Systematic Review on the Metabolic Interest of Glucosinolates and Their Bioactive Derivatives for Human Health
Abstract
1. Introduction
2. Objectives
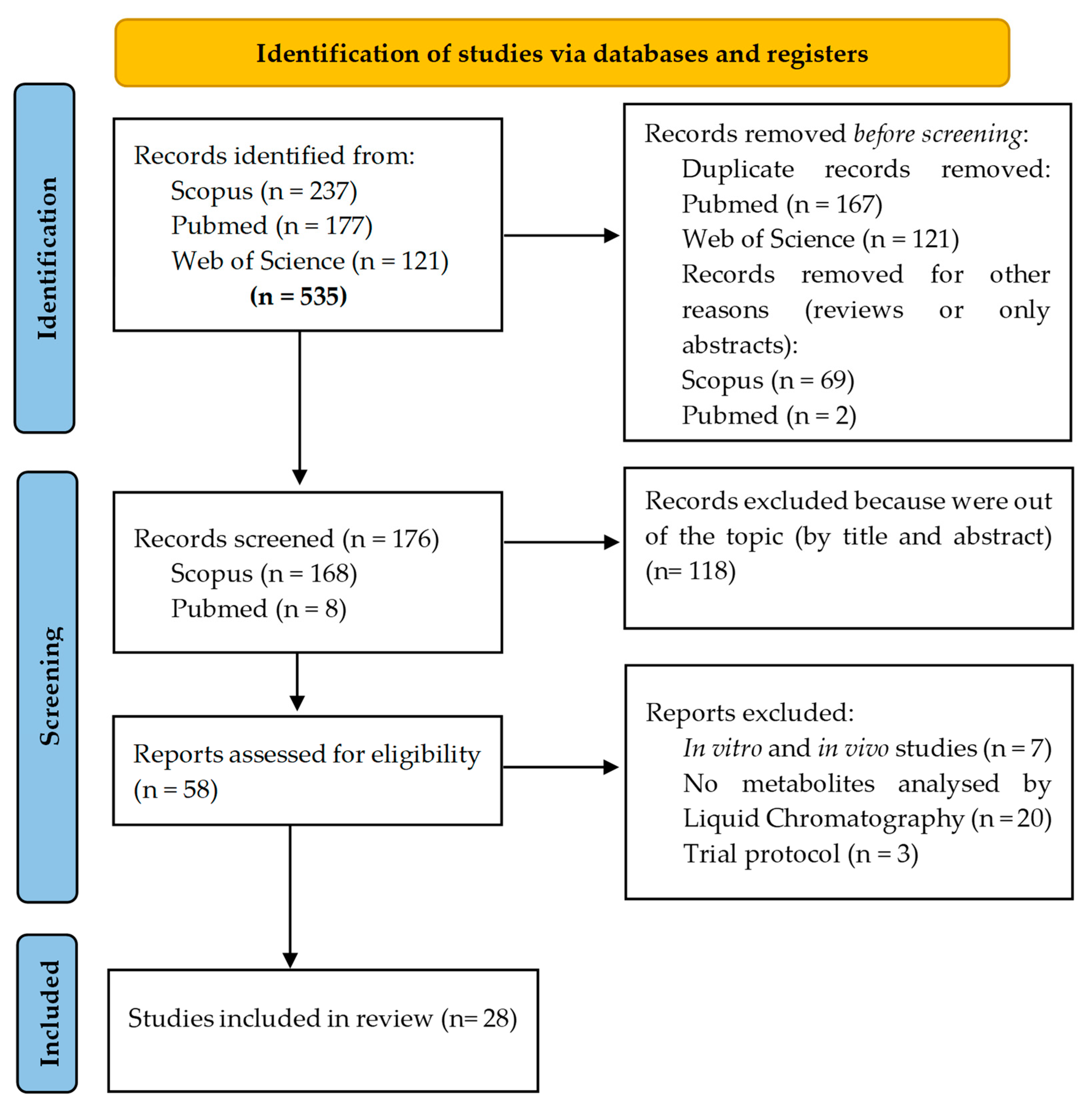
3. Methodology
4. Dietary Interventions with Cruciferous Sprouts
| Dietary Source–Chronic or Acute Intake | Sulfur-Nitrogen-Based Compounds-Dose | Biological Samples | Subjects Characteristics | Study Record Identifier (NCT Number) | Metabolites and Conjugates–Concentration Range | Analytical Technique | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Myrosinase-treated broccoli sprout extract 8 weeks | GR 200 µmol sulforaphane/day | Plasma and urine | Men prostate cancer risk: aged 65.7 ± 5.4 years who were scheduled for prostate biopsy | NCT01265953 | SFN: 0.0001/0.66 µM (plasma/urine) | LC-MS/MS | [58] | ||
| SFN-GSH: 0.03/0.0002 µM | |||||||||
| SFN-CysGly: 0.04/0.005 µM | |||||||||
| SFN-Cys: 0.02/1.23 µM | |||||||||
| SFN-NAC: 0.03/2.9 µM | |||||||||
| Beverages of Broccoli sprout powders (High, medium, low dosage) Nightly, 10 days | GR and SFN 100 mL of beverage/day 120–600 µmol GR 8–40 µmol SFN | Urine | Healthy adults (24–65 years old) | NCT02656420 | SFN (Mercapturic acid benzene deriv.) | 0.67–1.09 nM | LC-ESI-MS/MS-selected reaction monitoring (SRM) | [63] | |
| SFN-Cys | |||||||||
| SFN mercapturic | |||||||||
| Broccoli sprout powder 1 day | GR 1 g of broccoli sprout powder | Urine | 5 healthy adult participants (4 males and 1 female, between the ages of 40 and 50 years) | N.A. | ITC metabolites: 6.6 (powder), 8.2 (gel-cap), and 4.7 (enteric-cap) µM | HPLC-UV-VIS/DAD | [64] | ||
| Broccoli sprouts 10 weeks | GR 30 g broccoli sprouts/day | Urine | Healthy obese adults (35–55 years old) | NCT03390855 | SFN: 0.543 µM (day 70) | UHPLC-QqQ-MS/MS | [65] | ||
| SFN-Cys: 0.8 µM | |||||||||
| SFN-NAC: 2.301 µM | |||||||||
| 3,3′-DIM: 0.707 µM | |||||||||
| Broccoli sprouts 5 weeks | GR 30 g broccoli sprouts/ day | Urine | Healthy obese adults (men, non-menopausal women, and post-menopausal women; 40–60 years old) | NCT03390855 | SFN: men 0.4604; pre-menopausal 0.4989; post-menopausal 0.8937 nmol/mg creatinine (day 35) | UHPLC-QqQ-MS/MS | [66] | ||
| SFN-Cys: men 0.466; pre-menopausal 0.3727; post-menopausal 1.8191 nmol/mg creatinine | |||||||||
| SFN-NAC: men 1.5031; pre-menopausal 1.8079; post-menopausal 4.0647 nmol/mg creatinine | |||||||||
| 3,3′-DIM: men 0.7186; pre-menopausal 0.7467; post-menopausal 0.5544 nmol/mg creatinine | |||||||||
| Brassica carinata sprouts (AVRDC) 1 day | Epithionitrile 15.2 g B. carinata sprouts | Urine | Healthy adults | N.A. | N-acetyl-S-(3-cyano-2-(methylsulfonyl)propyl-cysteine 3 h: 37 µM | UHPLC-ESI-(Q)ToF-MS | [67] | ||
| Broccoli sprouts powder drink 24 days | GR or SFN (800 and 150 µmol, respectively) | Urine | Healthy volunteers | NCT01008826 | Day 6, TTA: 3.4/1.68 µmol/24 h (from GR/SFN matrix) | UHPLC-QqQ-MS/MS | [68] | ||
| Day 6, GR: 3.71/ND µmol/24h | |||||||||
| Day 6, SFN: 19.22/103.22 µmol/24 h | |||||||||
| Broccoli sprouts powder 1 day | GR | 0.64 g broccoli sprouts powder | Urine | Five participants (two males, and three females, aged 22–52) | N.A. | SFN: 2.06 µM | LC-MS/MS | [69] | |
| Glucoiberin | Iberin: 0.23 µM | ||||||||
| Broccoli sprouts powder with inactive myrosinase 1 day | GR | 0.64 g broccoli sprouts powder | Urine | Five participants (two males, and three females, aged 22–52) | N.A. | SFN: 0.66 µM | LC-MS/MS | [69] | |
| Glucoiberin | Iberin: 0.12 µM | ||||||||
| Gel capsule: oral formulation extracted from broccoli sprouts 28 days | GR 50, 100 or 200 µmol SFN | Plasma | 17 patients (Caucasian descent), 12 female and 5 males, 22–66 years old (mean 47), with skin melanoma | N.A. | SFN: 120 ng/mL (range 1–208 for the 50 μmol group), 206 (range 89–420 for the 100 μmol group), 656 ng/mL (range 396–1305 for the 200 μmol group) | LC-MS/MS | [70] | ||
| Gel capsule: oral formulation extracted from broccoli sprouts 28 days | GR 50, 100, or 200 µmol SFN | Skin | 17 patients (Caucasian descent), 12 female and 5 males, 22–66 years old (mean 47), with skin melanoma | N.A. | SFN: 0 ng/g (range 0–21.8 for the 50 μmol group); 0–18.9 ng/g (range:0–18.9 for 100 μmol group); 34.1 ng/g (range for the 200 μmol group) | LC-MS/MS | [70] | ||
5. Evidence of Dietary Interventions Using Cruciferous Vegetables and Derived Food Products
| Dietary Source-Intervention Time | Sulfur-nitrogen-Based Compounds-Dose | Biological Samples | Subjects’ Characteristics | Study Record Identifier (NCT Number) | Metabolites and Conjugates–Concentration Range | Analytical Technique | Reference |
|---|---|---|---|---|---|---|---|
| Commercially frozen broccoli 17 days once a day | GR and Glucoerucin 200 g of broccoli (providing 97.5 µmol of glucoraphanin and 5.8 µmol of glucoerucin) | Plasma and urine | Healthy subjects: 10 women and 8 men, 37–65 years of age | NCT02346812 | SFN | UHPLC-QqQ-MS/MS | [82] |
| SFN-GSH | |||||||
| SFN-CysGly | |||||||
| SFN-Cys | |||||||
| SFN-NAC * | |||||||
| Erucin-GSH | |||||||
| Erucin-CysGly > 37% | |||||||
| Erucin-Cys | |||||||
| Erucin-NAC * * (The two compounds represent > 41%) | |||||||
| SFN + sulforaphane metabolites | |||||||
| Blanched and frozen broccoli 26 days | GR and glucoerucin 200 g (the day before the study treated group eat only 100 g and on the day of the study all volunteers treated and non-treated eat 200 g) | Plasma and urine | Healthy women and men (between 40 and 70 years old) | NCT03013465 | SFN 13%/4% (% plasma/urine) | LC-MS | [86] |
| SFN-GSH 6% | |||||||
| SFN-CysGly 14% | |||||||
| SFN-Cys 2%/11% | |||||||
| SFN-NAC 5%/38% | |||||||
| Erucin-GSH < 1% | |||||||
| Erucin-CysGly 50% | |||||||
| Erucin-Cys 7%/7% | |||||||
| Erucin-NAC 2%/39% | |||||||
| Kale and daikon radish 1 day | GSL 250 g of baby kale (steamed weight 263 g), 25 g of uncooked daikon radish | Urine | Healthy adults (32–71 years old) | NCT03449849 | I3C: 6 arbitrary units/hour | UHPLC- HRAM-MS | [89] |
