Antioxidant Activity of Leaf Extracts from Stevia rebaudiana Bertoni Exerts Attenuating Effect on Diseased Experimental Rats: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy and Eligibility Criteria
2.2. Data Extraction and Antioxidant Markers
2.3. Statistical Analysis
3. Results
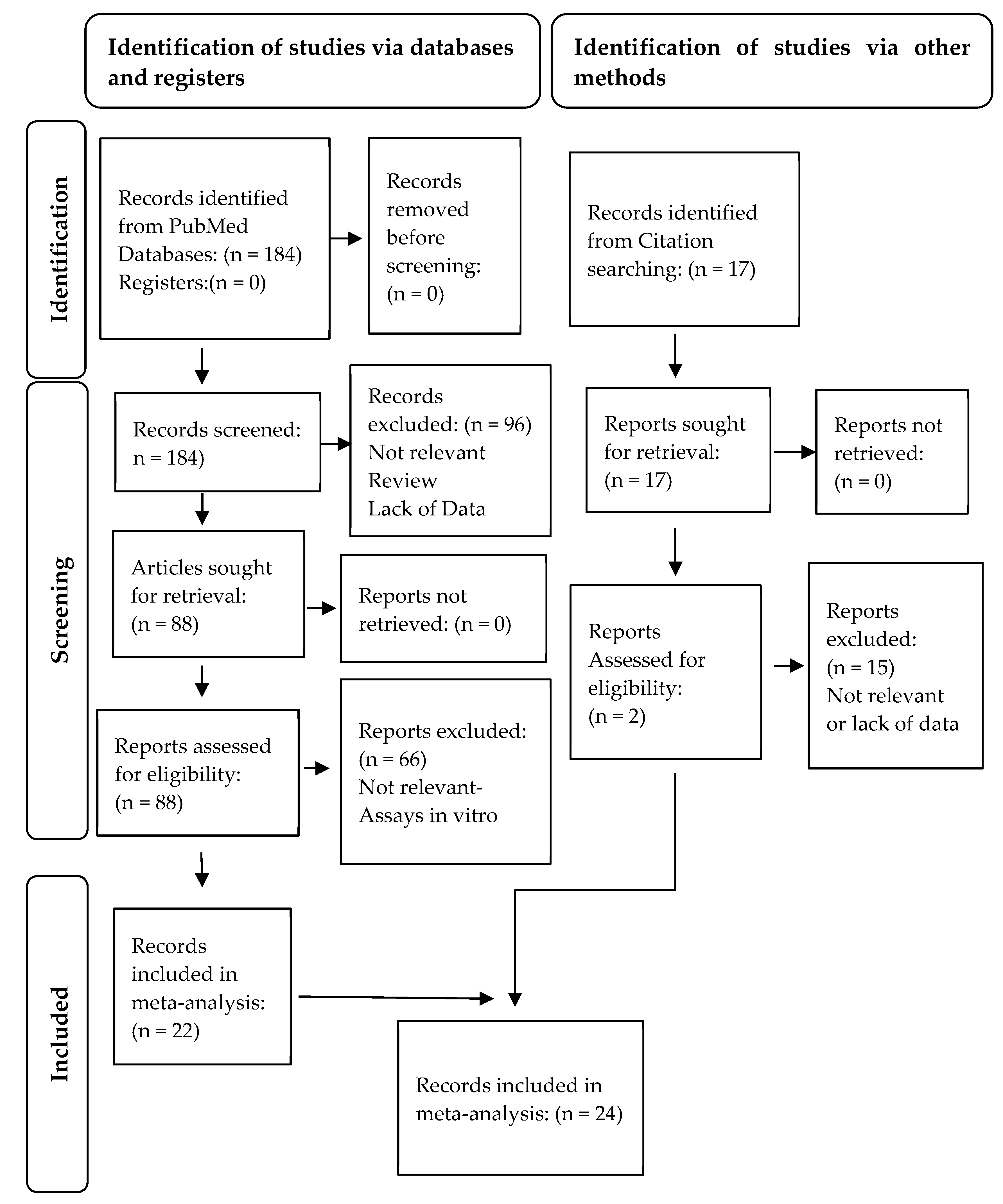
3.1. Study Selection and Characteristics
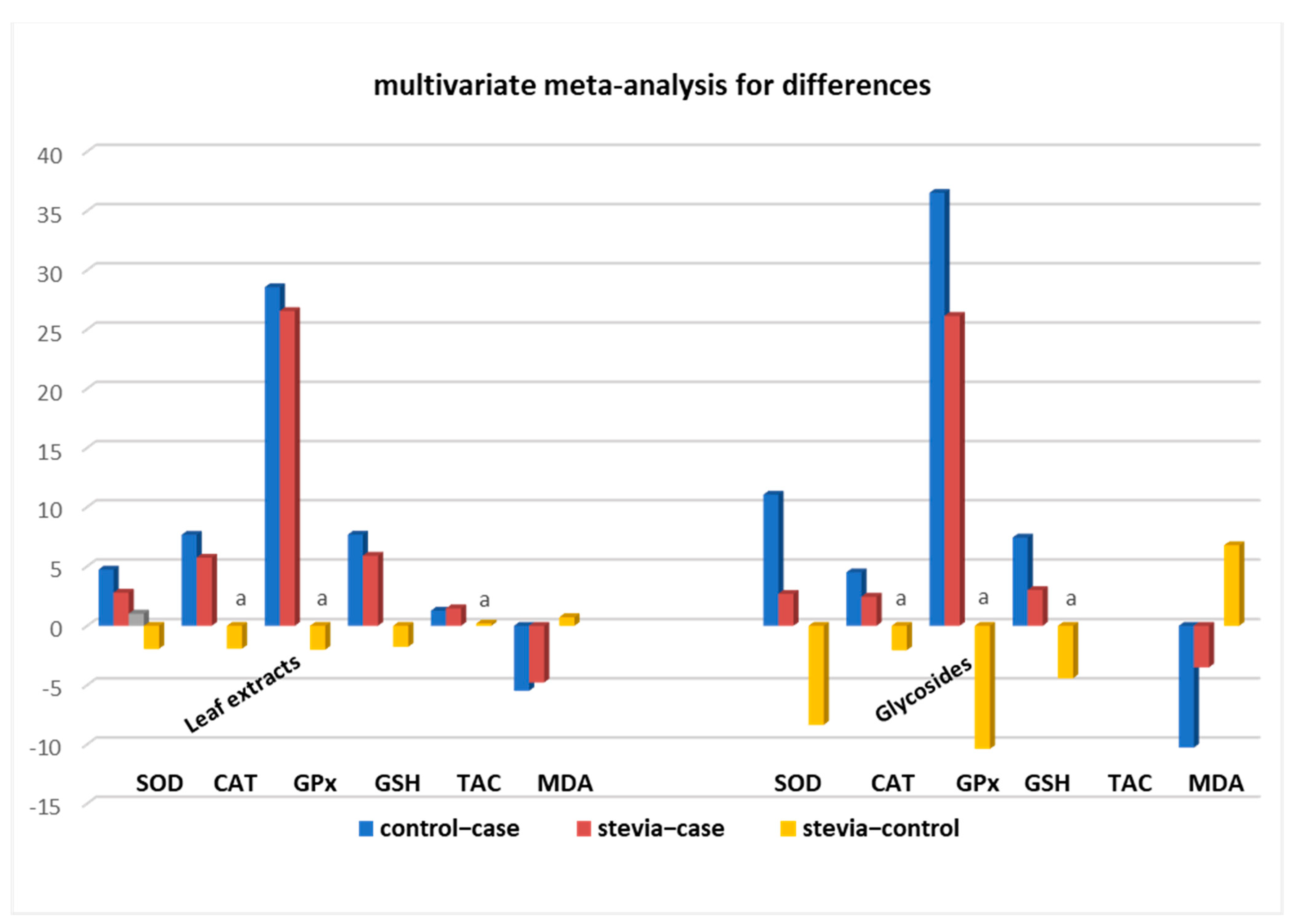
3.2. Bioactive Compounds from Stevia Leaf Extracts Exert Significantly Higher Antioxidant Activity Compared to Stevia Glycosides
3.3. Datasets from SOD, CAT, GSH, and TAC Assays Can Be Combined for Meta-Analysis: Meta-Regression Analysis
3.4. Stratification Meta-Analysis for Datasets from Leaf Extracts
3.5. Meta-Analysis of Lipid Peroxidation (MDA) Assay Datasets
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rojas, E.; Bermúdez, V.; Motlaghzadeh, Y.; Mathew, J.; Fidilio, E.; Faria, J.; Rojas, J.; de Bravo, M.C.; Contreras, J.; Mantilla, L.P.; et al. Stevia rebaudiana Bertoni and its effects in human disease: Emphasizing its role in inflammation, atherosclerosis and metabolic syndrome. Curr. Nutr. Rep. 2018, 7, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Peteliuk, V.; Rybchuk, L.; Bayliak, M.; Storey, K.B.; Lushchak, O. Natural sweetener Stevia rebaudiana: Functionalities, health benefits and potential risks. EXCLI J. 2021, 20, 1412. [Google Scholar]
- Orellana-Paucar, A.M. Steviol Glycosides from Stevia rebaudiana: An Updated Overview of Their Sweetening Activity, Pharmacological Properties, and Safety Aspects. Molecules 2023, 28, 1258. [Google Scholar] [CrossRef]
- Geuns, J.M.C. Stevioside. Phytochemistry 2003, 64, 913–921. [Google Scholar] [CrossRef]
- De Oliveira, A.J.B.; Gonçalves, R.A.C.; Chierrito, T.P.C.; Dos Santos, M.M.; De Souza, L.M.; Gorin, P.A.J.; Sassaki, G.L.; Iacomini, M. Structure and degree of polymerisation of fructooligosaccharides present in roots and leaves of Stevia rebaudiana (Bert.) Bertoni. Food Chem. 2011, 129, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedula, V.S.P.; Zamora, J. Reversed-Phase HPLC Analysis of Steviol Glycosides Isolated from Stevia rebaudiana Bertoni. Food Nutr. Sci. 2014, 5, 1711–1716. [Google Scholar] [CrossRef]
- Tavarini, S.; Angelini, L.G. Stevia rebaudiana Bertoni as a source of bioactive compounds: The effect of harvest time, experimental site and crop age on steviol glycoside content and antioxidant properties. J. Sci. Food Agric. 2013, 93, 2121–2129. [Google Scholar] [CrossRef]
- Wölwer-Rieck, U. The leaves of Stevia rebaudiana (Bertoni), their constituents and the analyses thereof: A review. J. Agric. Food Chem. 2012, 60, 886–895. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.C.; Moguel-Ordoñez, Y.B.; Segura-Campos, M.R. Biological activity of Stevia rebaudiana Bertoni and their relationship to health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2680–2690. [Google Scholar] [CrossRef]
- Borgo, J.; Laurella, L.C.; Martini, F.; Catalán, C.A.N.; Sülsen, V.P. Stevia genus: Phytochemistry and biological activities update. Molecules 2021, 26, 2733. [Google Scholar] [CrossRef]
- Pacifico, S.; Piccolella, S.; Nocera, P.; Tranquillo, E.; Dal Poggetto, F.; Catauro, M. New insights into phenol and polyphenol composition of Stevia rebaudiana leaves. J. Pharm. Biomed. Anal. 2019, 163, 45–57. [Google Scholar] [CrossRef]
- Karaköse, H.; Jaiswal, R.; Kuhnert, N. Characterization and quantification of hydroxycinnamate derivatives in Stevia rebaudiana leaves by LC-MS n. J. Agric. Food Chem. 2011, 59, 10143–10150. [Google Scholar] [CrossRef]
- Myint, K.Z.; Wu, K.; Xia, Y.; Fan, Y.; Shen, J.; Zhang, P.; Gu, J. Polyphenols from Stevia rebaudiana (Bertoni) leaves and their functional properties. J. Food Sci. 2020, 85, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishnan, B.; Bawane, A.A.; Akki, K.S.; Hukkeri, V.I. Free radical scavenging activity of flavonoid containing leaf extracts of Stevia rebaudiana Bert. Anc. Sci. Life 2006, 25, 44–48. [Google Scholar]
- Karakose, H.; Muller, A.; Kuhnert, N. Profiling and quantification of phenolics in Stevia rebaudiana leaves. J. Agric. Food Chem. 2015, 63, 9188–9198. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Culotta, V.C.; Yang, M.; O’Halloran T, V. Activation of superoxide dismutases: Putting the metal to the pedal. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2006, 1763, 747–758. [Google Scholar] [CrossRef] [Green Version]
- Alfonso-Prieto, M.; Biarnes, X.; Vidossich, P.; Rovira, C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, O.; Olawumi, J.; Opeyemi, A.; Christiania, O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist. Reprod. 2018, 22, 61. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Jové, M.; Mota-Martorell, N.; Pamplona, R.; Pradas, I.; Martín-Gari, M.; Ayala, V. The advanced lipoxidation end-product malondialdehyde-lysine in aging and longevity. Antioxidants 2020, 9, 1132. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- Cichoz-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Forero, D.A.; Lopez-Leon, S.; González-Giraldo, Y.; Bagos, P.G. Ten simple rules for carrying out and writing meta-analyses. PLoS Comput. Biol. 2019, 15, e1006922. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Hopewell, S.; McDonald, S.; Clarke, M.J.; Egger, M. Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst. Rev. 2007, 2010, MR000010. [Google Scholar] [CrossRef]
- Simpson, T.; Pase, M.; Stough, C. Bacopa monnieri as an Antioxidant Therapy to Reduce Oxidative Stress in the Aging Brain. Evid.-Based Complement. Altern. Med. 2015, 2015, 615384. [Google Scholar] [CrossRef] [Green Version]
