6-Gingerol Ameliorates Adiposity and Inflammation in Adipose Tissue in High Fat Diet-Induced Obese Mice: Association with Regulating of Adipokines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Biochemical Analysis
2.3. Histological Analysis
2.4. Western Blot Analysis
2.5. Geme Expression Analysis by Quantitative Real-Time RT-PCR
2.6. Statistical Analysis
3. Results
3.1. 6-Gingerol Reduces Body Weight Gain and Adipose Hypertrophy
3.2. 6-Gingerol Reduces Serum Lipids, Insulin, and Leptin Levels and Improves Insulin Resistance
3.3. 6-Gingerol Modulates the Adipogenesis Involved Gene Expression in WAT
3.4. 6-Gingerol Modulates the Inflammation Involved Gene Expression in WAT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, U.J.; Choi, M.-S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorotnikov, A.V.; Stafeev, I.S.; Menshikov, M.Y.; Shestakova, M.V.; Parfyonova, Y.V. Latent Inflammation and Defect in Adipocyte Renewal as a Mechanism of Obesity-Associated Insulin Resistance. Biochem. Mosc. 2019, 84, 1329–1345. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef] [PubMed]
- Young, H.-Y.; Luo, Y.-L.; Cheng, H.-Y.; Hsieh, W.-C.; Liao, J.-C.; Peng, W.-H. Analgesic and Anti-Inflammatory Activities of [6]-Gingerol. J. Ethnopharmacol. 2005, 96, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, P.; Liu, M.; Wei, Z.; Fan, S.; Wang, X.; Sun, S.; Chu, L. [6]-Gingerol Ameliorates ISO-Induced Myocardial Fibrosis by Reducing Oxidative Stress, Inflammation, and Apoptosis through Inhibition of TLR4/MAPKs/NF-ΚB Pathway. Mol. Nutr. Food Res. 2020, 64, e2000003. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Zhi, F.X.; Kun, T.H.; Hua, F.J.; Huan Ling, L.; Fang, F.; Wen, C.; Jie, W.; Yang, L.C. 6-Gingerol Attenuates Ventilator-Induced Lung Injury via Anti-Inflammation and Antioxidative Stress by Modulating the PPARγ/NF-ΚBsignalling Pathway in Rats. Int. Immunopharmacol. 2021, 92, 107367. [Google Scholar] [CrossRef]