| MI3C: 1.2 arbitrary units/hour | |||||||
| I3-CAL: 3.5 arbitrary units/hour | |||||||
| MI3-CAL: 15 arbitrary units/hour | |||||||
| I3-CA: 22 arbitrary units/hour | |||||||
| MI3-CA: 15 arbitrary units/hour | |||||||
| AITC-Cys: 0.5 arbitrary units/hour | |||||||
| AITC-NAC: 3 arbitrary units/hour | |||||||
| 4-methylsulfinyl-3-butenyl isothiocyanate: 23 arbitrary unit/hour | |||||||
| 4-methylsulfinyl-3-butenyl isothiocyanate-cysteine: 7 arbitrary unit/hour | |||||||
| 4-methylsulfinyl-3-butenyl isothiocyanate-N-acetyl cysteine: 23 arbitrary units/hour | |||||||
| Ascorbigen: 2.5 arbitrary unit/hour | |||||||
| HABG: 5 arbitrary units/hour | |||||||
| MABG: 0.7 arbitrary units/hour | |||||||
| Raw broccoli 12 day | GR 200 g of uncooked broccoli florets | Plasma and urine | Healthy adults (28–67 years old) | NCT03287115 | I3C: 2200 (nmol/mmol cretinine) | UHPLC- HRAM MS | [90] |
| I3-CAL: 80 | |||||||
| I3-CA: 50 | |||||||
| ABG: 8000 | |||||||
| SFN: 800 | |||||||
| SFN-GSH: 5.5 | |||||||
| SFN-Cys: 150 | |||||||
| SFN-NAC: 700 | |||||||
| MI3C: 4500 | |||||||
| MI3-CAL600 | |||||||
| 4-methylsulfinyl-3-butenyl isothiocyanate 6500 | |||||||
| MABG 10,000 | |||||||
| HABG 1000 | |||||||
| Cooked broccoli 1 day | GR 200 g 1 g powdered brown mustard (Brassica juncea) | Urine | 12 Healthy adults between 18 and 64 years | N.A. | SFN-NAC 44.7 ± 33.9 μmol SFN-NAC per gram creatinine (9.8 ± 5.1 µmol SF-NAC per gram creatinine within 24 h without mustard) | HPLC-UV | [90] |
| Cooked B. carinata leaves (ethiopian kale) 4 days | Sinigrin 269 µmol sinigrin per serving (15 g) | Urine | 22 Participants (5 males and 17 females), aged 22.7 ± 2.4 years | DRKS00010836 | AITC-NAC 9.36 ± 9.81 (24 h after 4 days intake) mol/L urine | LC-ESI-MS/MS | [91] |
| Raw B. carinata leaves 5 days | AITC 177 µmol of AITC per serving (15 g) | Plasma and urine | 22 Participants (5 males and 17 females), aged 22.7 ± 2.4 years | DRKS00010836 | AITC-NAC 38.07 ± 21.00 (24 h after 4 days intake) mol/L urine | LC-ESI-MS/MS | [91] |
| AITC-GSH 53.90 ± 10.17 (2 h after day-5 intake) nmol/L plasma | |||||||
| AITC-CysGly 233.07 ± 167.55 (2 h after day-5 intake) nmol/L plasma | |||||||
| AITC-Cys 92.71 ± 71.811 (2 h after day-5 intake) nmol/L plasma | |||||||
| AITC-NAC 23.32 ± 10.21 (2 h after day-5 intake) nmol/L plasma |
6. Other Dietary Sources of Glucosinolates: Seeds, Extracts, or Formulas Enriched in GSL
| Dietary Source- Intervention Time | Sulfur-Nitrogen-Based Compounds-Dose | Biological Samples | Subjects Characteristics | Study Record Identifier (NCT Number) | Metabolites and Conjugates-Concentration Range | Analytical Technique | Reference | |
|---|---|---|---|---|---|---|---|---|
| Broccoli seed extract (BroccoMax®) and broccoli sprout extract 1 day | GR 32/64 mg sulforaphane | Plasma | Healthy women aged 18–35 years | N.A. | SFN: 125/150 nM (non-active/active) | LC-MS | [30] | |
| SFN-GSH: 140/280 nM | ||||||||
| SFN-CysGly: 300/550 nM | ||||||||
| SFN-Cys: 100/160 nM | ||||||||
| SFN-NAC: 48/66 nM | ||||||||
| Broccoli seed extract (BroccoMax®) 1 day | GR 32/64 mg sulforaphane | Plasma | Women with a singleton pregnancy and a diagnosis of preeclampsia or gestational hypertension, >18 years old | N.A. | SFN: 44/80 nM | LC-MS | [30] | |
| SFN-GSH: 60/160 nM | ||||||||
| SFN-CysGly: 110/180 | ||||||||
| SFN-Cys: 50/60 nM | ||||||||
| SFN-NAC: 60/120 nM | ||||||||
| Broccoli seed extract (BroccoMax®) 1 day | GR 32/64 mg sulforaphane | Plasma | Healthy nulliparous women aged between 20 and 23 years | N.A. | SFN: 183.5/206.5 nM | LC-MS | [106] | |
| SFN-GSH: 150.1/240.8 nM | ||||||||
| SFN-CysGly: 408/419.2 nM | ||||||||
| SFN-Cys 113.8/112.2 nM | ||||||||
| SFN-NAC: 74.3/35.6 nM | ||||||||
| Broccoli powder in soup and mustard seeds 1 day | GSL 200 mL soup | Ileal fluid | Ileostomates 53.3 ± 9.2 years | NCT04113928 | SFN: 1.05 µM | HPLC–UV/GC–MS | [107] | |
| Glucoiberin: 4–22 µM | ||||||||
| Sinigrin: 0 µM | ||||||||
| Gluconapin: 6–46 µM | ||||||||
| Glucoerucin: 0–32 µM | ||||||||
| GB: 2–23 µM | ||||||||
| Gluconasturtiin: 0–3 µM | ||||||||
| GR: 30–60 µM | ||||||||
| Glucoalysin: 1–4 µM | ||||||||
| HGB: 0–2 µM | ||||||||
| NeoGB: 3–24 µM | ||||||||
| MGB: 4–48 µM | ||||||||
| Broccoli seed and sprout extract supplement Avmacol® 1 day | GR 8 tablets per day per subject, estimated to deliver about 369 μmol/subject/day of GR | Urine | Healthy adults (24–69 years old) | N.A. | 25.67% (uncoated and no omeprazole); 35.48% (coated and no omeprazole); 33.59% (uncoated and omeprazole); 36.41% (coated and omeprazole) conversion efficiency | cyclocondensation reaction-HPLC assay | [108] | |
| Broccoli seed and sprout extract supplement Avmacol® 15 weeks | GR 2.2 μmol/kg/day | Plasma | Children 3–12 with autism spectrum disorder | NCT02561481 | Dithiocarbamates: SFN group week 0: 0.007; SFN group week 7: 0.299; SFN group week 15: 0.329 nmol/ml | Cyclocondensation reaction-HPLC assay | [109] | |
| Broccoli soup and broccoli soup with mustard 1 day | GSL 200 ml | Ileal fluid | Ileostomy subjects | NCT04113928 | Kynurenine: 99.5 (without mustard seeds) and 42.8 (with mustard seeds) ng | UHPLC-QqQ | [110] | |
| Tryptamine: Ileal fluid content: 11.7 (without mustard seeds) and 13.2 (with mustard seeds) ng | ||||||||
| Indole-3-lactic acid: Ileal fluid content: 88.6 (without mustard seeds) and 308.8 (with mustard seeds) ng | ||||||||
| Indole-3-aldehyde: Ileal fluid content: 34.4 (without mustard seeds) and 103.9 (with mustard seeds) ng | ||||||||
| Indole-3-acetic acid: Ileal fluid content: 18.2 (without mustard seeds) and 28.0 (with mustard seeds) ng | ||||||||
| Nasturtium leaves suspension made from freeze-dried leaves 48 h | Benzyl glucosinolate (Glucotropaeolin) 1.71 µmol of Benzyl-GSL and 191 µmol of BITC | Plasma and urine | Four healthy women aged between 26 and 61 | N.A. | BITC-GSH:-(in urine) | Total metabolites in plasma: 0.36–1.06 in plama µmol/L | LC–ESI–MS/MS/GC-MS/MS | [111] |