- Somogyi, A.; Rosta, K.; Pusztai, P.; Tulassay, Z.; Nagy, G. Antioxidant measurements. Physiol. Meas. 2007, 28, R41–R55. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, P.M.; Das, B.; Viswanathan, P.N. A Modified Spectrophotometric Assay of Superoxide Dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar] [PubMed]
- Hadwan, M.H. New method for assessment of serum catalase activity. Indian J. Sci. Technol. 2016, 9, 1–5. [Google Scholar] [CrossRef]
- Singh, S.; Yadav, D.; Garg, V. Antihyperglycemic and antioxidative ability of Stevia rebaudiana (Bertoni) leaves in diabetes induced mice Nano-encapsulation of various herbal remedies View project antihyperglycemic and antioxidative ability of Stevia rebaudiana (bertoni) leaves in diabetes induced mice. Int. J. Pharm. Pharm. Sci. 2013, 5, 297–302. [Google Scholar]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Ranjbar, T.; Nekooeian, A.A.; Tanideh, N.; Koohi-Hosseinabadi, O.; Masoumi, S.J.; Amanat, S.; Azarpira, N.; Monabati, A. A comparison of the effects of Stevia extract and metformin on metabolic syndrome indices in rats fed with a high-fat, high-sucrose diet. J. Food Biochem. 2020, 44, e13242. [Google Scholar] [CrossRef]
- El Nashar, E.M.; Obydah, W.; Alghamdi, M.A.; Saad, S.; Yehia, A.; Maryoud, A.; Kiwan, N.A.; Alasmari, W.A.; Hussein, A.M. Effects of Stevia rebaudiana Bertoni extracts in the rat model of epilepsy induced by pentylenetetrazol: Sirt-1, at the crossroads between inflammation and apoptosis. J. Integr. Neurosci. 2022, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tovar, E.; Flores-Beltrán, R.E.; Galindo-Gómez, S.; Vera-Aguilar, E.; Diaz-Ruiz, A.; Montes, S.; Camacho, J.; Tsutsumi, V.; Muriel, P. Stevia rebaudiana tea prevents experimental cirrhosis via regulation of NF-κB, Nrf2, transforming growth factor beta, Smad7, and hepatic stellate cell activation. Phytother. Res. 2018, 32, 2568–2576. [Google Scholar] [CrossRef]
- Ramos-Tovar, E.; Flores-Beltrán, R.E.; Galindo-Gómez, S.; Camacho, J.; Tsutsumi, V.; Muriel, P. An aqueous extract of Stevia rebaudiana variety Morita II prevents liver damage in a rat model of cirrhosis that mimics the human disease. Ann. Hepatol. 2019, 18, 472–479. [Google Scholar] [CrossRef]
- Assaei, R.; Mokarram, P.; Dastghaib, S.; Darbandi, S.; Darbandi, M.; Zal, F.; Akmali, M.; Omrani, G.H.R. Hypoglycemic effect of aquatic extract of Stevia in pancreas of diabetic rats: PPARγ-dependent regulation or antioxidant potential. Avicenna J. Med. Biotechnol. 2016, 8, 65. [Google Scholar]
- El-Mesallamy, A.; Mahmoud, S.A.; Elazab, K.M.; Hussein, S.A.M.; Hussein, A.M. Attenuation of metabolic dysfunctions in the skeletal muscles of type 1 diabetic rats by Stevia rebaudiana extracts, via AMPK upregulation and antioxidant activities. Acta Sci. Pol. Technol. Aliment. 2018, 17, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Riley, R.; White, I.R. Multivariate meta-analysis: Potential and promise. Stat. Med. 2011, 30, 2481–2498. [Google Scholar] [CrossRef] [Green Version]
- Mavridis, D.; Salanti, G. A practical introduction to multivariate meta-analysis. Stat. Methods Med. Res. 2013, 22, 133–158. [Google Scholar] [CrossRef]
- Van Houwelingen, H.C.; Arends, L.R.; Stijnen, T. Advanced methods in meta-analysis: Multivariate approach and meta-regression. Stat. Med. 2002, 21, 589–624. [Google Scholar] [CrossRef] [PubMed]
- Vennou, K.E.; Piovani, D.; Kontou, P.I.; Bonovas, S.; Bagos, P.G. Multiple outcome meta-analysis of gene-expression data in inflammatory bowel disease. Genomics 2020, 112, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Kapoula, G.V.; Kontou, P.I.; Bagos, P.G. The impact of pneumatic tube system on routine laboratory parameters: A systematic review and meta-analysis. Clin. Chem. Lab. Med. 2017, 55, 1834–1844. [Google Scholar] [CrossRef]
- Nikolopoulos, G.K.; Bagos, P.G.; Tsangaris, I.; Tsiara, C.G.; Kopterides, P.; Vaiopoulos, A.; Kapsimali, V.; Bonovas, S.; Tsantes, A.E. The association between plasminogen activator inhibitor type 1 (PAI-1) levels, PAI-1 4G/5G polymorphism, and myocardial infarction: A mendelian randomization meta-analysis. Clin. Chem. Lab. Med. 2014, 52, 937–950. [Google Scholar] [CrossRef]
- Harbord, R.M.; Higgins, J.P.T. Meta-regression in Stata. Stata J. 2008, 8, 493–519. [Google Scholar] [CrossRef] [Green Version]
- White, I.R.; Barrett, J.K.; Jackson, D.; Higgins, J.P.T. Consistency and inconsistency in network meta-analysis: Model estimation using multivariate meta-regression. Res. Synth Methods 2012, 3, 111–125. [Google Scholar] [CrossRef] [Green Version]
- StataCorp LP. Stata Multilevel Mixed-Effects Reference Manual; StataCorp LP: College Station, TX, USA, 2013; Volume 9. [Google Scholar]
- Abdallah, S.H.; Mostafa, N.M.; Mohamed, M.A.E.H.; Nada, A.S.; Singab, A.N.B. UPLC-ESI-MS/MS profiling and hepatoprotective activities of Stevia leaves extract, butanol fraction and stevioside against radiation-induced toxicity in rats. Nat. Prod. Res. 2022, 36, 5619–5625. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, R.A.; Abdel-Rahman, M.S.; Al Bayoumi, S.; Ali, L.A. Effect of stevia aqueous extract on the antidiabetic activity of saxagliptin in diabetic rats. J. Ethnopharmacol. 2021, 265, 113188. [Google Scholar] [CrossRef] [PubMed]
- Casas-Grajales, S.; Reyes-Gordillo, K.; Cerda-García-Rojas, C.M.; Tsutsumi, V.; Lakshman, M.R.; Muriel, P. Rebaudioside A administration prevents experimental liver fibrosis: An in vivo and in vitro study of the mechanisms of action involved. J. Appl. Toxicol. 2019, 39, 1118–1131. [Google Scholar] [CrossRef]