- Alsahli, M.A.; Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Anwar, S.; Almutary, A.G.; Alrumaihi, F.; Rahmani, A.H. 6-Gingerol, a Major Ingredient of Ginger Attenuates Diethylnitrosamine-Induced Liver Injury in Rats through the Modulation of Oxidative Stress and Anti-Inflammatory Activity. Mediat. Inflamm. 2021, 2021, 6661937. [Google Scholar] [CrossRef]
- Son, M.J.; Miura, Y.; Yagasaki, K. Mechanisms for Antidiabetic Effect of Gingerol in Cultured Cells and Obese Diabetic Model Mice. Cytotechnology 2015, 67, 641–652. [Google Scholar] [CrossRef]
- Samad, M.B.; Mohsin, M.N.A.B.; Razu, B.A.; Hossain, M.T.; Mahzabeen, S.; Unnoor, N.; Muna, I.A.; Akhter, F.; Kabir, A.U.; Hannan, J.M.A. [6]-Gingerol, from Zingiber Officinale, Potentiates GLP-1 Mediated Glucose-Stimulated Insulin Secretion Pathway in Pancreatic β-Cells and Increases RAB8/RAB10-Regulated Membrane Presentation of GLUT4 Transporters in Skeletal Muscle to Improve Hyperglycemia in Lepr(Db/Db) Type 2 Diabetic Mice. BMC Complement. Altern. Med. 2017, 17, 395. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Lee, H.; Jung, C.H.; Ha, S.Y.; Seo, H.-D.; Kim, Y.I.; Ha, T. 6-Gingerol Ameliorates Hepatic Steatosis via HNF4α/MiR-467b-3p/GPAT1 Cascade. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1201–1213. [Google Scholar] [CrossRef]
- Kim, J.-K.; Kim, Y.; Na, K.-M.; Surh, Y.-J.; Kim, T.-Y. [6]-Gingerol Prevents UVB-Induced ROS Production and COX-2 Expression in Vitro and in Vivo. Free Radic. Res. 2007, 41, 603–614. [Google Scholar] [CrossRef]
- Lee, H.S.; Seo, E.Y.; Kang, N.E.; Kim, W.K. [6]-Gingerol Inhibits Metastasis of MDA-MB-231 Human Breast Cancer Cells. J. Nutr. Biochem. 2008, 19, 313–319. [Google Scholar] [CrossRef]