| BITC-CysGly: - | ||||||||
| BITC-Cys: 1–2 µmol/L, maximum after 4 h | ||||||||
| BITC-NAC: 60 µmol/L maximum after 4 h consumption | ||||||||
| Bread enriched with nasturtium leaves 48 h | 4.3 µmol of Benzyl-GSL and 2.48 µmol of BITC | Plasma and urine | Three healthy women aged between 26 and 61 | N.A. | BITC-GSH: - | Total metabolites in plasma: 0.24–0.35 µmol/L | LC–ESI–MS/MS/GC-MS/MS | [111] |
| BITC-CysGly: - | ||||||||
| BITC-Cys: 0.2–0.5 µmol/L | ||||||||
| BITC-NAC: 10–20 µmol/L, maximum after 4–6 h | ||||||||
| Nasturtium leaves suspension made from freeze-dried leaves 48 h | 4.3 µmol of Benzyl-GSL and 2.48 µmol of BITC | Plasma | Healthy women aged between 26 and 61 | N.A. | BITC-Lys: <0.2 µmol/L | LC–ESI–MS/MS/GC-MS/MS | [111] | |
| BITC-Cys: <0.2 µmol/L | ||||||||
| Nasturtium leaves suspension made from freeze-dried leaves 48 h | 1.71 µmol of Benzyl-GSL and 191 µmol of BITC | Breath | Healthy women aged between 26 and 61 | N.A. | BITC: Individual time courses of exhaling both breakdown products among subjects, 0.03–5.89 nmol L−1 | LC–ESI–MS/MS/GC-MS/MS | [111] | |
| Bread enriched with nasturtium leaves 48 h | 4.3 µmol of Benzyl-GSL and 2.48 µmol of BITC | Breath | Healthy women aged between 26 and 61 | N.A. | BITC: Individual time courses of exhaling both breakdown products among subjects, 0.03–5.89 nmol L−1 | LC–ESI–MS/MS/GC-MS/MS | [111] | |
| Nasturtium leaves suspension made from freeze-dried leaves | 1.71 µmol of Benzyl-GSL and 191 µmol of BITC. | Urine | Healthy women aged between 26 and 61 | N.A. | BITC: 2.0–8.0 µmol/L | LC–ESI–MS/MS/GC-MS/MS | [111] | |
| Bread enriched with nasturtium leaves | 4.3 µmol of Benzyl-GSL and 2.48 µmol of BITC | Urine | Healthy women aged between 26 and 61 | N.A. | BITC: 2.0–6.0 µmol/L | LC–ESI–MS/MS/GC-MS/MS | [111] | |
| Cooked broccoli, with powdered brown mustard (Brassica juncea) 1 day | GR 200 g broccoli, 1 g powdered brown mustard (Brassica juncea) | Urine | 12 healthy adults between 18 and 64 years | N.A. | SFN-NAC: 44.7 ± 33.9 µmol SFN-NAC per gram creatinine within 24 h (without mustard: 9.8 ± 5.1 µmol SF-NAC per gram creatinine within 24 h) | HPLC-UV | [90] | |
| Baked snack food containing equivalent phytochemicals1 day | GR 12.7 (glucoraphanin) mg | Urine | Healthy adults (18 females and 10 were premenopausal), average 42 years (age range 20–68 year) | NCT02231502 | SFN: 36.25 ± 27.9 nmol/mg intake | HPLC-QTrap | [112] | |
| Microwaved vegetables 1 day | 12.6 (glucoraphanin) mg | Urine | Healthy adults (18 females and 10 were premenopausal), average 42 years (age range 20–68 year) | NCT02231502 | SFN-NAC: 272.17 ± 280.8 nmol/mg intake | HPLC-QTrap | [112] | |
| Baked snack food containing equivalent phytochemicals1 day | 12.7 (glucoraphanin) mg | Urine | Healthy adults (18 females and 10 were premenopausal), average 42 years (age range 20–68 year) | NCT02231502 | SFN: 43.72 ± 44.2 nmol/mg intake | HPLC-QTrap | [112] | |
| Microwaved vegetables 1 day | 12.6 (glucoraphanin) mg | Urine | Healthy adults (18 females and 10 were premenopausal), average 42 years (age range 20–68 year) | NCT02231502 | SFN-NAC: 508.54 ± 450.9 nmol/mg intake | HPLC-QTrap | [112] | |
| Standard broccoli soup 1 day | GSL: 84 ± 2.8 µmoles glucoraphanin per broccoli soup | Plasma | 10 participants (3 Men and 7 women) aged 18–65 years | NCT02300324 | GR: 0.01 ± 0.01 μmol L−1 | UPLC–MS/MS | [29] | |
| BENEFORTE broccoli soup 1 day | 280 ± 8.8 µmoles glucoraphanin per broccoli soup | GR: 0.03 ± 0.01 μmol L−1 | ||||||
| Broccoli soup 1 day | 452 ± 10.6 µmoles glucoraphanin per broccoli soup | GR: 0.04 ± 0.02 μmol L−1 | ||||||
| Standard broccoli soup 1 day | GR: 84 ± 2.8 µmoles glucoraphanin per broccoli soup | Urine | GR and glucoerucin: 0.54 ± 0.29 μmol/24 h | |||||
| BENEFORTE broccoli soup 1 day | 280 ± 8.8 µmoles glucoraphanin per broccoli soup | GR and glucoerucin: 1.44 ± 0.66 μmol/24 h | ||||||
| Broccoli soup 1 day | GR and glucoerucin: 2.12 ± 0.98 μmol/24 h | |||||||
7. Conclusions and Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamal, R.M.; Razis, A.F.A.; Sukri, N.S.M.; Perimal, E.K.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases. Molecules 2022, 27, 624. [Google Scholar] [CrossRef]
- Bischoff, K.L. Glucosinolates. Nutraceuticals Effic. Saf. Toxic. 2016, 40, 551–554. [Google Scholar] [CrossRef]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional Ingredients From Brassicaceae Species: Overview and Perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef]
- Capuano, E.; Dekker, M.; Verkerk, R.; Oliviero, T. Food as Pharma? The Case of Glucosinolates. Curr. Pharm. Des. 2017, 23, 2697–2721. [Google Scholar] [CrossRef]
- Burow, M. Complex Environments Interact With Plant Development to Shape Glucosinolate Profiles. Adv. Bot. Res. 2016, 80, 15–30. [Google Scholar] [CrossRef]
- Basu, N.; Maity, S.K.; Chaudhury, A.; Ghosh, R. Trichloroisocyanuric acid (TCCA): An efficient green reagent for activation of thioglycosides toward hydrolysis. Carbohydr. Res. 2013, 369, 10–13. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and Stability of Glucosinolates and Their Breakdown Products in Foods. Angew. Chemie Int. Ed. 2014, 53, 11430–11450. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology And Biochemistry of Glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014, 64, 48. [Google Scholar] [CrossRef] [PubMed]
- Agerbirk, N.; De Vos, M.; Kim, J.H.; Jander, G. Indole glucosinolate breakdown and its biological effects. Phytochem. Rev. 2009, 8, 101–120. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Polat, U. The Effects on Metabolism of Glucosinolates and Theirs Hydrolysis Products. J. Biol. Environ. Sci. 2010, 4, 39–42. [Google Scholar]