- Casas-Grajales, S.; Ramos-Tovar, E.; Chávez-Estrada, E.; Alvarez-Suarez, D.; Hernández-Aquino, E.; Reyes-Gordillo, K.; Cerda-García-Rojas, C.M.; Camacho, J.; Tsutsumi, V.; Lakshman, M.R.; et al. Antioxidant and immunomodulatory activity induced by stevioside in liver damage: In vivo, in vitro and in silico assays. Life Sci. 2019, 224, 187–196. [Google Scholar] [CrossRef]
- Deenadayalan, A.; Subramanian, V.; Paramasivan, V.; Veeraraghavan, V.P.; Rengasamy, G.; Sadagopan, J.C.; Rajagopal, P.; Jayaraman, S. Stevioside attenuates insulin resistance in skeletal muscle by facilitating IR/IRS-1/Akt/GLUT 4 signaling pathways: An in vivo and in silico approach. Molecules 2021, 26, 7689. [Google Scholar] [CrossRef] [PubMed]
- El-Hadary, A.; Sitohy, M. Safely effective hypoglycemic action of stevia and turmeric extracts on diabetic Albino rats. J. Food Biochem. 2021, 45, e13549. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, F.H.; Khalil, A.A.; Ibrahim, E.M.; Mansour, A.; Hussein, A.M. Effects of exercise and stevia on renal ischemia/reperfusion injury in rats. Acta Sci. Pol. Technol. Aliment. 2019, 18, 317–332. [Google Scholar]
- Hussein, A.M.; Eid, E.A.; Bin-Jaliah, I.; Taha, M.; Lashin, L.S. Exercise and Stevia Rebaudiana (R) Extracts Attenuate Diabetic Cardiomyopathy in Type 2 Diabetic Rats: Possible Underlying Mechanisms. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1117–1132. [Google Scholar] [CrossRef]
- Mehmood, A.; Zhao, L.; Wang, C.; Hossen, I.; Nadeem, M. Stevia residue extract alone and combination with allopurinol attenuate hyperuricemia in fructose–PO-induced hyperuricemic mice. J. Food Biochem. 2020, 44, e13087. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Zhao, L.; Wang, C.; Hossen, I.; Raka, R.N.; Zhang, H. Stevia residue extract increases intestinal uric acid excretion via interactions with intestinal urate transporters in hyperuricemic mice. Food Funct. 2019, 10, 7900–7912. [Google Scholar] [CrossRef] [PubMed]
- Morsi, A.A.; Mersal, E.A.; Farrag, A.R.H.; Abdelmoneim, A.M.; Abdelmenem, A.M.; Salim, M.S. Histomorphological Changes in a Rat Model of Polycystic Ovary Syndrome and the Contribution of Stevia Leaf Extract in Modulating the Ovarian Fibrosis, VEGF and TGF-β Immunoexpressions: Comparison with Metformin. Acta Histochem. Cytochem. 2022, 55, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Moselhy, S.S.; Ghoneim, M.A.; Khan, J.A. Vivo evaluation of antimicrobial and antioxidant potential of stevia extract. Afr. J. Tradit. Complement Altern Med. 2016, 6, 18–21. [Google Scholar] [CrossRef] [Green Version]
- Perumal, V.; Manickam, T.; Bang, K.S.; Velmurugan, P.; Oh, B.T. Antidiabetic potential of bioactive molecules coated chitosan nanoparticles in experimental rats. Int. J. Biol. Macromol. 2016, 92, 63–69. [Google Scholar] [CrossRef]
- Ramos-Tovar, E.; Hernández-Aquino, E.; Casas-Grajales, S.; Buendia-Montaño, L.D.; Galindo-Gómez, S.; Camacho, J.; Tsutsumi, V.; Muriel, P. Stevia prevents acute and chronic liver injury induced by carbon tetrachloride by blocking oxidative stress through Nrf2 upregulation. Oxid Med. Cell Longev. 2018, 2018, 3823426. [Google Scholar] [CrossRef] [Green Version]
- Ranjan, R.; Jena, J.; Ranjan, R.; Jaiswal, J. Stevia as a natural sweetener. IJRPC 2011, 1, 1199–1202. [Google Scholar]
- S, L.; Chaudhary, S.; R S, R. Hydroalcoholic extract of Stevia rebaudiana bert. leaves and stevioside ameliorates lipopolysaccharide induced acute liver injury in rats. Biomed. Pharmacother. 2017, 95, 1040–1050. [Google Scholar] [CrossRef]
- Shivanna, N.; Naika, M.; Khanum, F.; Kaul, V.K. Antioxidant, anti-diabetic and renal protective properties of Stevia rebaudiana. J. Diabetes Complicat. 2013, 27, 103–113. [Google Scholar] [CrossRef]
- Mostafa, A.F.; Elalfy, M.M.; Shata, A.; Elhadidy, M.G. Prophylactic effect of aquatic extract of stevia on acetic acid induced-ulcerative colitis in male rats: A possible role of Nrf2 and PPARγ. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 1093–1104. [Google Scholar] [CrossRef]
- Myint, K.Z.; Chen, J.M.; Zhou, Z.Y.; Xia, Y.M.; Lin, J.; Zhang, J. Structural dependence of antidiabetic effect of steviol glycosides and their metabolites on streptozotocin-induced diabetic mice. J. Sci. Food Agric. 2020, 100, 3841–3849. [Google Scholar] [CrossRef]
- Arumugam, B.; Subramaniam, A.; Alagaraj, P. Stevia as a natural sweetener: A review. Cardiovasc. Hematol. Agents Med. Chem. 2020, 18, 94–103. [Google Scholar] [CrossRef]
- Raspe, D.T.; da Silva, C.; Cláudio da Costa, S. Compounds from Stevia rebaudiana Bertoni leaves: An overview of non-conventional extraction methods and challenges. Food Biosci. 2022, 46, 101593. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, M.; Altman, D.G. Statistics Notes Interaction revisited: The difference between two estimates. BMJ 2003, 326, 219. [Google Scholar] [CrossRef] [Green Version]
- Borenstien, M.; Hedges, L.; Higgins, J.P.; Rothstein, H. Introduction to Meta-Analysis; John Wiley & Sons: West Sussex, UK, 2009. [Google Scholar]
- Schisterman, E.F.; Cole, S.R.; Platf, R.W. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009, 20, 488–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.; Gong, X.; Li, X.; Wang, C.; Li, M. Advanced research on the antioxidant activity and mechanism of polyphenols from Hippophae species—A review. Molecules 2020, 25, 917. [Google Scholar] [CrossRef] [Green Version]