- Farombi, E.O.; Ajayi, B.O.; Adedara, I.A. 6-Gingerol Delays Tumorigenesis in Benzo[a]Pyrene and Dextran Sulphate Sodium-Induced Colorectal Cancer in Mice. Food Chem. Toxicol. 2020, 142, 111483. [Google Scholar] [CrossRef]
- Tzeng, T.-F.; Liu, I.-M. 6-Gingerol Prevents Adipogenesis and the Accumulation of Cytoplasmic Lipid Droplets in 3T3-L1 Cells. Phytomedicine 2013, 20, 481–487. [Google Scholar] [CrossRef]
- Tzeng, T.-F.; Chang, C.J.; Liu, I.-M. 6-Gingerol Inhibits Rosiglitazone-Induced Adipogenesis in 3T3-L1 Adipocytes. Phytother. Res. 2014, 28, 187–192. [Google Scholar] [CrossRef]
- Li, C.; Zhou, L. Inhibitory Effect 6-Gingerol on Adipogenesis through Activation of the Wnt/β-Catenin Signaling Pathway in 3T3-L1 Adipocytes. Toxicol. Vitr. 2015, 30, 394–401. [Google Scholar] [CrossRef]
- Beattie, J.H.; Nicol, F.; Gordon, M.-J.; Reid, M.D.; Cantlay, L.; Horgan, G.W.; Kwun, I.-S.; Ahn, J.-Y.; Ha, T.-Y. Ginger Phytochemicals Mitigate the Obesogenic Effects of a High-Fat Diet in Mice: A Proteomic and Biomarker Network Analysis. Mol. Nutr. Food Res. 2011, 55 (Suppl. 2), S203–S213. [Google Scholar] [CrossRef]
- Saravanan, G.; Ponmurugan, P.; Deepa, M.A.; Senthilkumar, B. Anti-Obesity Action of Gingerol: Effect on Lipid Profile, Insulin, Leptin, Amylase and Lipase in Male Obese Rats Induced by a High-Fat Diet. J. Sci. Food Agric. 2014, 94, 2972–2977. [Google Scholar] [CrossRef]
- Okamoto, M.; Irii, H.; Tahara, Y.; Ishii, H.; Hirao, A.; Udagawa, H.; Hiramoto, M.; Yasuda, K.; Takanishi, A.; Shibata, S.; et al. Synthesis of a New [6]-Gingerol Analogue and Its Protective Effect with Respect to the Development of Metabolic Syndrome in Mice Fed a High-Fat Diet. J. Med. Chem. 2011, 54, 6295–6304. [Google Scholar] [CrossRef]