- Almushayti, A.Y.; Brandt, K.; Carroll, M.A.; Scotter, M.J. Current analytical methods for determination of glucosinolates in vegetables and human tissues. J. Chromatogr. A 2021, 1643, 462060. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Klopsch, R.; Oliviero, T.; Schreiner, M.; Verkerk, R.; Dekker, M. Optimizing isothiocyanate formation during enzymatic glucosinolate breakdown by adjusting pH value, temperature and dilution in Brassica vegetables and Arabidopsis thaliana. Sci. Rep. 2017, 7, 40807. [Google Scholar] [CrossRef] [PubMed]
- Gu, E.H.; Su’udi, M.; Han, N.R.; Kwon, B.; Lim, S.; Kim, J. Increase in aliphatic glucosinolates synthesis during early seedling growth and insect herbivory in radish (Raphanus sativus L.) plant. Hortic. Environ. Biotechnol. 2015, 56, 255–262. [Google Scholar] [CrossRef]
- Baenas, N.; Wagner, A.E. Drosophila melanogaster as an alternative model organism in nutrigenomics. Genes Nutr. 2019, 14, 14. [Google Scholar] [CrossRef]
- Parchem, K.; Piekarska, A.; Bartoszek, A. Enzymatic activities behind degradation of glucosinolates. Glucosinolates Prop. Recover. Appl. 2019, 79–106. [Google Scholar] [CrossRef]
- Luang-In, V.; Narbad, A.; Nueno-Palop, C.; Mithen, R.; Bennett, M.; Rossiter, J.T. The metabolism of methylsulfinylalkyl- and methylthioalkyl-glucosinolates by a selection of human gut bacteria. Mol. Nutr. Food Res. 2014, 58, 875–883. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Yuan, J.M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the power of plants to people. Mol. Nutr. Food Res. 2018, 62, e1700965. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, T.; Lamers, S.; Capuano, E.; Dekker, M.; Verkerk, R. Bioavailability of Isothiocyanates From Broccoli Sprouts in Protein, Lipid, and Fiber Gels. Mol. Nutr. Food Res. 2018, 62, 1700837. [Google Scholar] [CrossRef]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef]
- Baenas, N.; Medina, S.; García-Viguera, C.; Moreno, D.A. Bioavailability and new biomarkers of cruciferous sprouts consumption. Food Res. Int. 2017, 100, 497–503. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Sarwar, N.; Gao, P.; Kondapally Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Sivapalan, T.; Melchini, A.; Saha, S.; Needs, P.W.; Traka, M.H.; Tapp, H.; Dainty, J.R.; Mithen, R.F. Bioavailability of Glucoraphanin and Sulforaphane from High-Glucoraphanin Broccoli. Mol. Nutr. Food Res. 2018, 62, 1700911. [Google Scholar] [CrossRef]
- Langston-Cox, A.G.; Anderson, D.; Creek, D.J.; Palmer, K.R.; Marshall, S.A.; Wallace, E.M. Sulforaphane Bioavailability and Effects on Blood Pressure in Women with Pregnancy Hypertension. Reprod. Sci. 2021, 28, 1489–1497. [Google Scholar] [CrossRef]
- Felker, P.; Bunch, R.; Leung, A.M. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutr. Rev. 2016, 74, 248. [Google Scholar] [CrossRef]
- Collett, M.G.; Stegelmeier, B.L.; Tapper, B.A. Could nitrile derivatives of turnip (Brassica rapa) glucosinolates be hepato- or cholangiotoxic in cattle? J. Agric. Food Chem. 2014, 62, 7370–7375. [Google Scholar] [CrossRef] [PubMed]
- Herr, I.; Büchler, M.W. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat. Rev. 2010, 36, 377–383. [Google Scholar] [CrossRef]
- Yanaka, A.; Fahey, J.W.; Fukumoto, A.; Nakayama, M.; Inoue, S.; Zhang, S.; Tauchi, M.; Suzuki, H.; Hyodo, I.; Yamamoto, M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev. Res. 2009, 2, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Bonvicini, F.; Pagnotta, E.; Punzo, A.; Calabria, D.; Simoni, P.; Mirasoli, M.; Passerini, N.; Bertoni, S.; Ugolini, L.; Lazzeri, L.; et al. Effect of Lactobacillus acidophilus Fermented Broths Enriched with Eruca sativa Seed Extracts on Intestinal Barrier and Inflammation in a Co-Culture System of an Enterohemorrhagic Escherichia coli and Human Intestinal Cells. Nutrients 2020, 12, 3064. [Google Scholar] [CrossRef] [PubMed]
- Riso, P.; Martini, D.; Møller, P.; Loft, S.; Bonacina, G.; Moro, M.; Porrini, M. DNA damage and repair activity after broccoli intake in young healthy smokers. Mutagenesis 2010, 25, 595–602. [Google Scholar] [CrossRef]
- Egner, P.A.; Chen, J.G.; Zarth, A.T.; Ng, D.K.; Wang, J.B.; Kensler, K.H.; Jacobson, L.P.; Muñoz, A.; Johnson, J.L.; Groopman, J.D.; et al. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: Results of a randomized clinical trial in China. Cancer Prev. Res. 2014, 7, 813–823. [Google Scholar] [CrossRef]
- Armah, C.N.; Derdemezis, C.; Traka, M.H.; Dainty, J.R.; Doleman, J.F.; Saha, S.; Leung, W.; Potter, J.F.; Lovegrove, J.A.; Mithen, R.F. Diet rich in high glucoraphanin broccoli reduces plasma LDL cholesterol: Evidence from randomised controlled trials. Mol. Nutr. Food Res. 2015, 59, 918–926. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Golzarand, M.; Zojaji, H.; Azizi, F. A comparative study of broccoli sprouts powder and standard triple therapy on cardiovascular risk factors following H.pylori eradication: A randomized clinical trial in patients with type 2 diabetes. J. Diabetes Metab. Disord. 2014, 13, 64. [Google Scholar] [CrossRef]
- Kuchernig, J.C.; Burow, M.; Wittstock, U. Evolution of specifier proteins in glucosinolate-containing plants. BMC Evol. Biol. 2012, 12, 127. [Google Scholar] [CrossRef]
- Shiina, A.; Kanahara, N.; Sasaki, T.; Oda, Y.; Hashimoto, T.; Hasegawa, T.; Yoshida, T.; Iyo, M.; Hashimoto, K. An Open Study of Sulforaphane-rich Broccoli Sprout Extract in Patients with Schizophrenia. Clin. Psychopharmacol. Neurosci. 2015, 13, 62. [Google Scholar] [CrossRef]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, J.M.J.; Wollheim, C.B.; Wierup, N.; et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Transl. Med. 2017, 9, eaah4477. [Google Scholar] [CrossRef] [PubMed]
- Almuhayawi, M.S.; AbdElgawad, H.; Al Jaouni, S.K.; Selim, S.; Hassan, A.H.A.; Khamis, G. Elevated CO2 improves glucosinolate metabolism and stimulates anticancer and anti-inflammatory properties of broccoli sprouts. Food Chem. 2020, 328, 127102. [Google Scholar] [CrossRef]