- Shahat, A.A.; Ibrahim, A.Y.; Elsaid, M.S. Polyphenolic content and antioxidant activity of some wild Saudi Arabian asteraceae plants. Asian Pac. J. Trop. Med. 2014, 7, 545–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Bayat, E.; Rahpeima, Z.; Dastghaib, S.; Gholizadeh, F.; Erfani, M.; Asadikaram, G.; Mokarram, P. Stevia rebaudiana extract attenuate metabolic disorders in diabetic rats via modulation of glucose transport and antioxidant signaling pathways and aquaporin-2 expression in two extrahepatic tissues. J. Food Biochem. 2020, 44, e13252. [Google Scholar] [CrossRef] [PubMed]
- Mejia, E.; Pearlman, M. Natural alternative sweeteners and diabetes management. Curr. Diab. Rep. 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prata, C.; Zambonin, L.; Rizzo, B.; Maraldi, T.; Angeloni, C.; Vieceli Dalla Sega, F.; Fiorentini, D.; Hrelia, S. Glycosides from Stevia rebaudiana Bertoni possess insulin-mimetic and antioxidant activities in rat cardiac fibroblasts. Oxid. Med. Cell Longev. 2017, 2017, 3724545. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, Ł.; Marszałek, K.; Skapska, S. Influence of steviol glycosides on the stability of vitamin C and anthocyanins. J. Agric. Food Chem. 2014, 62, 11264–11269. [Google Scholar] [CrossRef] [PubMed]
- Šic Žlabur, J.; Dobričević, N.; Brnčić, M.; Barba, F.J.; Lorenzo, J.M.; Franco, D.; Atanasov, A.G.; Voća, S.; Brnčić, S.R. Evaluation of the behavior of phenolic compounds and steviol glycosides of sonicated strawberry juice sweetened with stevia (Stevia rebaudiana Bertoni). Molecules 2019, 24, 1202. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Aceves, L.M.; Dublán-García, O.; López-Martínez, L.X.; Novoa-Luna, K.A.; Islas-Flores, H.; Galar-Martínez, M.; García-Medina, S.; Hernández-Navarro, M.D.; Gómez-Oliván, L.M. Reduction of the oxidative stress status using steviol glycosides in a fish model (Cyprinus carpio). Biomed. Res. Int. 2017, 2017, 2352594. [Google Scholar] [CrossRef] [Green Version]
- Suckling, J.; Morse, S.; Murphy, R.; Astley, S.; Halford, J.C.G.; Harrold, J.A.; Le-Bail, A.; Koukouna, E.; Musinovic, H.; Perret, J.; et al. Environmental life cycle assessment of production of the high intensity sweetener steviol glycosides from Stevia rebaudiana leaf grown in Europe: The SWEET project. Int. J. Life Cycle Assess 2023, 28, 221–233. [Google Scholar] [CrossRef]




| Author | Year | Country | Assay | After Treatment (A) Pre-Treatment (P) with Stevia | # Controls | Control Value | Control SD | # Cases | Case Value | Case SD | # Stevia | Stevia Value | Stevia SD | Extract/Compound | Type of Tissue Tested | Type of Disease |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mostafa et al. [72] | 2020 | Egypt | SOD | 80 mg/kg/day orally, 1 week (A) | 10 | 3.6 | 0.11 | 10 | 1.6 | 0.1 | 10 | 2.66 | 0.09 | Aqueous | Colon | Ulcerative colitis |
| Mehmood et al. [64] | 2019 | China | SOD | 400 mg/kg/day orally, 8 weeks (A) | 8 | 118 | 6.1 | 8 | 53 | 2.8 | 8 | 107 | 12 | Hydroalcoholic | Duodenum | Hyperuricemia |
| Mehmood et al. [64] | 2019 | China | SOD | 400 mg/kg/day orally, 8 weeks (A) | 8 | 70 | 7.9 | 8 | 30 | 7 | 8 | 51 | 12 | Hydroalcoholic | Jejunum | Hyperuricemia |
| Mehmood et al. [64] | 2019 | China | SOD | 400 mg/kg/day orally, 8 weeks (A) | 8 | 55 | 8.1 | 8 | 21 | 4.5 | 8 | 40 | 4.5 | Hydroalcoholic | Ileum | Hyperuricemia |
| Mehmood et al. [63] | 2020 | China | SOD | 200 mg/kg/day orally, 4 weeks (A) | 8 | 118 | 4.9 | 8 | 91 | 5.9 | 8 | 115 | 5 | Hydroalcoholic | Serum | Hyperuricemia |
| El-Mesallamy et al. [45] | 2018 | Egypt | SOD | 200 mg/kg/day orally, 4 weeks (A) | 10 | 48 | 4 | 10 | 25 | 7.5 | 10 | 41 | 4 | Hydroalcoholic | Skeletal muscles | Diabetes mellitus |
| El-Mesallamy et al. [45] | 2018 | Egypt | SOD | 2 mg/kg/day orally, 4 weeks (A) | 10 | 48 | 4 | 10 | 25 | 7.5 | 10 | 30 | 4 | Stevioside | Skeletal muscles | Diabetes mellitus |
| Latha et al. [70] | 2017 | India | SOD | 500 mg/kg/day orally, 1 week (A) | 8 | 21 | 4.24 | 8 | 4 | 4.52 | 8 | 16.5 | 2.54 | Hydroalcoholic | Liver | Acute liver injury |
| Latha et al. [70] | 2017 | India | SOD | 250 mg/kg/day orally, 1 week (A) | 8 | 21 | 4.24 | 8 | 4 | 4.52 | 8 | 17 | 4.52 | Stevioside | Liver | Acute liver injury |
| Moselhy et al. [66] | 2016 | Saudi Arabia | SOD | 200 mg/kg/day orally, 2 weeks (A) | 10 | 0.25 | 0.0023 | 10 | 0.18 | 0.03 | 10 | 0.21 | 0.029 | Organic | Liver | Hepatotoxic |
| Perumal et al. [67] | 2016 | India | SOD | 100 mg/kg/day orally, 3 weeks (A) | 6 | 10.15 | 1.05 | 6 | 4.63 | 0.95 | 6 | 6.75 | 1.25 | Hydroalcoholic | Liver | Diabetes mellitus |
| Perumal et al. [67] | 2016 | India | SOD | 100 mg/kg/day orally, 3 weeks (A) | 6 | 11.04 | 1.3 | 6 | 4.63 | 1.69 | 6 | 6.74 | 1.18 | Hydroalcoholic | Kidney | Diabetes mellitus |
| Shivanna et al. [71] | 2012 | India | SOD | NR orally, 4 weeks (P) | 10 | 2.41 | 0.91 | 10 | 1.21 | 0.05 | 10 | 2.72 | 0.52 | Fraction methanol | Liver | Diabetes mellitus |
| Myint et al. [73] | 2020 | China | SOD | 12 mg/kg/day orally, 6 weeks (A) | 6 | 74.53 | 1.82 | 6 | 36.61 | 1.44 | 6 | 39.23 | 1.28 | Rebaudioside A | Liver | Diabetes mellitus |
| Myint et al. [73] | 2020 | China | SOD | 10 mg/kg/day orally, 6 weeks (A) | 6 | 74.53 | 1.82 | 6 | 36.61 | 1.44 | 6 | 44.46 | 1.28 | Stevioside | Liver | Diabetes mellitus |
| Singh et al. [37] | 2013 | India | SOD | 300 mg/kg/day orally, 3 weeks (A) | 7 | 50.5 | 32.80 | 7 | 37.5 | 18.79 | 7 | 50 | 10.85 | Organic | Liver | Diabetes mellitus |
| Singh et al. [37] | 2013 | India | SOD | 300 mg/kg/day orally, 3 weeks (A) | 7 | 190.2 | 50.27 | 7 | 40 | 19.84 | 7 | 10 | 14.02 | Organic | Pancreas | Diabetes mellitus |