- Brahma Naidu, P.; Uddandrao, V.V.S.; Ravindar Naik, R.; Suresh, P.; Meriga, B.; Begum, M.S.; Pandiyan, R.; Saravanan, G. Ameliorative Potential of Gingerol: Promising Modulation of Inflammatory Factors and Lipid Marker Enzymes Expressions in HFD Induced Obesity in Rats. Mol. Cell. Endocrinol. 2016, 419, 139–147. [Google Scholar] [CrossRef]
- Cheng, Z.; Xiong, X.; Zhou, Y.; Wu, F.; Shao, Q.; Dong, R.; Liu, Q.; Li, L.; Chen, G. 6-Gingerol Ameliorates Metabolic Disorders by Inhibiting Hypertrophy and Hyperplasia of Adipocytes in High-Fat-Diet Induced Obese Mice. Biomed. Pharmacother. 2022, 146, 112491. [Google Scholar] [CrossRef] [PubMed]
- Hashem, R.M.; Rashed, L.A.; Hassanin, K.M.A.; Hetta, M.H.; Ahmed, A.O. Effect of 6-Gingerol on AMPK- NF-ΚB Axis in High Fat Diet Fed Rats. Biomed. Pharmacother. 2017, 88, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, K.-J.; Kim, B.-H.; Koh, E.-J.; Seo, M.-J.; Lee, B.-Y. 6-Gingerol Suppresses Adipocyte-Derived Mediators of Inflammation In Vitro and in High-Fat Diet-Induced Obese Zebra Fish. Planta Med. 2017, 83, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Singh, A.; Mittal, M.; Sharan, K.; Singh, N.; Dixit, P.; Sanyal, S.; Maurya, R.; Chattopadhyay, N. [6]-Gingerol Induces Bone Loss in Ovary Intact Adult Mice and Augments Osteoclast Function via the Transient Receptor Potential Vanilloid 1 Channel. Mol. Nutr. Food Res. 2012, 56, 1860–1873. [Google Scholar] [CrossRef]
- Mlinar, B.; Marc, J.; Janez, A.; Pfeifer, M. Molecular Mechanisms of Insulin Resistance and Associated Diseases. Clin. Chim. Acta 2007, 375, 20–35. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Madsen, M.S.; Siersbæk, R.; Boergesen, M.; Nielsen, R.; Mandrup, S. Peroxisome Proliferator-Activated Receptor γ and C/EBPα Synergistically Activate Key Metabolic Adipocyte Genes by Assisted Loading. Mol. Cell. Biol. 2014, 34, 939–954. [Google Scholar] [CrossRef] [Green Version]