- Li, Y.Z.; Yang, Z.Y.; Gong, T.T.; Liu, Y.S.; Liu, F.H.; Wen, Z.Y.; Li, X.Y.; Gao, C.; Luan, M.; Zhao, Y.H.; et al. Cruciferous vegetable consumption and multiple health outcomes: An umbrella review of 41 systematic reviews and meta-analyses of 303 observational studies. Food Funct. 2022, 13, 4247–4259. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Burman, S.; Nair, A.B.; Chauhan, S.; Sircar, D.; Roy, P.; Dhanwat, M.; Lahiri, D.; Mehta, D.; Das, R.; et al. Brassica oleracea Extracts Prevent Hyperglycemia in Type 2 Diabetes Mellitus. Prev. Nutr. Food Sci. 2022, 27, 50–62. [Google Scholar] [CrossRef]
- Li, Z.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of Phenolic Compounds and Antioxidant Activity of 12 Cruciferous Vegetables. Molecules 2018, 23, 1139. [Google Scholar] [CrossRef]
- Baptista, F.I.; Henriques, A.G.; Silva, A.M.S.; Wiltfang, J.; Da Cruz E Silva, O.A.B. Flavonoids as therapeutic compounds targeting key proteins involved in Alzheimer’s disease. ACS Chem. Neurosci. 2014, 5, 83–92. [Google Scholar] [CrossRef]
- Eun, J.C.; Lee, Y.A.; Hye, H.Y.; Yokozawa, T. Protective effects of broccoli (Brassica oleracea) against oxidative damage in vitro and in vivo. J. Nutr. Sci. Vitaminol. 2006, 52, 437–444. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Peñas, E.; Ciska, E.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C.; Frias, J. Time dependence of bioactive compounds and antioxidant capacity during germination of different cultivars of broccoli and radish seeds. Food Chem. 2010, 120, 710–716. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 2010, 119, 1485–1490. [Google Scholar] [CrossRef]
- Soengas, P.; Cartea, M.E.; Francisco, M.; Sotelo, T.; Velasco, P. New insights into antioxidant activity of Brassica crops. Food Chem. 2012, 134, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.; Moreno, D.A.; Cartea, M.E.; Ferreres, F.; García-Viguera, C.; Velasco, P. Simultaneous identification of glucosinolates and phenolic compounds in a representative collection of vegetable Brassica rapa. J. Chromatogr. A 2009, 1216, 6611–6619. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, G.R.; Bagatta, M.; Pagnotta, E.; Angelino, D.; Gennari, L.; Ninfali, P.; Rollin, P.; Iori, R. Comparison of bioactive phytochemical content and release of isothiocyanates in selected brassica sprouts. Food Chem. 2013, 141, 297–303. [Google Scholar] [CrossRef] [PubMed]
- San Vicente Mártir Valencia, V.; Baenas, N.; Moreno, D.A.; García-Viguera, C. Estudio de la bioactividad in vitro e in vivo de brotes de brócoli ricos en glucosinolatos/isotiocianatos. Nereis 2018, 10, 69–78. [Google Scholar]
- Marino, M.; Martini, D.; Venturi, S.; Tucci, M.; Porrini, M.; Riso, P.; Del Bo’, C. An Overview of Registered Clinical Trials on Glucosinolates and Human Health: The Current Situation. Front. Nutr. 2021, 8, 798. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Xiao, D.; Lew, K.L.; Dhir, R.; Singh, S.V. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis 2004, 25, 83–90. [Google Scholar] [CrossRef]
- Zhang, Z.; Garzotto, M.; Davis, E.W.; Mori, M.; Stoller, W.A.; Farris, P.E.; Wong, C.P.; Beaver, L.M.; Thomas, G.V.; Williams, D.E.; et al. Sulforaphane Bioavailability and Chemopreventive Activity in Men Presenting for Biopsy of the Prostate Gland: A Randomized Controlled Trial. Nutr. Cancer 2020, 72, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Janobi, A.A.A.; Mithen, R.F.; Gasper, A.V.; Shaw, P.N.; Middleton, R.J.; Ortori, C.A.; Barrett, D.A. Quantitative measurement of sulforaphane, iberin and their mercapturic acid pathway metabolites in human plasma and urine using liquid chromatography-tandem electrospray ionisation mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 844, 223–234. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Gaeini, Z. Common Limitations and Challenges of Dietary Clinical Trials for Translation into Clinical Practices. Int. J. Endocrinol. Metab. 2021, 19, e108170. [Google Scholar] [CrossRef]
- Castro, N.P.; Rangel, M.C.; Merchant, A.S.; MacKinnon, G.; Cuttitta, F.; Salomon, D.S.; Kim, Y.S. Sulforaphane suppresses the growth of triplenegative breast cancer stem-like cells in vitro and in vivo. Cancer Prev. Res. 2019, 12, 147–158. [Google Scholar] [CrossRef]
- Bozic, D.; Baralić, K.; Živančević, K.; Miljaković, E.A.; Ćurčić, M.; Antonijević, B.; Djordjević, A.B.; Bulat, Z.; Zhang, Y.; Yang, L.; et al. Predicting sulforaphane-induced adverse effects in colon cancer patients via in silico investigation. Biomed. Pharmacother. 2022, 146, 112598. [Google Scholar] [CrossRef]
- Chen, J.G.; Johnson, J.; Egner, P.; Ng, D.; Zhu, J.; Wang, J.B.; Xue, X.F.; Sun, Y.; Zhang, Y.H.; Lu, L.L.; et al. Dose-dependent detoxication of the airborne pollutant benzene in a randomized trial of broccoli sprout beverage in Qidong, China. Am. J. Clin. Nutr. 2019, 110, 675–684. [Google Scholar] [CrossRef]
- Zawari, M.; Poller, B.; Walker, G.; Pearson, A.; Hampton, M.; Carr, A.C. Formulation of Broccoli Sprout Powder in Gastro-Resistant Capsules Protects against the Acidic pH of the Stomach In Vitro but Does Not Increase Isothiocyanate Bioavailability In Vivo. Antioxidants 2019, 8, 359. [Google Scholar] [CrossRef]
- López-Chillón, M.T.; Carazo-Díaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villaño, D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin. Nutr. 2019, 38, 745–752. [Google Scholar] [CrossRef]