| Singh et al. [37] | 2013 | India | SOD | 300 mg/kg/day orally, 3 weeks (A) | 7 | 55 | 9.26 | 7 | 25 | 9.26 | 7 | 9 | 9.26 | Organic | Kidney | Diabetes mellitus |
| El-Hadary et al. [60] | 2021 | Egypt | SOD | 300 mg/kg/day orally, 8 weeks (A) | 10 | 54.3 | 2.3 | 10 | 47.2 | 1.2 | 10 | 58.8 | 0.9 | Hydroalcoholic | Liver | Diabetes mellitus |
| Morsi et al. [65] | 2022 | Egypt | SOD | 300 mg/kg/day orally, 4 weeks (A) | 7 | 36.25 | 1.25 | 7 | 21.25 | 3 | 7 | 29 | 1.75 | Glycosides-sweetener | Ovary | Polycystic ovary syndrome |
| Deenadayalan et al. [59] | 2021 | India | SOD | 20 mg/kg/day orally, 45 days (A) | 6 | 32.5 | 3.06 | 6 | 13 | 1.84 | 6 | 20 | 1.84 | Stevioside | Skeletal muscles | Diabetes mellitus |
| Mostafa et al. [72] | 2020 | Egypt | CAT | 80 mg/kg/day orally, 1 week (P) | 10 | 7.95 | 0.11 | 10 | 3.9 | 0.08 | 10 | 6.6 | 0.09 | Aqueous | Colon | Ulcerative colitis |
| Elsaid et al. [61] | 2019 | Egypt | CAT | 200 mg/kg/day orally, 5 weeks (P) | 12 | 34.66 | 3.14 | 12 | 14 | 2.36 | 12 | 27.66 | 3.72 | Hydroalcoholic | Kidney | Renal ischemia/reperfusion |
| Abdallah et al. [55] | 2022 | Egypt | CAT | 500 mg/kg/day orally, 1 week (A) | 7 | 36 | 0.5 | 7 | 21 | 5 | 7 | 30 | 0.5 | Organic | Liver | Liver disease |
| Abdallah et al. [55] | 2022 | Egypt | CAT | 250 mg/kg/day orally, 1 week (A) | 7 | 36 | 0.5 | 7 | 21 | 5 | 7 | 35 | 5 | Stevioside | Liver | Liver disease |
| Mehmood et al. [63] | 2020 | China | CAT | 200 mg/kg/day orally, 4 weeks (A) | 8 | 17.5 | 1.5 | 8 | 8 | 1.1 | 8 | 10.5 | 1.2 | Hydroalcoholic | Serum | Hyperuricemia |
| Moselhy et al. [66] | 2016 | Saudi Arabia | CAT | 200 mg/kg/day orally, 2 weeks (A) | 10 | 0.89 | 0.07 | 10 | 0.32 | 0.05 | 10 | 0.76 | 0.05 | Organic | Liver | Hepatotoxic |
| El Nashar et al. [41] | 2022 | Egypt | CAT | 200 mg/kg/day orally, 4 weeks (P and A) | 10 | 67.67 | 31.53 | 10 | 52.08 | 29.6 | 10 | 95.25 | 35.45 | Organic | Brain | Epilepsy |
| Hussein et al. [62] | 2020 | Egypt | CAT | 400 mg/kg/day orally, 4 weeks (A) | 8 | 17 | 0.45 | 8 | 7 | 0.19 | 8 | 22.5 | 0.98 | Hydroalcoholic | Heart | Diabetes mellitus |
| Shivanna et al. [71] | 2012 | India | CAT | NR orally, 4 weeks (P) | 10 | 1.02 | 0.05 | 10 | 0.52 | 0.07 | 10 | 0.78 | 0.17 | Fraction methanol | Liver | Diabetes mellitus |
| Assaei et al. [44] | 2016 | Iran | CAT | 400 mg/kg/day orally, 4 weeks (A) | 10 | 29.4 | 8.85 | 10 | 9.9 | 6.96 | 10 | 33.7 | 6.33 | Aqueous | Pancreas | Diabetes mellitus |
| Deenadayalan et al. [59] | 2021 | India | CAT | 20 mg/kg/day orally, 45 days (A) | 6 | 11.5 | 3.06 | 6 | 6.25 | 3.18 | 6 | 8.75 | 1.83 | Stevioside | Skeletal muscles | Diabetes mellitus |
| Perumal et al. [67] | 2016 | India | CAT | 100 mg/kg/day orally, 3 weeks (A) | 6 | 42.8 | 6.2 | 6 | 25.8 | 3.72 | 6 | 35.69 | 6.42 | Hydroalcoholic | Liver | Diabetes mellitus |
| Perumal et al. [67] | 2016 | India | CAT | 100 mg/kg/day orally, 3 weeks (A) | 6 | 34.04 | 5.27 | 6 | 23.56 | 1.54 | 6 | 25.3 | 1.71 | Hydroalcoholic | Kidney | Diabetes mellitus |
| El-Mesallamy et al. [45] | 2018 | Egypt | CAT | 200 mg/kg/day orally, 4 weeks (A) | 10 | 90 | 1 | 10 | 55 | 3 | 10 | 80 | 4 | Hydroalcoholic | Skeletal muscles | Diabetes mellitus |
| El-Mesallamy et al. [45] | 2018 | Egypt | CAT | 2 mg/kg/day orally, 4 weeks (A) | 10 | 90 | 1 | 10 | 55 | 3 | 10 | 72 | 4 | Stevioside | Skeletal muscles | Diabetes mellitus |
| Latha et al. [70] | 2017 | India | CAT | 500 mg/kg/day orally, 1 week (A) | 8 | 0.87 | 0.73 | 8 | 0.21 | 0.03 | 8 | 0.51 | 0.31 | Hydroalcoholic | Liver | Acute liver injury |
| Latha et al. [70] | 2017 | India | CAT | 250 mg/kg/day orally, 1 week (A) | 8 | 0.87 | 0.73 | 8 | 0.21 | 0.03 | 8 | 0.3 | 0.28 | Stevioside | Liver | Acute liver injury |
| El-Mesallamy et al. [45] | 2018 | Egypt | GPx | 200 mg/kg/day orally, 4 weeks (A) | 10 | 515 | 2 | 10 | 280 | 4 | 10 | 480 | 7 | Hydroalcoholic | Skeletal muscles | Diabetes mellitus |
| El-Mesallamy et al. [45] | 2018 | Egypt | GPx | 2 mg/kg/day Orally, 4 weeks (A) | 10 | 515 | 2 | 10 | 280 | 4 | 10 | 450 | 3.5 | Stevioside | Skeletal muscles | Diabetes mellitus |
| Deenadayalan et al. [59] | 2021 | India | GPx | 20 mg/kg/day orally, 45 days (A) | 6 | 26 | 6.12 | 6 | 14 | 3.68 | 6 | 20 | 3.68 | Stevioside | Skeletal muscles | Diabetes mellitus |
| El-Hadary et al. [60] | 2021 | Egypt | GPx | 300 mg/kg/day orally, 8 weeks (A) | 10 | 165.6 | 0.7 | 10 | 137.8 | 1.4 | 10 | 175.8 | 5.3 | Hydroalcoholic | Liver | Diabetes mellitus |
| Mostafa et al. [72] | 2020 | Egypt | GSH | 80 mg/kg/day orally, 1 week (A) | 10 | 5.7 | 0.09 | 10 | 2.2 | 0.05 | 10 | 4.9 | 0.03 | Aqueous | Colon | Ulcerative colitis |
| Abdel-Aal et al. [56] | 2021 | Egypt | GSH | 400 mg/kg/day orally, 3 weeks (A) | 8 | 22 | 3.11 | 8 | 3 | 0.70 | 8 | 12.5 | 0.70 | Aqueous | Liver | Diabetes mellitus |
| Abdel-Aal et al. [56] | 2021 | Egypt | GSH | 400 mg/kg/day orally, 3 weeks (A) | 8 | 22 | 1.41 | 8 | 7 | 0.71 | 8 | 17 | 1.27 | Aqueous | Kidney | Diabetes mellitus |
| Hussein et al. [62] | 2020 | Egypt | GSH | 400 mg/kg/day orally, 4 weeks (A) | 8 | 11.25 | 0.4 | 8 | 3.1 | 0.23 | 8 | 11.7 | 0.97 | Hydroalcoholic | Heart | Diabetes mellitus |
| Mehmood et al. [64] | 2019 | China | GSH | 400 mg/kg/day orally, 8 weeks (A) | 8 | 125 | 37.5 | 8 | 40 | 7.5 | 8 | 100 | 27 | Hydroalcoholic | Duodenum | Hyperuricemia |
| Mehmood et al. [64] | 2019 | China | GSH | 400 mg/kg/day orally, 8 weeks (A) | 8 | 100 | 27 | 8 | 40 | 2 | 8 | 80 | 17.5 | Hydroalcoholic | Jejunum | Hyperuricemia |
