.; Bayraktar, A.; Ercan, F.B.; Gundogdu, A.; Velioglu, H.A.; Gol, M.F.; Soylu, A.E.; Koc, F.; Gulpinar, E.A.; et al. Stratification of the Gut Microbiota Composition Landscape across the Alzheimer’s Disease Continuum in a Turkish Cohort. mSystems 2022, 7, e0000422. [Google Scholar] [CrossRef]
- Woo, V.; Alenghat, T. Epigenetic regulation by gut microbiota. Gut Microbes 2022, 14, 2022407. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Goto, S.; Tsuji, H.; Okuno, T.; Asahara, T.; Nomoto, K.; Shibata, A.; Fujisawa, Y.; Minato, T.; Okamoto, A.; et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS ONE 2015, 10, e0142164. [Google Scholar] [CrossRef]
- Petrov, V.A.; Saltykova, I.V.; Zhukova, I.A.; Alifirova, V.M.; Zhukova, N.G.; Dorofeeva, Y.B.; Tyakht, A.V.; Kovarsky, B.A.; Alekseev, D.G.; Kostryukova, E.S.; et al. Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bull. Exp. Biol. Med. 2017, 162, 734–737. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.D.; Xiao, Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Barichella, M.; Severgnini, M.; Cilia, R.; Cassani, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cancello, R.; Ceccarani, C.; Faierman, S.; et al. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 2019, 34, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, H.B.; Lutz, H.L.; Weigle, I.Q.; Patel, P.; Michalkiewicz, J.; Roman-Santiago, C.J.; Zhang, C.M.; Liang, Y.; Srinath, A.; Zhang, X.; et al. Gut microbiota-driven brain Abeta amyloidosis in mice requires microglia. J. Exp. Med. 2022, 219, e20200895. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Yang, B.; Chen, K.; Kong, Y.; Fang, N.; Gong, T.; Wang, F.; Ling, Z.; Liu, J. Effect of Clostridium butyricum against Microglia-Mediated Neuroinflammation in Alzheimer’s Disease via Regulating Gut Microbiota and Metabolites Butyrate. Mol. Nutr. Food Res. 2020, 64, e1900636. [Google Scholar] [CrossRef]
- Lekchand Dasriya, V.; Samtiya, M.; Dhewa, T.; Puniya, M.; Kumar, S.; Ranveer, S.; Chaudhary, V.; Vij, S.; Behare, P.; Singh, N.; et al. Etiology and management of Alzheimer’s disease: Potential role of gut microbiota modulation with probiotics supplementation. J. Food Biochem. 2022, 46, e14043. [Google Scholar] [CrossRef]
- Vaiserman, A.; Koliada, A.; Lushchak, O. Neuroinflammation in pathogenesis of Alzheimer’s disease: Phytochemicals as potential therapeutics. Mech. Ageing Dev. 2020, 189, 111259. [Google Scholar] [CrossRef]
- Wang, Y.; Lim, Y.Y.; He, Z.; Wong, W.T.; Lai, W.F. Dietary phytochemicals that influence gut microbiota: Roles and actions as anti-Alzheimer agents. Crit. Rev. Food Sci. Nutr. 2022, 62, 5140–5166. [Google Scholar] [CrossRef]