- Villaño, D.; López-Chillón, M.T.; Zafrilla, P.; Moreno, D.A. Bioavailability of broccoli sprouts in different human overweight populations. J. Funct. Foods 2019, 59, 337–344. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Baldermann, S.; Brobrowski, A.; Maikath, A.; Wiesner-Reinhold, M.; Rohn, S.; Schreiner, M. Identification of N-Acetyl-S-(3-Cyano-2-(Methylsulfanyl)Propyl-Cysteine as a Major Human Urine Metabolite from the Epithionitrile 1-Cyano-2,3-Epithiopropane, the Main Glucosinolate Hydrolysis Product from Cabbage. Nutrients 2019, 11, 908. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Salvatore, S.R.; Schopfer, F.J.; Cheng, X.; Zhou, J.; Kensler, T.W.; Wendell, S.G. Evaluation of 2-Thiothiazolidine-4-Carboxylic Acid, a Common Metabolite of Isothiocyanates, as a Potential Biomarker of Cruciferous Vegetable Intake. Mol. Nutr. Food Res. 2019, 63, 1801029. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.N.O.; Oliveira, A.P.; Wiecikowski, A.F.; Carvalho, R.S.; Castro, J.d.L.; de Oliveira, F.A.G.; Pereira, H.M.G.; da Veiga, V.F.; Capella, M.M.A.; Rocha, L.; et al. Phenolic compounds from Viscum album tinctures enhanced antitumor activity in melanoma murine cancer cells. Saudi Pharm. J. 2018, 26, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Tahata, S.; Singh, S.V.; Lin, Y.; Hahm, E.R.; Beumer, J.H.; Christner, S.M.; Rao, U.N.; Sander, C.; Tarhini, A.A.; Tawbi, H.; et al. Evaluation of Biodistribution of Sulforaphane after Administration of Oral Broccoli Sprout Extract in Melanoma Patients with Multiple Atypical Nevi. Cancer Prev. Res. 2018, 11, 429–437. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed]
- Platz, S.; Kühn, C.; Schiess, S.; Schreiner, M.; Mewis, I.; Kemper, M.; Pfeiffer, A.; Rohn, S. Determination of benzyl isothiocyanate metabolites in human plasma and urine by LC-ESI-MS/MS after ingestion of nasturtium (Tropaeolum majus L.). Anal. Bioanal. Chem. 2013, 405, 7427–7436. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, nitriles, and epithionitriles from glucosinolates are affected by genotype and developmental stage in Brassica oleracea varieties. Front. Plant Sci. 2017, 8, 1095. [Google Scholar] [CrossRef] [PubMed]
- Brocker, E.R.; Benn, M.H.; Lüthy, J.; von Däniken, A. Metabolism and distribution of 3,4-epithiobutanenitrile in the rat. Food Chem. Toxicol. 1984, 22, 227–232. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, P.; Piślewska-Bednarek, M.; Svatoš, A.; Schneider, B.; Doubský, J.; Mansurova, M.; Humphry, M.; Consonni, C.; Panstruga, R.; Sanchez-Vallet, A.; et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 2009, 323, 101–106. [Google Scholar] [CrossRef]
- Pilipczuk, T.; Kusznierewicz, B.; Chmiel, T.; Przychodzeń, W.; Bartoszek, A. Simultaneous determination of individual isothiocyanates in plant samples by HPLC-DAD-MS following SPE and derivatization with N-acetyl-l-cysteine. Food Chem. 2017, 214, 587–596. [Google Scholar] [CrossRef]
- Corstens, M.N.; Berton-Carabin, C.C.; Elichiry-Ortiz, P.T.; Hol, K.; Troost, F.J.; Masclee, A.A.M.; Schroën, K. Emulsion-alginate beads designed to control in vitro intestinal lipolysis: Towards appetite control. J. Funct. Foods 2017, 34, 319–328. [Google Scholar] [CrossRef]
- Nestorov, I.A.; Aarons, L.J.; Arundel, P.A.; Rowland, M. Lumping of whole-body physiologically based pharmacokinetic models. J. Pharmacokinet. Biopharm. 1998, 26, 21–46. [Google Scholar] [CrossRef]
- Casarett and Doull’s Toxicology: The Basic Science of Poisons. Chapter 7: Toxicokinetics, 8th ed.; McGraw Hill Education: New York, NY, USA, 2019; Available online: https://accesspharmacy.mhmedical.com/book.aspx?bookid=2462 (accessed on 14 March 2023).
- Gerami, P.; Yao, Z.; Polsky, D.; Jansen, B.; Busam, K.; Ho, J.; Martini, M.; Ferris, L.K. Development and validation of a noninvasive 2-gene molecular assay for cutaneous melanoma. J. Am. Acad. Dermatol. 2017, 76, 114–120.e2. [Google Scholar] [CrossRef]
- Charron, C.S.; Vinyard, B.T.; Ross, S.A.; Seifried, H.E.; Jeffery, E.H.; Novotny, J.A. Absorption and metabolism of isothiocyanates formed from broccoli glucosinolates: Effects of BMI and daily consumption in a randomised clinical trial. Br. J. Nutr. 2018, 120, 1370–1379. [Google Scholar] [CrossRef]
- Petri, N.; Tannergren, C.; Holst, B.; Mellon, F.A.; Bao, Y.; Plumb, G.W.; Bacon, J.; O’Leary, K.A.; Kroon, P.A.; Knutson, L.; et al. Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metab. Dispos. 2003, 31, 805–813. [Google Scholar] [CrossRef]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortés, J.A.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.S.; Vinyard, B.T.; Jeffery, E.H.; Ross, S.A.; Seifried, H.E.; Novotny, J.A. BMI Is Associated With Increased Plasma and Urine Appearance of Glucosinolate Metabolites After Consumption of Cooked Broccoli. Front. Nutr. 2020, 7, 174. [Google Scholar] [CrossRef]
- Conaway, C.C.; Getahun, S.M.; Liebes, L.L.; Pusateri, D.J.; Topham, D.K.W.; Botero-Omary, M.; Chung, F.L. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr. Cancer 2000, 38, 168–178. [Google Scholar] [CrossRef]
- Li, F.; Hullar, M.A.J.; Beresford, S.A.A.; Lampe, J.W. Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Br. J. Nutr. 2011, 106, 408–416. [Google Scholar] [CrossRef]
- Sun, J.; Charron, C.S.; Liu, Z.; Novotny, J.A.; Harrington, P.D.B.; Ross, S.A.; Seifried, H.E.; Chen, P. Study on Human Urinary Metabolic Profiles after Consumption of Kale and Daikon Radish using a High-resolution Mass Spectrometry-Based Non-targeted and Targeted Metabolomic Approach. J. Agric. Food Chem. 2020, 68, 14307–14318. [Google Scholar] [CrossRef]
- Okunade, O.; Niranjan, K.; Ghawi, S.K.; Kuhnle, G.; Methven, L. Supplementation of the Diet by Exogenous Myrosinase via Mustard Seeds to Increase the Bioavailability of Sulforaphane in Healthy Human Subjects after the Consumption of Cooked Broccoli. Mol. Nutr. Food Res. 2018, 62, e1700980. [Google Scholar] [CrossRef] [PubMed]
- Schlotz, N.; Odongo, G.A.; Herz, C.; Waßmer, H.; Kühn, C.; Hanschen, F.S.; Neugart, S.; Binder, N.; Ngwene, B.; Schreiner, M.; et al. Are Raw Brassica Vegetables Healthier Than Cooked Ones? A Randomized, Controlled Crossover Intervention Trial on the Health-Promoting Potential of Ethiopian Kale. Nutrients 2018, 10, 1622. [Google Scholar] [CrossRef]