| Mehmood et al. [64] | 2019 | China | GSH | 400 mg/kg/day orally, 8 weeks (A) | 8 | 100 | 8 | 8 | 35 | 10 | 8 | 84 | 10 | Hydroalcoholic | Ileum | Hyperuricemia |
| Elsaid et al. [61] | 2019 | Egypt | GSH | 200 mg/kg/day orally, 5 weeks (P) | 12 | 8.65 | 0.57 | 12 | 3.38 | 0.36 | 12 | 7.17 | 0.64 | Hydroalcoholic | Kidney | Renal ischemia/reperfusion |
| Ramos-Tovar et al. [43] | 2019 | Mexico | GSH | 100 mg/kg/day orally, 12 weeks (A) | 8 | 10 | 1.98 | 8 | 3.75 | 1.95 | 8 | 8.55 | 1.27 | Aqueous | Liver | Liver cirrhosis |
| Casas-Grajales et al. [58] | 2019 | Mexico | GSH | 20 mg/kg/twice daily intraperitoneally, 8 weeks (A) | 8 | 13 | 0.85 | 8 | 10 | 0.28 | 8 | 13.5 | 1.13 | Stevioside | Liver | Liver fibrosis |
| Casas-Grajales et al. [57] | 2019 | Mexico | GSH | 20 mg/kg/twice daily intraperitoneally, 8 weeks (A) | 8 | 13.3 | 0.85 | 8 | 10 | 0.28 | 8 | 12.2 | 0.57 | Rebaudioside A | Liver | Liver fibrosis |
| Ramos-Tovar et al. [42] | 2018 | Mexico | GSH | 100 mg/kg/day orally, 10 weeks (A) | 8 | 5.6 | 1.41 | 8 | 3 | 0.28 | 8 | 4.5 | 1.56 | Aqueous | Liver | Liver cirrhosis |
| Ramos-Tovar et al. [68] | 2018 | Mexico | GSH | 100 mg/kg/day orally, 1 week (A) | 8 | 11.5 | 1.27 | 8 | 6 | 2.55 | 8 | 11 | 2.55 | Aqueous | Liver | Liver cirrhosis |
| Latha et al. [70] | 2017 | India | GSH | 500 mg/kg/day orally, 1 week (A) | 8 | 230 | 14.14 | 8 | 95 | 28.29 | 8 | 212 | 98.99 | Hydroalcoholic | Liver | Acute Liver injury |
| Latha et al. [70] | 2017 | India | GSH | 250 mg/kg/day orally, 1 week (A) | 8 | 230 | 14.14 | 8 | 95 | 28.28 | 8 | 181 | 14.14 | Stevioside | Liver | Acute Liver injury |
| Perumal et al. [67] | 2016 | India | GSH | 100 mg/kg/day orally, 3 weeks (A) | 6 | 43.4 | 7.20 | 6 | 11.83 | 8.23 | 6 | 21.4 | 6.74 | Hydroalcoholic | Liver | Diabetes mellitus |
| Perumal et al. [67] | 2016 | India | GSH | 100 mg/kg/day orally, 3 weeks (A) | 6 | 41.4 | 9.48 | 6 | 19.04 | 5.07 | 6 | 27.43 | 8.01 | Hydroalcoholic | Kidney | Diabetes mellitus |
| Shivanna et al. [71] | 2012 | India | GSH | NR orally, 4 weeks (P) | 10 | 24.58 | 0.51 | 10 | 13.58 | 0.4 | 10 | 21.11 | 0.51 | Fraction methanol | Plasma | Diabetes mellitus |
| Myint et al. [73] | 2020 | China | GSH | 12 mg/kg/day orally, 6 weeks (A) | 6 | 56.75 | 1.27 | 6 | 35.78 | 1.26 | 6 | 36.6 | 1.24 | Rebaudioside A | Liver | Diabetes mellitus |
| Myint et al. [73] | 2020 | China | GSH | 10 mg/kg/day orally, 6 weeks (A) | 6 | 56.75 | 1.27 | 6 | 35.78 | 1.26 | 6 | 39.89 | 1.24 | Stevioside | Liver | Diabetes mellitus |
| Singh et al. [37] | 2013 | India | GSH | 300 mg/kg/day orally, 3 weeks(A) | 7 | 25.2 | 10.05 | 7 | 7.1 | 6.09 | 7 | 28.1 | 19.31 | Organic | Liver | Diabetes mellitus |
| Singh et al. [37] | 2013 | India | GSH | 300 mg/kg/day orally, 3 weeks (A) | 7 | 7.5 | 6.09 | 7 | 4.1 | 1.32 | 7 | 22.1 | 10.05 | Organic | Pancreas | Diabetes mellitus |
| Singh et al. [37] | 2013 | India | GSH | 300 mg/kg/day orally, 3 weeks (A) | 7 | 22.4 | 8.73 | 7 | 3.5 | 0.26 | 7 | 18.1 | 8.73 | Organic | Kidney | Diabetes mellitus |
| El-Hadary et al. [60] | 2021 | Egypt | GSH | 300 mg/kg/day orally, 8 weeks (A) | 10 | 80.8 | 0.9 | 10 | 57.2 | 1.7 | 10 | 81.6 | 1.9 | Hydroalcoholic | Liver | Diabetes mellitus |
| Abdallah et al. [55] | 2022 | Egypt | GSH | 500 mg/kg/day orally, 1 week (A) | 7 | 50 | 1 | 7 | 42.5 | 2.5 | 7 | 48 | 1.5 | Organic | Liver | Liver disease |
| Abdallah et al. [55] | 2022 | Egypt | GSH | 250 mg/kg/day orally, 1 week (A) | 7 | 50 | 1 | 7 | 42.5 | 2.5 | 7 | 51 | 2 | Stevioside | Liver | Liver disease |
| Deenadayalan et al. [59] | 2021 | India | GSH | 20 mg/kg/day orally, 45 days (A) | 6 | 13.25 | 2.45 | 6 | 6 | 2.21 | 6 | 9 | 1.22 | Stevioside | Skeletal muscles | Diabetes mellitus |
| Ranjbar et al. [40] | 2020 | Iran | TAC | 400 mg/kg/day orally, 14 weeks (A) | 10 | 0.36 | 0.16 | 10 | 0.19 | 0.19 | 10 | 0.28 | 0.13 | Hydroalcoholic | Serum | Metabolic syndrome |
| El Nashar et al. [41] | 2022 | Egypt | TAC | 200 mg/kg/day orally, 4 weeks (P and A) | 10 | 4.65 | 1.28 | 10 | 1.91 | 1.27 | 10 | 6.3 | 2.63 | Organic | Brain | Epilepsy |
| Abdel-Aal et al. [56] | 2021 | Egypt | MDA | 400 mg/kg/day orally, 3 weeks (A) | 8 | 0.2 | 0.06 | 8 | 0.58 | 0.06 | 8 | 0.24 | 0.04 | Aqueous | Liver | Diabetes mellitus |
| Abdel-Aal et al. [56] | 2021 | Egypt | MDA | 400 mg/kg/day orally, 3 weeks (A) | 8 | 0.21 | 0.02 | 8 | 0.46 | 0.03 | 8 | 0.24 | 0.21 | Aqueous | Kidney | Diabetes mellitus |
| Ranjbar et al. [40] | 2020 | Iran | MDA | 400 mg/kg/day orally, 14 weeks (A) | 10 | 34 | 17.39 | 10 | 45 | 23.72 | 10 | 38 | 12.33 | Hydroalcoholic | Serum | Metabolic syndrome |
| Hussein et al. [62] | 2020 | Egypt | MDA | 400 mg/kg/day orally, 4 weeks (A) | 8 | 1.49 | 0.03 | 8 | 9.9 | 0.26 | 8 | 2 | 0.13 | Hydroalcoholic | Heart | Diabetes mellitus |
| Mehmood et al. [64] | 2019 | China | MDA | 400 mg/kg/day orally, 8 weeks(A) | 8 | 1.4 | 0.1 | 8 | 5.75 | 0.9 | 8 | 1.75 | 0.49 | Hydroalcoholic | Duodenum | Hyperuricemia |
| Mehmood et al. [64] | 2019 | China | MDA | 400 mg/kg/day orally, 8 weeks (A) | 8 | 1.2 | 0.25 | 8 | 4.4 | 0.79 | 8 | 1.2 | 0.95 | Hydroalcoholic | Jejunum | Hyperuricemia |
| Mehmood et al. [64] | 2019 | China | MDA | 400 mg/kg/day orally, 8 weeks (A) | 8 | 1.1 | 0.37 | 8 | 4.1 | 1.21 | 8 | 1.5 | 0.49 | Hydroalcoholic | Ileum | Hyperuricemia |
| Mehmood et al. [63] | 2020 | China | MDA | 200 mg/kg/day orally, 4 weeks (A) | 8 | 4.6 | 1.95 | 8 | 9.15 | 1 | 8 | 5.75 | 1.25 | Hydroalcoholic | Serum | Hyperuricemia |
| Elsaid et al. [61] | 2019 | Egypt | MDA | 200 mg/kg/day orally, 5 weeks (A) | 12 | 1.89 | 0.31 | 12 | 5.2 | 1.07 | 12 | 2.92 | 0.16 | Hydroalcoholic | Kidney | Renal ischemia/reperfusion |