- Kuri-Harcuch, W.; Velez-delValle, C.; Vazquez-Sandoval, A.; Hernández-Mosqueira, C.; Fernandez-Sanchez, V. A Cellular Perspective of Adipogenesis Transcriptional Regulation. J. Cell. Physiol. 2019, 234, 1111–1129. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Young-Cheul, K. Adipocyte Differentiation and Gene Expression. J. Nutr. 2000, 130, 3122S–3126S. [Google Scholar] [CrossRef]
- Farmer, S.R. Transcriptional Control of Adipocyte Formation. Cell. Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-E.; Ge, K. Transcriptional and Epigenetic Regulation of PPARγ Expression during Adipogenesis. Cell. Biosci. 2014, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Ferré, P.; Foufelle, F. SREBP-1c Transcription Factor and Lipid Homeostasis: Clinical Perspective. Horm. Res. 2007, 68, 72–82. [Google Scholar] [CrossRef]
- Ferré, P.; Foufelle, F. Hepatic Steatosis: A Role for de Novo Lipogenesis and the Transcription Factor SREBP-1c. Diabetes Obes. Metab. 2010, 12 (Suppl. 2), 83–92. [Google Scholar] [CrossRef]
- Nomiyama, T.; Perez-Tilve, D.; Ogawa, D.; Gizard, F.; Zhao, Y.; Heywood, E.B.; Jones, K.L.; Kawamori, R.; Cassis, L.A.; Tschöp, M.H.; et al. Osteopontin Mediates Obesity-Induced Adipose Tissue Macrophage Infiltration and Insulin Resistance in Mice. J. Clin. Investig. 2007, 117, 2877–2888. [Google Scholar] [CrossRef] [Green Version]
- van Niekerk, E.; Mels, C.M.C.; Swanepoel, M.; Delles, C.; Welsh, P.; Botha-Le Roux, S. The Inflammatory Score and Cardiovascular Risk in Young Adults with Overweight or Obesity: The African-PREDICT Study. Cytokine 2023, 163, 156121. [Google Scholar] [CrossRef]