- Nakamura, Y.; Iwahashi, T.; Tanaka, A.; Koutani, J.; Matsuo, T.; Okamoto, S.; Sato, K.; Ohtsuki, K. 4-(Methylthio)-3-butenyl isothiocyanate, a principal antimutagen in daikon (Raphanus sativus; Japanese white radish). J. Agric. Food Chem. 2001, 49, 5755–5760. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Charron, C.S.; Novotny, J.A.; Peng, B.; Yu, L.; Chen, P. Profiling glucosinolate metabolites in human urine and plasma after broccoli consumption using non-targeted and targeted metabolomic analyses. Food Chem. 2020, 309, 125660. [Google Scholar] [CrossRef] [PubMed]
- Van Haard, P.M.M.; Pavel, S. Chromatography of urinary indole derivatives. J. Chromatogr. B Biomed. Sci. Appl. 1988, 429, 59–94. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Juvik, J.A.; Jeffery, E.H. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry 2004, 65, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Matusheski, N.V.; Swarup, R.; Juvik, J.A.; Mithen, R.; Bennett, M.; Jeffery, E.H. Epithiospecifier protein from broccoli (Brassica oleracea L. ssp. italica) inhibits formation of the anticancer agent sulforaphane. J. Agric. Food Chem. 2006, 54, 2069–2076. [Google Scholar] [CrossRef]
- Okunade, O.A.; Ghawi, S.K.; Methven, L.; Niranjan, K. Thermal and pressure stability of myrosinase enzymes from black mustard (Brassica nigra L. W.D.J. Koch. var. nigra), brown mustard (Brassica juncea L. Czern. var. juncea) and yellow mustard (Sinapsis alba L. subsp. maire) seeds. Food Chem. 2015, 187, 485–490. [Google Scholar] [CrossRef]
- Eylen, D.; Oey, I.; Hendrickx, M.; Loey, A. Behavior of mustard seed (Sinapis alba L.) myrosinase during temperature/pressure treatments: A case study on enzyme activity and stability. Eur. Food Res. Technol. 2008, 226, 545–553. [Google Scholar] [CrossRef]
- Wang, G.C.; Farnham, M.; Jeffery, E.H. Impact of Thermal Processing on Sulforaphane Yield from Broccoli (Brassica oleracea L. ssp. italica). J. Agric. Food Chem. 2012, 60, 6743–6748. [Google Scholar] [CrossRef]
- Xin, H.; Khan, N.A.; Falk, K.C.; Yu, P. Mid-infrared spectral characteristics of lipid molecular structures in Brassica carinata seeds: Relationship to oil content, fatty acid and glucosinolate profiles, polyphenols, and condensed tannins. J. Agric. Food Chem. 2014, 62, 7977–7988. [Google Scholar] [CrossRef]
- Zhang, Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol. Nutr. Food Res. 2010, 54, 127. [Google Scholar] [CrossRef]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef] [PubMed]
- Sturm, C.; Wagner, A.E. Molecular Sciences Brassica-Derived Plant Bioactives as Modulators of Chemopreventive and Inflammatory Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 1890. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, T.; Pugalendhi, P.; Jayaganesh, R.; Ananthakrishnan, D.; Gunasekaran, K. Effect of allyl isothiocyanate on NF-κB signaling in 7,12-dimethylbenz(a)anthracene and N-methyl-N-nitrosourea-induced mammary carcinogenesis. Breast Cancer 2018, 25, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Legler, D.F.; Bruckner, M.; Uetz-von Allmen, E.; Krause, P. Prostaglandin E2 at new glance: Novel insights in functional diversity offer therapeutic chances. Int. J. Biochem. Cell Biol. 2010, 42, 198–201. [Google Scholar] [CrossRef]
- Langston-Cox, A.; Anderson, D.; Creek, D.J.; Palmer, K.; Wallace, E.M.; Marshall, S.A. Measuring Sulforaphane and Its Metabolites in Human Plasma: A High Throughput Method. Molecules 2020, 25, 829. [Google Scholar] [CrossRef]
- Abukhabta, S.; Khalil Ghawi, S.; Karatzas, K.A.; Charalampopoulos, D.; McDougall, G.; Allwood, J.W.; Verrall, S.; Lavery, S.; Latimer, C.; Pourshahidi, L.K.; et al. Sulforaphane-enriched extracts from glucoraphanin-rich broccoli exert antimicrobial activity against gut pathogens in vitro and innovative cooking methods increase in vivo intestinal delivery of sulforaphane. Eur. J. Nutr. 2021, 60, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Panjwani, A.A.; Liu, H.; Cornblatt, G.; Cornblatt, B.S.; Ownby, S.L.; Fuchs, E.; Holtzclaw, W.D.; et al. Bioavailability of Sulforaphane Following Ingestion of Glucoraphanin-Rich Broccoli Sprout and Seed Extracts with Active Myrosinase: A Pilot Study of the Effects of Proton Pump Inhibitor Administration. Nutrients 2019, 11, 1489. [Google Scholar] [CrossRef]
- Zimmerman, A.W.; Singh, K.; Connors, S.L.; Liu, H.; Panjwani, A.A.; Lee, L.C.; Diggins, E.; Foley, A.; Melnyk, S.; Singh, I.N.; et al. Randomized controlled trial of sulforaphane and metabolite discovery in children with Autism Spectrum Disorder. Mol. Autism 2021, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Koper, J.E.B.; Kortekaas, M.; Loonen, L.M.P.; Huang, Z.; Wells, J.M.; Gill, C.I.R.; Pourshahidi, L.K.; McDougall, G.; Rowland, I.; Pereira-Caro, G.; et al. Aryl hydrocarbon Receptor activation during in vitro and in vivo digestion of raw and cooked broccoli (brassica oleracea var. Italica). Food Funct. 2020, 11, 4026–4037. [Google Scholar] [CrossRef]
- Kühn, C.; Kupke, F.; Baldermann, S.; Klopsch, R.; Lamy, E.; Hornemann, S.; Pfeiffer, A.F.H.; Schreiner, M.; Hanschen, F.S.; Rohn, S. Diverse Excretion Pathways of Benzyl Glucosinolate in Humans after Consumption of Nasturtium (Tropaeolum majus L.)—A Pilot Study. Mol. Nutr. Food Res. 2018, 62, 1800588. [Google Scholar] [CrossRef]
- Perez-Moral, N.; Saha, S.; Philo, M.; Hart, D.J.; Winterbone, M.S.; Hollands, W.J.; Spurr, M.; Bows, J.; van der Velpen, V.; Kroon, P.A.; et al. Comparative bio-accessibility, bioavailability and bioequivalence of quercetin, apigenin, glucoraphanin and carotenoids from freeze-dried vegetables incorporated into a baked snack versus minimally processed vegetables: Evidence from in vitro models and a human bioavailability study. J. Funct. Foods 2018, 48, 410–419. [Google Scholar] [CrossRef]
- Fahey, J.W.; Wade, K.L.; Wehage, S.L.; Holtzclaw, W.D.; Liu, H.; Talalay, P.; Fuchs, E.; Stephenson, K.K. Stabilized sulforaphane for clinical use: Phytochemical delivery efficiency. Mol. Nutr. Food Res. 2017, 61, 1600766. [Google Scholar] [CrossRef] [PubMed]