| Ramos-Tovar et al. [43] | 2019 | Mexico | MDA | 100 mg/kg/day orally, 12 weeks (A) | 8 | 0.19 | 0.03 | 8 | 0.29 | 0.06 | 8 | 0.2 | 0.04 | Aqueous | Liver | Liver cirrhosis |
| Casas-Grajales et al. [58] | 2019 | Mexico | MDA | 20 mg/kg/twice daily intraperitoneally, 8 weeks (A) | 8 | 0.2 | 0.03 | 8 | 0.67 | 0.09 | 8 | 0.4 | 0.07 | Stevioside | Liver | Liver fibrosis |
| Casas-Grajales et al. [57] | 2019 | Mexico | MDA | 20 mg/kg twice daily intraperitoneally, 8 weeks (A) | 8 | 0.22 | 0.06 | 8 | 0.67 | 0.11 | 8 | 0.32 | 0.09 | Rebaudioside A | Liver | Liver fibrosis |
| El-Mesallamy et al. [45] | 2018 | Egypt | MDA | 200 mg/kg/day orally, 4 weeks (A) | 10 | 4 | 1.1 | 10 | 11.5 | 0.9 | 10 | 6 | 0.95 | Hydroalcoholic | Skeletal muscles | Diabetes mellitus |
| El-Mesallamy et al. [45] | 2018 | Egypt | MDA | 2 mg/kg/day orally, 4 weeks (A) | 10 | 4 | 1.1 | 10 | 11.5 | 0.9 | 10 | 8 | 0.95 | Stevioside | Skeletal muscles | Diabetes mellitus |
| Ramos-Tovar et al. [42] | 2018 | Mexico | MDA | 100 mg/kg/day orally, 10 weeks (A) | 8 | 0.17 | 0.03 | 8 | 0.34 | 0.09 | 8 | 0.24 | 0.11 | Aqueous | Liver | Liver cirrhosis |
| Ramos-Tovar et al. [68] | 2018 | Mexico | MDA | 100 mg/kg/day orally, 1 week (A) | 8 | 0.10 | 0.03 | 8 | 0.31 | 0.03 | 8 | 0.15 | 0.06 | Aqueous | Liver | Liver cirrhosis |
| Latha et al. [70] | 2017 | India | MDA | 500 mg/kg/day orally, 1 week (A) | 8 | 35 | 2.83 | 8 | 160 | 19.8 | 8 | 85 | 5.66 | Hydroalcoholic | Liver | Acute liver injury |
| Latha et al. [70] | 2017 | India | MDA | 250 mg/kg/day orally, 1 week (A) | 8 | 35 | 2.83 | 8 | 160 | 19.78 | 8 | 115 | 2.83 | Stevioside | Liver | Acute liver injury |
| Moselhy et al. [66] | 2016 | Saudi Arabia | MDA | 200 mg/kg/day orally, 2 weeks (A) | 10 | 5.11 | 0.14 | 10 | 10.14 | 0.37 | 10 | 6.94 | 0.47 | Organic | Liver | Hepatotoxic |
| Perumal et al. [67] | 2016 | India | MDA | 100 mg/kg/day orally, 3 weeks (A) | 6 | 0.9 | 0.22 | 6 | 2.33 | 0.56 | 6 | 1.06 | 0.42 | Hydroalcoholic | Liver | Diabetes mellitus |
| Perumal et al. [67] | 2016 | India | MDA | 100 mg/kg/day orally, 3 weeks (A) | 6 | 0.53 | 0.220 | 6 | 2.07 | 1.13 | 6 | 1.49 | 0.61 | Hydroalcoholic | Kidney | Diabetes mellitus |
| Assaei et al. [44] | 2016 | Iran | MDA | 400 mg/kg/day orally, 4 weeks (A) | 10 | 0.4 | 0.13 | 10 | 1.4 | 0.25 | 10 | 0.45 | 0.13 | Aqueous | Pancreas | Diabetes mellitus |
| Shivanna et al. [71] | 2012 | India | MDA | NR, 4 weeks (P) | 10 | 0.06 | 0.01 | 10 | 0.16 | 0.03 | 10 | 0.07 | 0.01 | Fraction methanol | Liver | Diabetes mellitus |
| Myint et al. [73] | 2020 | China | MDA | 12 mg/kg/day orally, 6 weeks (A) | 6 | 7.12 | 0.11 | 6 | 12.63 | 0.32 | 6 | 12.43 | 0.23 | Rebaudioside A | Liver | Diabetes mellitus |
| Myint et al. [73] | 2020 | China | MDA | 10 mg/kg/day orally, 6 weeks (A) | 6 | 7.12 | 0.11 | 6 | 12.63 | 0.32 | 6 | 11.89 | 0.23 | Stevioside | Liver | Diabetes mellitus |
| Singh et al. [37] | 2013 | India | MDA | 300 mg/kg/day orally, 3 weeks (A) | 7 | 25 | 66.14 | 7 | 410 | 66.14 | 7 | 10.2 | 1.32 | Organic | Liver | Diabetes mellitus |
| Singh et al. [37] | 2013 | India | MDA | 300 mg/kg/day orally, 3 weeks (A) | 7 | 11.1 | 2.64 | 7 | 75.2 | 47.62 | 7 | 4.8 | 1.32 | Organic | Pancreas | Diabetes mellitus |
| Singh et al. [37] | 2013 | India | MDA | 30 mg/kg/day orally, 3 weeks (A) | 7 | 50.2 | 31.75 | 7 | 415 | 165.36 | 7 | 11 | 1.32 | Organic | Kidney | Diabetes mellitus |
| El-Hadary et al. [60] | 2021 | Egypt | MDA | 300 mg/kg/day orally, 8 weeks (A) | 10 | 5.33 | 0.2 | 10 | 11.9 | 0.5 | 10 | 4.9 | 0.1 | Hydroalcoholic | Liver | Diabetes mellitus |
| Morsi et al. [65] | 2022 | Egypt | MDA | 300 mg/kg/day orally, 4 weeks (A) | 7 | 50 | 1.5 | 7 | 160 | 2 | 7 | 76 | 1.5 | Glycosides-sweetener | Ovary | Polycystic ovary syndrome |
| Abdallah et al. [55] | 2022 | Egypt | MDA | 500 mg/kg/day orally, 1 week (A) | 7 | 4.1 | 0.5 | 7 | 10.9 | 1.75 | 7 | 4.8 | 0.8 | Organic | Liver | Liver disease |
| Abdallah et al. [55] | 2022 | Egypt | MDA | 250 mg/kg/day orally, 1 week (A) | 7 | 4.1 | 0.5 | 7 | 10.9 | 1.75 | 7 | 5 | 1 | Stevioside | Liver | Liver disease |
| El Nashar et al. [41] | 2022 | Egypt | MDA | 200 mg/kg/day orally, 4 weeks (P and A) | 10 | 50.76 | 5.58 | 10 | 81.79 | 5.82 | 10 | 42.68 | 14.6 | Organic | Brain | Epilepsy |
| SOD | CAT | GPx | GSH | TAC | SOD | CAT | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference | Coef. | p-value | 95% CI | # Studies | Coef. | p-value | 95% CI | # Studies | Coef. | p-value | 95% CI | # Studies | Coef. | p-value | 95% CI | # Studies | Coef. | p-value | 95% CI | # Studies | Coef. | p-value | 95% CI | # Studies |
| Overall | ||||||||||||||||||||||||
| control–case | 6.09 | 0.00 | 3.99, 8.18 | 21 | 6.61 | 0.00 | 3.24, 9.99 | 17 | 31.70 | 0.05 | −0.16, 63.56 | 4 | 7.06 | 0.00 | 4.46, 9.67 | 27 | 1.25 | 0.00 | 0.46, 2.03 | 2 | −6.25 | 0.00 | −7.87, −4.63 | 33 |
| stevia–case | 2.73 | 0.00 | 1.66, 3.79 | 21 | 4.65 | 0.00 | 2.02, 7.28 | 17 | 25.67 | 0.03 | 2.47, 48.88 | 4 | 4.92 | 0.00 | 2.87, 6.98 | 27 | 1.45 | 0.12 | −0.39, 3.27 | 2 | −4.66 | 0.00 | −6.05, −3.27 | 33 |
| stevia–control | −3.36 | 0.00 | −5.24, −1.48 | 21 | 1.96 | 0.01 | −3.51, −0.41 | 17 | −6.03 | 0.22 a | −15.62, 3.56 | 4 | −2.14 | 0.00 | −3.33, −0.95 | 27 | 0.19 | 0.79 a | −1.24, 1.62 | 2 | 1.56 | 0.00 | 0.72, 2.46 | 33 |
| Leaf extract | ||||||||||||||||||||||||
| control–case | 4.72 | 0.00 | 3.08, 6.37 | 15 | 7.66 | 0.00 | 3.01, 12.33 | 13 | 28.55 | 0.16 | −11.17, 68.28 | 2 | 7.67 | 0.00 | 3.72, 11.61 | 20 | 1.25 | 0.00 | 0.46, 2.03 | 2 | −5.49 | 0.00 | −7.15, 0.83 | 25 |