- Subash-Babu, P.; Mohammed Alowaidh, H.; Al-Harbi, L.N.; Shamlan, G.; Aloud, A.A.; AlSedairy, S.A.; Alshatwi, A.A. Ocimum basilicum L. Methanol Extract Enhances Mitochondrial Efficiency and Decreases Adipokine Levels in Maturing Adipocytes Which Regulate Macrophage Systemic Inflammation. Molecules 2022, 27, 1388. [Google Scholar] [CrossRef]
- Nehme, R.; Chervet, A.; Decombat, C.; Longechamp, L.; Rossary, A.; Boutin, R.; Rousset, A.; Senejoux, F.; Vachias, C.; Auxenfans, C.; et al. Aspalathus Linearis (Rooibos) Targets Adipocytes and Obesity-Associated Inflammation. Nutrients 2023, 15, 1751. [Google Scholar] [CrossRef]
- Santamarina, A.B.; Calder, P.C.; Estadella, D.; Pisani, L.P. Anthocyanins Ameliorate Obesity-Associated Metainflammation: Preclinical and Clinical Evidence. Nutr. Res. 2023, 114, 50–70. [Google Scholar] [CrossRef]
- Xue, F.; Cheng, J.; Liu, Y.; Cheng, C.; Zhang, M.; Sui, W.; Chen, W.; Hao, P.; Zhang, Y.; Zhang, C. Cardiomyocyte-specific knockout of ADAM17 ameliorates left ventricular remodeling and function in diabetic cardiomyopathy of mice. Signal Transduct. Target Ther. 2022, 7, 259. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Z.; Qiao, G.; Tang, B.; Li, P. Visualization of endoplasmic reticulum viscosity in the liver of mice with nonalcoholic fatty liver disease by a near-infrared fluorescence probe. Chin. Chem. Lett. 2021, 32, 3641–3645. [Google Scholar] [CrossRef]
- Glass, C.K.; Olefsky, J.M. Inflammation and Lipid Signaling in the Etiology of Insulin Resistance. Cell. Metab. 2012, 15, 635–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Zhang, F.; Du, Y.; Cui, W.; Dou, Y.; Lin, Y.; Zhao, Z.; Ma, X. Effect of tetrahedral DNA nanostructures on LPS-induced neuroinflammation in mice. Chin. Chem. Lett. 2022, 33, 1901–1906. [Google Scholar] [CrossRef]
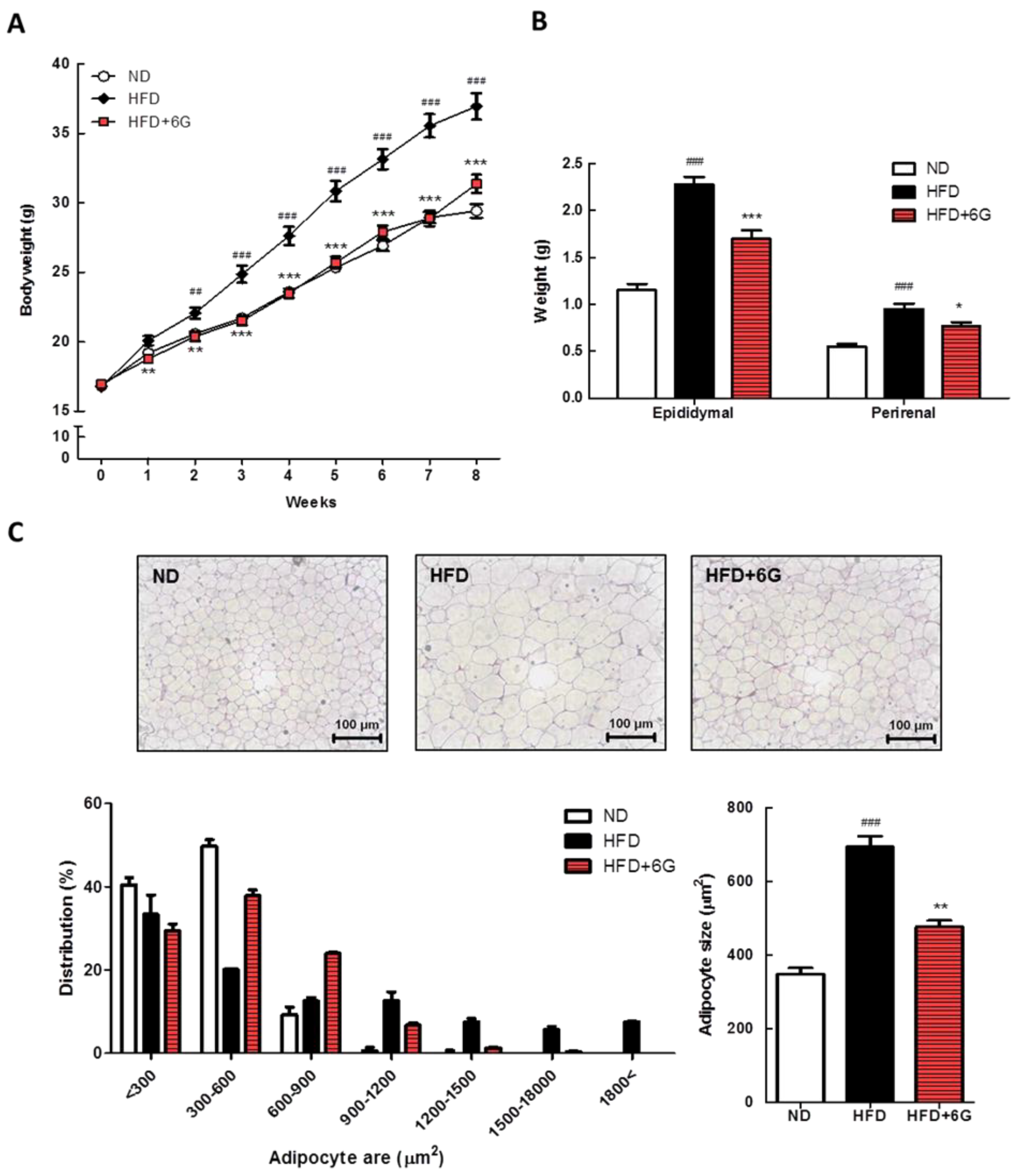
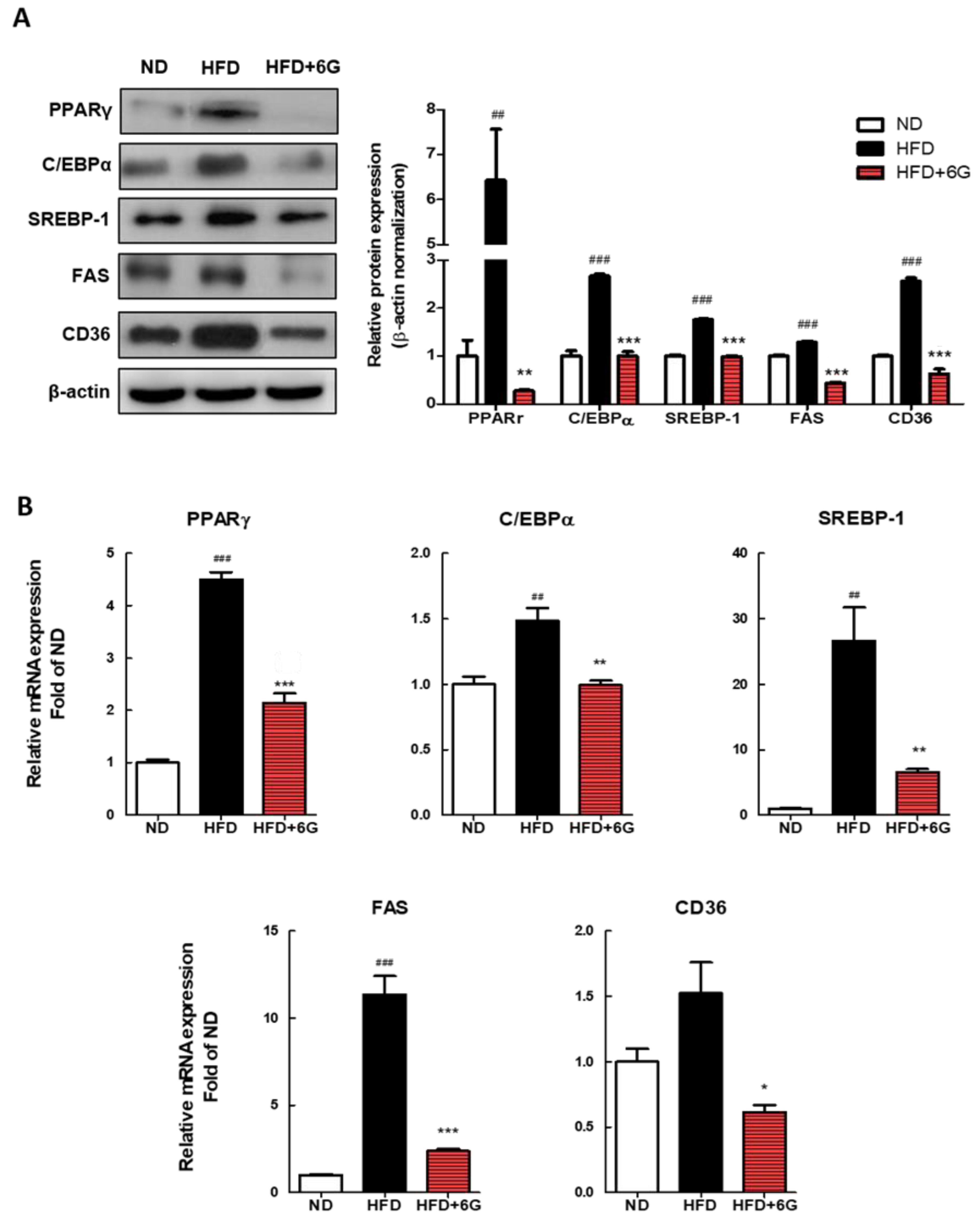
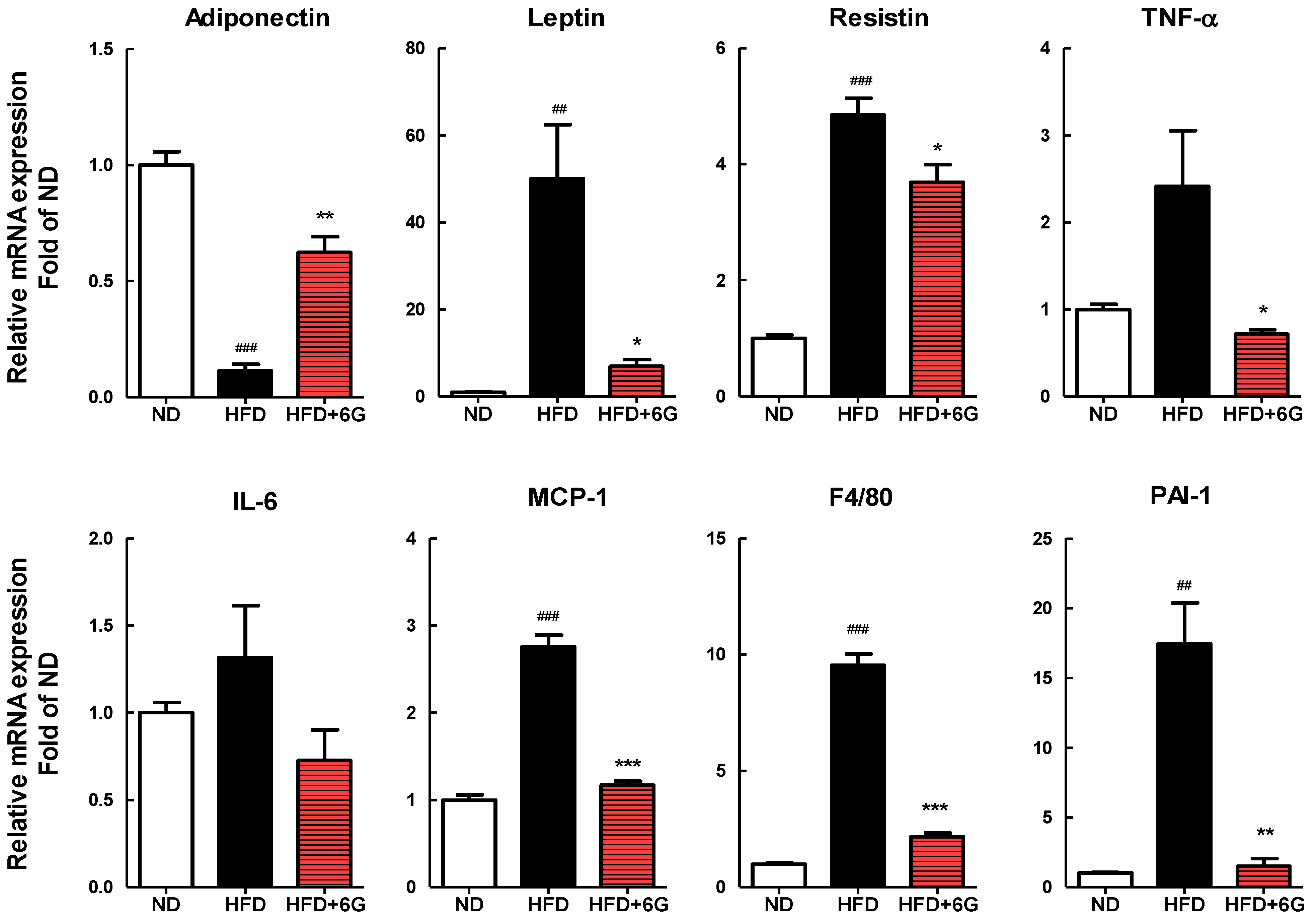
| ND | HFD | HFD + 6G | |
|---|---|---|---|