- Ghawi, S.K.; Methven, L.; Niranjan, K. The potential to intensify sulforaphane formation in cooked broccoli (Brassica oleracea var. italica) using mustard seeds (Sinapis alba). Food Chem. 2013, 138, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.M.; Teran-Garcia, M.; Jeffery, E.H. Enhancing sulforaphane absorption and excretion in healthy men through the combined consumption of fresh broccoli sprouts and a glucoraphanin-rich powder. Br. J. Nutr. 2012, 107, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Szabó, D.; Tod, P.; Gölöncsér, F.; Román, V.; Lendvai, B.; Otrokocsi, L.; Sperlágh, B. Maternal P2X7 receptor inhibition prevents autism-like phenotype in male mouse offspring through the NLRP3-IL-1β pathway. Brain Behav. Immun. 2022, 101, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Dayalan Naidu, S.; Suzuki, T.; Yamamoto, M.; Fahey, J.W.; Dinkova-Kostova, A.T. Phenethyl Isothiocyanate, a Dual Activator of Transcription Factors NRF2 and HSF1. Mol. Nutr. Food Res. 2018, 62, e1700908. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or sulforaphane: Is it the source or dose that matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.N.; Van de Water, J. Altered T cell responses in children with autism. Brain Behav. Immun. 2011, 25, 840–849. [Google Scholar] [CrossRef]
- Cotton, S.C.; Sharp, L.; Little, J.; Brockton, N. Glutathione S-Transferase Polymorphisms and Colorectal Cancer: A HuGE Review. Am. J. Epidemiol. 2000, 151, 7–32. [Google Scholar] [CrossRef]
- Liu, X.; Lv, K. Cruciferous vegetables intake is inversely associated with risk of breast cancer: A meta-analysis. Breast 2013, 22, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Mao, Q.; Cao, M.; Xie, L. Cruciferous vegetables intake and risk of prostate cancer: A meta-analysis. Int. J. Urol. 2012, 19, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shu, X.O.; Xiang, Y.B.; Yang, G.; Li, H.; Gao, J.; Cai, H.; Gao, Y.T.; Zheng, W. Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. Am. J. Clin. Nutr. 2011, 94, 240–246. [Google Scholar] [CrossRef] [PubMed]
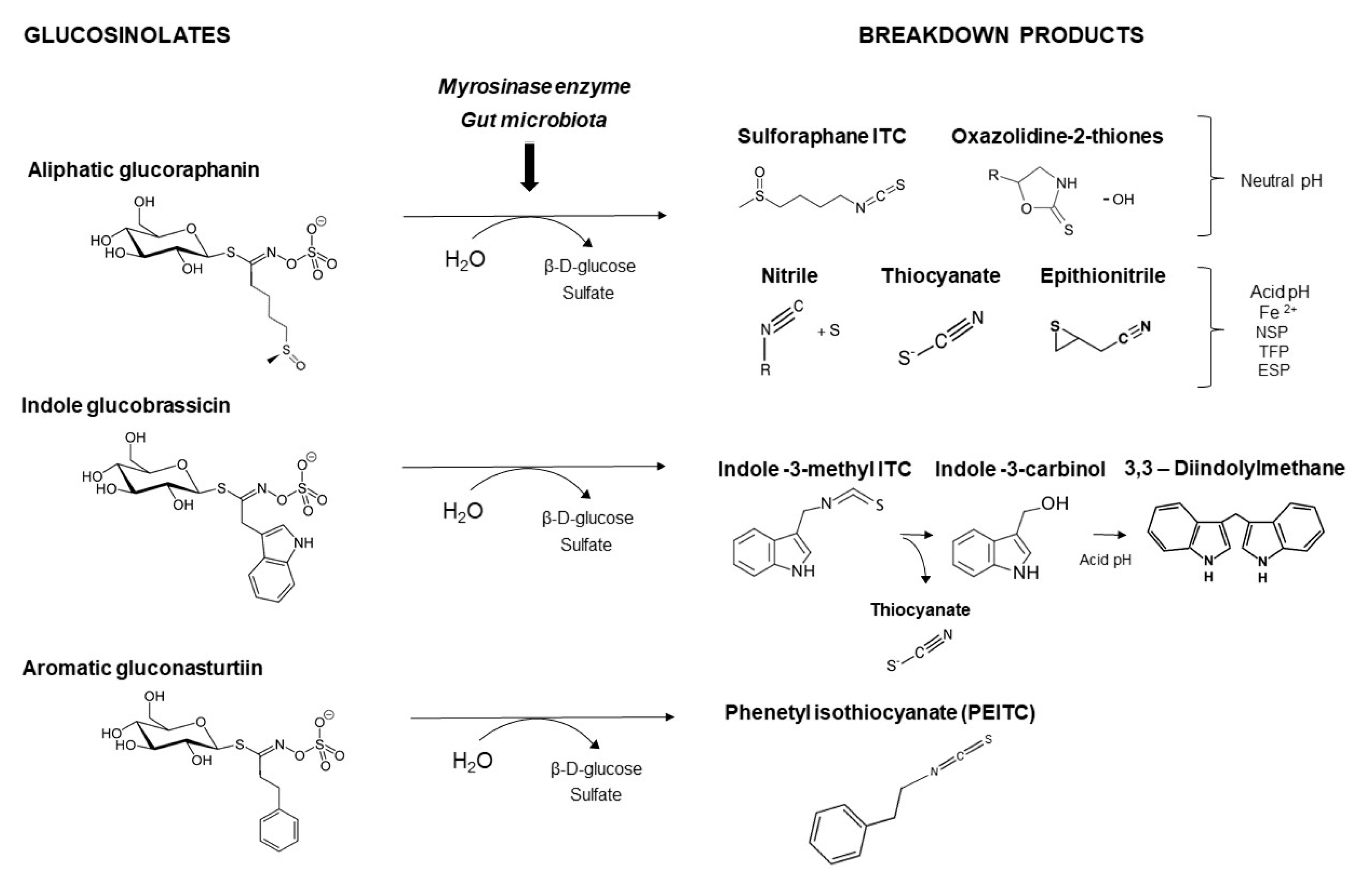
| Basic Chemical Structure | |||||
|---|---|---|---|---|---|
 | |||||
| Glucosinolate | Chemical Name | Side Chain (R) | Aminoacid Precursor | Reference | |
| Molecular Formula | 2D Structure | ||||
| Aliphatic glucosinolates | |||||
| Alkenyl | |||||
| Gluconapin | 3-butenyl-GSL | C4H7 |  | methionine (Met) | [9,10,11] |
| Glucobrassicanapin | 4-pentenyl-GSL | C5H9 | 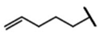 | Met | [9,10,11] |
| Sinigrin | 2-propenyl-GSL | C3H4 |  | Met | [9,10,11] |
| Hydroxyalkenyl | |||||
| Progoitrin | 2-hydroxy-3-butenyl-GSL | C4H6 |  | Met | [9,10,11] |
| Epiprogoitrin | 2(S)-2-hydroxy-3-butenyl-GSL | C4H6 | 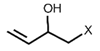 | Met | [10,11,12] |
| Gluconapoleiferin | 2-hydroxy-4-pentenyl-GSL | C5H8 | 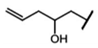 | Met | [9,10,11] |
| Sulfur containing | |||||
| Glucoiberverin | 3-methyltiopropyl-GSL | C3H14S |  | Met | [9,10,11] |
| Glucoerucin | 4-methylthiobutyl-GSL | C4H16S |  | Met | [9,10,11] |
| Dehydroerucin | 4-methylthio-3-butenyl-GSL | C4H14S | 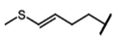 | Met | [9,10,11] |
| Glucoiberin | 3-methylsulfinylpropyl-GSL | C3H12SO | 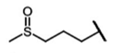 | Met | [9,10,11] |
| Glucoraphanin | 4-methylsufinylbutyl-GSL | C4H14SO | 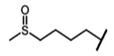 | Met | [9,10,11] |
| Glucoalyssin | 5-methylsulfinylpentyl-GSL | C5H16SO | 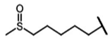 | Met | [9,10,11] |
| Glucoraphenin | 4-methylsulfinyl-3-butenyl-GSL | C4H12SO | 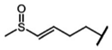 | Met | [9,10,11] |
| Glucoerysolin | 4-(methylsulfonyl)butyl-GSL | C6H12SO2 |  | Met | [9,10,11] |
| Indolic glucosinolates | |||||
| Glucobrassicin | 3-indolylmethyl-GSL | C9H9N |  | Tryptophan (Trp) | [9,10,11] |
| 4-Hydroxy-glucobrassicin | 4-hydroxy-3-indolylmethyl-GSL | C9H9NO | 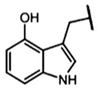 | Trp | [9,11] |
| 4-Methoxy-glucobrassicin | 4-methoxy-3-indolylmethyl-GSL | C10H11NO | 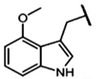 | Trp | [9,10,11] |
| 1-Methoxy-glucobrassicin | 1-methoxy-indolylmethyl-GSL | C10H11NO | 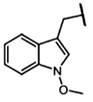 | Trp | [9] |
| Neoglucobrassicin | N-methoxy-3-indlymethyl-GSL | C10H11NO | 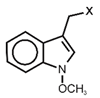 | Trp | [10,11,13] |
| Phenyl (aromatic) glucosinolates | |||||
| Glucotropaeolin | Benzyl-GSL | C7H8 | 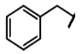 | Phenylalanine (Phe) | [9] |
| Gluconasturtiin | 2-phenetyl-GSL | C8H10 |  | Phe | [9,10,11] |
| Sinalbin | 4-hydroxybenzyl-GSL | C7H8O |  | Phe | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa-Pérez, A.; Núñez-Gómez, V.; Baenas, N.; Di Pede, G.; Achour, M.; Manach, C.; Mena, P.; Del Rio, D.; García-Viguera, C.; Moreno, D.A.; et al. Systematic Review on the Metabolic Interest of Glucosinolates and Their Bioactive Derivatives for Human Health. Nutrients 2023, 15, 1424. https://doi.org/10.3390/nu15061424
Costa-Pérez A, Núñez-Gómez V, Baenas N, Di Pede G, Achour M, Manach C, Mena P, Del Rio D, García-Viguera C, Moreno DA, et al. Systematic Review on the Metabolic Interest of Glucosinolates and Their Bioactive Derivatives for Human Health. Nutrients. 2023; 15(6):1424. https://doi.org/10.3390/nu15061424
Chicago/Turabian StyleCosta-Pérez, Antonio, Vanesa Núñez-Gómez, Nieves Baenas, Giuseppe Di Pede, Mariem Achour, Claudine Manach, Pedro Mena, Daniele Del Rio, Cristina García-Viguera, Diego A. Moreno, and et al. 2023. "Systematic Review on the Metabolic Interest of Glucosinolates and Their Bioactive Derivatives for Human Health" Nutrients 15, no. 6: 1424. https://doi.org/10.3390/nu15061424
APA StyleCosta-Pérez, A., Núñez-Gómez, V., Baenas, N., Di Pede, G., Achour, M., Manach, C., Mena, P., Del Rio, D., García-Viguera, C., Moreno, D. A., & Domínguez-Perles, R. (2023). Systematic Review on the Metabolic Interest of Glucosinolates and Their Bioactive Derivatives for Human Health. Nutrients, 15(6), 1424. https://doi.org/10.3390/nu15061424