- El Gaamouch, F.; Chen, F.; Ho, L.; Lin, H.Y.; Yuan, C.; Wong, J.; Wang, J. Benefits of dietary polyphenols in Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1019942. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Solch, R.J.; Aigbogun, J.O.; Voyiadjis, A.G.; Talkington, G.M.; Darensbourg, R.M.; O’Connell, S.; Pickett, K.M.; Perez, S.R.; Maraganore, D.M. Mediterranean diet adherence, gut microbiota, and Alzheimer’s or Parkinson’s disease risk: A systematic review. J. Neurol. Sci. 2022, 434, 120166. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, X.; Xu, Q.; Wei, G.; Zhang, L.; Fan, Y.; Wen, L.; Liu, Y.; Zhang, T.; Zhang, L.; et al. Qisheng Wan formula ameliorates cognitive impairment of Alzheimer’s disease rat via inflammation inhibition and intestinal microbiota regulation. J. Ethnopharmacol. 2022, 282, 114598. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Pi, Z.; Song, F.; Wu, J.; Liu, S. Poria cocos could ameliorate cognitive dysfunction in APP/PS1 mice by restoring imbalance of Abeta production and clearance and gut microbiota dysbiosis. Phytother. Res. 2021, 35, 2678–2690. [Google Scholar] [CrossRef]
- Terzo, S.; Amato, A.; Mule, F. From obesity to Alzheimer’s disease through insulin resistance. J. Diabetes Complicat. 2021, 35, 108026. [Google Scholar] [CrossRef]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Chen, S.; Gan, D.; Lin, S.; Zhong, Y.; Chen, M.; Zou, X.; Shao, Z.; Xiao, G. Metformin in aging and aging-related diseases: Clinical applications and relevant mechanisms. Theranostics 2022, 12, 2722–2740. [Google Scholar] [CrossRef]
- Wahab, A.; Gao, K.; Jia, C.; Zhang, F.; Tian, G.; Murtaza, G.; Chen, J. Significance of Resveratrol in Clinical Management of Chronic Diseases. Molecules 2017, 22, 1329. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Lai, C.Q.; Liu, D. Dietary Epicatechin, A Novel Anti-aging Bioactive Small Molecule. Curr. Med. Chem. 2021, 28, 3–18. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells 2022, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells 2022, 11, 359. [Google Scholar] [CrossRef]
- Hill, M.A.; Gammie, S.C. Alzheimer’s disease large-scale gene expression portrait identifies exercise as the top theoretical treatment. Sci. Rep. 2022, 12, 17189. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Alvarez-Alvarez, I.; Guillen-Grima, F.; Aguinaga-Ontoso, I. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurologia 2017, 32, 523–532. [Google Scholar] [CrossRef]
- Grubman, A.; Chew, G.; Ouyang, J.F.; Sun, G.; Choo, X.Y.; McLean, C.; Simmons, R.K.; Buckberry, S.; Vargas-Landin, D.B.; Poppe, D.; et al. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 2019, 22, 2087–2097. [Google Scholar] [CrossRef]
- Hajdarovic, K.H.; Yu, D.; Hassell, L.A.; Evans, S.; Packer, S.; Neretti, N.; Webb, A.E. Single-cell analysis of the aging female mouse hypothalamus. Nat. Aging 2022, 2, 662–678. [Google Scholar] [CrossRef]