| Food intake (g/day) | 3.29 ± 0.17 | 3.19 ± 0.05 | 3.18 ± 0.07 |
| Serum TG (mg/dL) | 50.37 ± 2.00 | 51.29 ± 1.97 | 42.16 ± 2.33 * |
| Serum FFA (μEg/L) | 590.23 ± 77.36 | 543.69 ± 66.70 | 404.96 ± 60.89 |
| Serum TC (mg/dL) | 81.24 ± 22.6 | 128.43 ± 3.63 ### | 134.79 ± 5.06 |
| Serum HDL-C (mg/dL) | 42.43 ± 2.14 | 59.30 ± 5.47 ## | 60.55 ± 2.80 |
| Serum leptin (ng/mL) | 28.47 ± 3.07 | 81.69 ± 8.35 ### | 46.03 ± 4.70 *** |
| Serum adiponectin (mg/dL) | 13.73 ± 0.05 | 11.21 ± 0.03 # | 14.88 ± 0.08 ** |
| Serum glucose (mg/dL) | 274.12 ± 17.73 | 386.77 ± 42.16 # | 315.76 ± 17.87 |
| Serum insulin (pg/mL) | 364.11 ± 17.38 | 520.86 ± 68.07 # | 348.67 ± 46.90 * |
| HOMA-IR | 6.18 ± 0.48 | 12.42 ± 2.32 # | 6.76 ± 0.98 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, K.H.; Um, M.Y.; Ahn, J.; Ha, T.Y. 6-Gingerol Ameliorates Adiposity and Inflammation in Adipose Tissue in High Fat Diet-Induced Obese Mice: Association with Regulating of Adipokines. Nutrients 2023, 15, 3457. https://doi.org/10.3390/nu15153457
Hong KH, Um MY, Ahn J, Ha TY. 6-Gingerol Ameliorates Adiposity and Inflammation in Adipose Tissue in High Fat Diet-Induced Obese Mice: Association with Regulating of Adipokines. Nutrients. 2023; 15(15):3457. https://doi.org/10.3390/nu15153457
Chicago/Turabian StyleHong, Kyung Hee, Min Young Um, Jiyun Ahn, and Tae Youl Ha. 2023. "6-Gingerol Ameliorates Adiposity and Inflammation in Adipose Tissue in High Fat Diet-Induced Obese Mice: Association with Regulating of Adipokines" Nutrients 15, no. 15: 3457. https://doi.org/10.3390/nu15153457
APA StyleHong, K. H., Um, M. Y., Ahn, J., & Ha, T. Y. (2023). 6-Gingerol Ameliorates Adiposity and Inflammation in Adipose Tissue in High Fat Diet-Induced Obese Mice: Association with Regulating of Adipokines. Nutrients, 15(15), 3457. https://doi.org/10.3390/nu15153457