- Yan, X.W.; Liu, H.J.; Hong, Y.X.; Meng, T.; Du, J.; Chang, C. lncRNA XIST induces Abeta accumulation and neuroinflammation by the epigenetic repression of NEP in Alzheimer’s disease. J. Neurogenet. 2022, 36, 11–20. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, X.; Shi, Z.; Wang, H.; Ren, D.; Zhang, Y.; Fan, Y.; Liu, Y.; Cui, Z. LncRNA XIST serves as a diagnostic biomarker in gestational diabetes mellitus and its regulatory effect on trophoblast cell via miR-497-5p/FOXO1 axis. Cardiovasc. Diagn. Ther. 2021, 11, 716–725. [Google Scholar] [CrossRef]
- Pineda, J.R.; Daynac, M.; Chicheportiche, A.; Cebrian-Silla, A.; Sii Felice, K.; Garcia-Verdugo, J.M.; Boussin, F.D.; Mouthon, M.A. Vascular-derived TGF-beta increases in the stem cell niche and perturbs neurogenesis during aging and following irradiation in the adult mouse brain. EMBO Mol. Med. 2013, 5, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Tian, F.; Xu, J.; Du, X.; Zhang, S.; Liu, L. New insight into dyslipidemia-induced cellular senescence in atherosclerosis. Biol. Rev. Camb. Philos. Soc. 2022, 97, 1844–1867. [Google Scholar] [CrossRef] [PubMed]
- Propson, N.E.; Roy, E.R.; Litvinchuk, A.; Kohl, J.; Zheng, H. Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging. J. Clin. Investig. 2021, 131, e144348. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Kumar, P.; Malik, J. Plants and phytochemicals for Huntington’s disease. Pharmacogn. Rev. 2013, 14, 81–91. [Google Scholar] [CrossRef]
- Chongtham, A.; Agrawal, N. Curcumin modulates cell death and is protective in Huntington’s disease model. Sci. Rep. 2016, 6, 18736. [Google Scholar] [CrossRef]
- Yoo, I.H.; Kim, M.J.; Kim, J.; Sung, J.J.; Park, S.T.; Ahn, S.W. The Anti-Inflammatory Effect of Sulforaphane in Mice with Experimental Autoimmune Encephalomyelitis. J. Korean Med. Sci. 2019, 28, e197. [Google Scholar] [CrossRef]
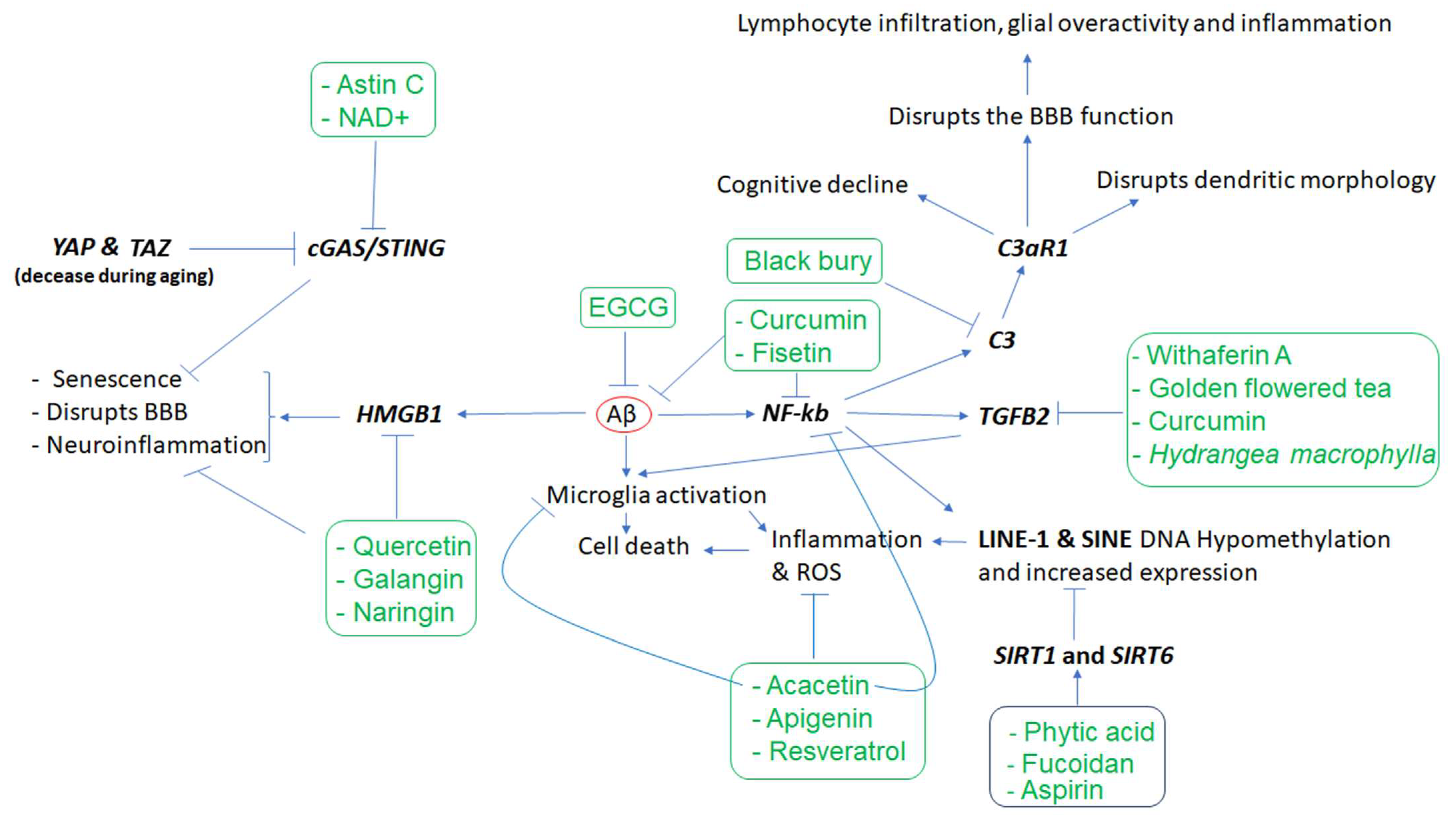

| Phytochemicals or Nutrients | In Vitro and/or Animal Models | Effects | Mechanisms of Action | References |
|---|---|---|---|---|
| Anthocyanins (bilberry) | APP/PSEN1 transgenic mouse model of AD |
|
| [22] |
| Cyanidin-3-O-glucoside (fruits and vegetables) | Mouse model of AD |
|
| [23] |
| Fucoidan (Atlantic brown algae) | D. melanogaster and mice |
|
| [39,40,42] |
| Bioactive compounds in black chokeberry (Aronia melanocapa L.) | Neuronal cells and mice brain |
|
| [45] |
| Curcumin | In vitro and in vivo |
|
| [76] |
| Curcumin | Animal model of spinal cord injury |
|
| [51] |
| Galangin | Rat brain |
|
| [58] |
| Astin C (a cyclopeptide from the Aster tataricus plant) | Mice |
|
| [68] |
| Apigenin (parsley and celery) | iPSC-derived neurons from patients with AD |
|
| [73] |
| Resveratrol (grapes and berries) | Yeast and flies |
|
| [74] |
| EGCG (tea polyphenol) | APP/PS1 mouse model of AD |
|
| [75] |
| Acacetin (Robinia pseudoacacia plant) | MPTP-induced mouse model of PD and LPS-induced mouse model of neuroinflammation |
|
| [77,78] |
| Phytic acid (plants and seeds) and aspirin | Neuronal cells and aged mice brain |
|
| [43,44] |
| Disease | Country | Increased | Decreased | Reference |
|---|---|---|---|---|
| PD | Finland | - | Prevotellaceae * | [119] |
| PD | USA | Blautia, Coprococcus, and Roseburia | [124] | |
| PD | Japan | Lactobacillus * | Clostridium coccoides * and Bacteroides fragilis * | [125] |
| PD | Russia | Lactobacillus *, Bifidobacterium, and Papillibacter cinnamivorans among others | Dorea, Bacteroides, Prevotella *, Coprococcus eutactus, and Ruminococcus callidus, among others | [126] |
| PD | China | Alistipes, Paraprevotella, Klebesiella, Sphingomonas, Acinetobacter, Aquabacterium, Desulfovibrio, Clostridium IV, Lachnospiracea incertae sedis, Butyricicoccus, Clostridium XVIII, and Nitrososphaera | Lactobacillus ¥ and Sediminibacterium | [127] |
| PD | Taiwan | Verrucomicrobia, Mucispirillum, Porphyromonas, Lactobacillus *, and Parabacteroides | Prevotella * (a genera of Prevotellaceae) * | [116] |
| PD | Italy | Lachnospiraceae | [128] | |
| AD | USA | Bacteroidetes *, Blautia®, and Alistipes© | Bifidobacterium ¥, Firmicutes, and Actinobacteria | [115] |
| AD | USA | Bacteroides *, Alistipes©, Odoribacter ¥, and Barnesiella | Lachnoclostridium, Butyrivibrio, and Eubacterium | [114] |
| AD | China | Bifidobacterium ¥, Sphingomonas, Lactobacillus, and Blautia® | Odoribacter ¥, Anaerobacterium, and Papillibacter | [121] |
| AD | Turkey | Bacteroides * and Prevotella | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdolmaleky, H.M.; Zhou, J.-R. Underlying Mechanisms of Brain Aging and Neurodegenerative Diseases as Potential Targets for Preventive or Therapeutic Strategies Using Phytochemicals. Nutrients 2023, 15, 3456. https://doi.org/10.3390/nu15153456
Abdolmaleky HM, Zhou J-R. Underlying Mechanisms of Brain Aging and Neurodegenerative Diseases as Potential Targets for Preventive or Therapeutic Strategies Using Phytochemicals. Nutrients. 2023; 15(15):3456. https://doi.org/10.3390/nu15153456
Chicago/Turabian StyleAbdolmaleky, Hamid Mostafavi, and Jin-Rong Zhou. 2023. "Underlying Mechanisms of Brain Aging and Neurodegenerative Diseases as Potential Targets for Preventive or Therapeutic Strategies Using Phytochemicals" Nutrients 15, no. 15: 3456. https://doi.org/10.3390/nu15153456
APA StyleAbdolmaleky, H. M., & Zhou, J.-R. (2023). Underlying Mechanisms of Brain Aging and Neurodegenerative Diseases as Potential Targets for Preventive or Therapeutic Strategies Using Phytochemicals. Nutrients, 15(15), 3456. https://doi.org/10.3390/nu15153456