| stevia–case | 2.77 | 0.00 | 1.39, 4.15 | 15 | 5.73 | 0.00 | 1.97, 9.5 | 13 | 26.54 | 0.08 | −2.99, 56.06 | 2 | 5.89 | 0.00 | 2.79, 8.99 | 20 | 1.45 | 0.12 | −0.39, 3.27 | 2 | −4.78 | 0.00 | −6.37, −3.19 | 25 |
| stevia–control | −1.95 | 0.00 | −3.18, −0.72 | 15 | −1.93 | 0.08 a | −4.07, 0.19 | 13 | −2.02 | 0.70 a | −12.30, 8.27 | 2 | −1.77 | 0.00 | −2.92, −0.62 | 20 | 0.19 | 0.79 a | −1.24, 1.62 | 2 | 0.71 | 0.00 | 0.24, 1.17 | 25 |
| Glycosides | ||||||||||||||||||||||||
| control–case | 11.04 | 0.00 | 3.83, 18.25 | 6 | 4.49 | 0.06 | −0.09, 9.08 | 4 | 36.52 | 0.29 | −31.09, 104.13 | 2 | 7.42 | 0.00 | 3.41, 11.43 | 7 | −10.28 | 0.00 | −15.02, −5.55 | 8 | ||||
| stevia–case | 2.67 | 0.00 | 1.54, 3.81 | 6 | 2.43 | 0.05 | 0.03, 4.83 | 4 | 26.13 | 0.30 | −23.34, 75.60 | 2 | 2.99 | 0.00 | 1.84, 4.13 | 7 | −3.51 | 0.00 | −4.53, −2.49 | 8 | ||||
| stevia–control | −8.36 | 0.01 | −15.34, −1.39 | 6 | −2.06 | 0.11 a | −4.58, 0.45 | 4 | −10.39 | 0.26 a | −28.55, 7.77 | 2 | −4.43 | 0.06 a | −9.07, 0.21 | 7 | 6.77 | 0.01 | 1.27, 12.27 | 8 | ||||
| SOD, CAT, GSH, TAC | SOD | CAT | GSH | TAC | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference | Coef. | p-value | 95% CI | # Studies | Coef. | p-value | 95% CI | # Studies | Coef. | p-value | 95% CI | # Studies | Coef. | p-value | 95% CI | # Studies | Coef. | p-value | 95% CI | # Studies |
| Combined leaf extract | ||||||||||||||||||||
| control−case | 6.13 | 0.00 | 4.42, 7.84 | 50 | 4.72 | 0.00 | 3.08, 6.37 | 15 | 7.66 | 0.00 | 3.01, 12.33 | 13 | 7.67 | 0.00 | 3.72, 11.61 | 20 | 1.25 | 0.00 | 0.46, 2.03 | 2 |
| stevia–case | 4.39 | 0.00 | 3.03, 5.74 | 50 | 2.77 | 0.00 | 1.39, 4.15 | 15 | 5.73 | 0.00 | 1.97, 9.5 | 13 | 5.85 | 0.00 | 2.79, 8.99 | 20 | 1.48 | 0.12 | −0.39, 3.27 | 2 |
| stevia–-control | −1.74 | 0.00 | −2.46, −1.02 | 50 | −1.95 | 0.00 | −3.18, −0.72 | 15 | −1.93 | 0.07 a | −4.07, 0.19 | 13 | −1.77 | 0.00 | −2.92, −0.62 | 20 | 0.19 | 0.79 a | −1.24, 1.62 | 2 |
| Aqueous | ||||||||||||||||||||
| control–case | 15.66 | 0.00 | 3.92, 27.40 | 9 | 22.47 | 0.26 | −17.18, 62.12 | 2 | 13.53 | 0.09 | −2.03, 29.07 | 6 | ||||||||
| stevia–case | 10.79 | 0.01 | 2.17, 19.40 | 9 | 15.70 | 0.22 | −9.34, 40.75 | 2 | 9.78 | 0.11 | −2.34, 21.91 | 6 | ||||||||
| stevia–control | −4.87 | 0.00 | −8.34, −1.41 | 9 | −6.76 | 0.36 a | −21.39, 7.86 | 2 | −3.74 | 0.04 | −7.30, −0.17 | 6 | ||||||||
| Organic | ||||||||||||||||||||
| control–case | 2.73 | 0.00 | 1.49, 3.98 | 13 | 2.45 | 0.00 | 1.18, 3.73 | 5 | 5.18 | 0.06 | −0.35, 10.71 | 3 | 2.08 | 0.01 | 0.59, 3.55 | 4 | ||||
| stevia–case | 1.85 | 0.00 | 0.77, 2.94 | 13 | 0.33 | 0.65 | −1.11, 1.78 | 5 | 4.09 | 0.03 | 0.29, 7.88 | 3 | 2.22 | 0.00 | 1.57, 2.87 | 4 | ||||
| stevia–control | −0.88 | 0.13 a | −2.03, 0.27 | 13 | −2.12 | 0.08 a | −4.52, 0.28 | 5 | −1.09 | 0.29 a | −3.15, 0.97 | 3 | 0.14 | 0.83 a | −1.16, 1.45 | 4 | ||||
| Hydroalcoholic | ||||||||||||||||||||
| control–case | 5.49 | 0.00 | 4.20, 6.76 | 26 | 4.75 | 0.00 | 4.17, 5.33 | 9 | 6.58 | 0.00 | 3.03, 10.12 | 7 | 6.38 | 0.00 | 3.35, 9.39 | 9 | ||||
| stevia–case | 4.26 | 0.00 | 2.64, 5.87 | 26 | 3.44 | 0.00 | 2.28, 4.60 | 9 | 5.48 | 0.06 | −0.25, 11.21 | 7 | 5.29 | 0.00 | 1.83, 8.72 | 9 | ||||
| stevia–control | −1.23 | 0.00 | −2.03, −0.43 | 26 | −1.31 | 0.03 | −2.49, −0.11 | 9 | −1.09 | 0.49 a | −4.19, 1.99 | 7 | −1.09 | 0.01 | −1.92, −0.27 | 9 | ||||
| SOD, CAT, GSH, TAC | SOD | CAT | GSH | TAC | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference | Coef. | p- value | 95% CI | # Studies | Coef. | p- value | 95% CI | # Studies | Coef. | p- value | 95% CI | # Studies | Coef. | p- value | 95% CI | # Studies | Coef. | p- value | 95% CI | # Studies |
| Overall | ||||||||||||||||||||
| control–case | 6.13 | 0.00 | 4.42, 7.84 | 50 | 4.72 | 0.00 | 3.08, 6.37 | 15 | 7.67 | 0.00 | 3.01, 12.33 | 13 | 7.67 | 0.00 | 3.72, 11.61 | 20 | 1.24 | 0.00 | 0.46, 2.03 | 2 |
| stevia–case | 4.39 | 0.00 | 3.03, 5.74 | 50 | 2.77 | 0.00 | 1.39, 4.15 | 15 | 5.73 | 0.00 | 1.97, 9.5 | 13 | 5.89 | 0.00 | 2.79, 8.99 | 20 | 1.44 | 0.12 | −0.39, 3.27 | 2 |
| stevia–control | −1.74 | 0.00 | −2.46, −1.02 | 50 | −1.95 | 0.00 | −3.18, −0.72 | 15 | −1.93 | 0.07 a | −4.07, 0.19 | 13 | −1.77 | 0.00 | −2.92, −0.62 | 20 | 0.19 | 0.79 a | −1.24, 1.62 | 2 |
| Diabetes mellitus | ||||||||||||||||||||
| control–case | 6.85 | 0.00 | 4.92, 8.80 | 34 | 3.41 | 0.00 | 2.28, 4.54 | 8 | 6.35 | 0.00 | 2.28, 10.43 | 6 | 8.04 | 0.00 | 3.70, 12.37 | 10 | ||||
| stevia–case | 3.75 | 0.00 | 2.22, 5.29 | 34 | 1.67 | 0.07 | −0.16, 3.49 | 8 | 6.22 | 0.07 | −0.43, 12.86 | 6 | 6.31 | 0.00 | 2.69, 9.92 | 10 | ||||
| stevia–control | −3.1 | 0.00 | −4.73, −1.48 | 34 | −1.74 | 0.08 a | −3.67, 0.19 | 8 | −0.14 | 0.94 a | −3.46, 3.19 | 6 | −1.73 | 0.06 a | −3.51, 0.05 | 10 | ||||
| Liver injury | ||||||||||||||||||||
| control–case | 3.65 | 0.00 | 2.76, 4.55 | 17 | 3.31 | 0.00 | 1.25, 5.37 | 2 | 5.49 | 0.04 | 0.35, 10.63 | 3 | 2.77 | 0.00 | 2.02, 3.52 | 5 | ||||
| stevia–case | 2.74 | 0.00 | 1.98, 3.49 | 17 | 2.13 | 0.04 | 0.08, 4.17 | 2 | 3.82 | 0.08 | −0.44, 8.09 | 3 | 2.14 | 0.00 | 1.48, 2.79 | 5 | ||||
| stevia–control | −0.92 | 0.00 | −1.3, −0.54 | 17 | −1.18 | 0.00 | −1.88, −0.48 | 2 | −1.67 | 0.00 | −2.72, −0.62 | 3 | −0.63 | 0.01 | −1.09, −0.17 | 5 | ||||
| Renal disorder | ||||||||||||||||||||
| control–case | 5.73 | 0.00 | 4.43, 7.03 | 10 | 5.53 | 0.00 | 4.05, 7.02 | 4 | 6.96 | 0.00 | 5.31, 8.61 | 2 | 5.65 | 0.00 | 2.52, 8.77 | 4 | ||||
| stevia–case | 3.80 | 0.00 | 2.63, 4.96 | 10 | 3.97 | 0.00 | 2.21, 5.73 | 4 | 3.15 | 0.01 | 0.69, 5.61 | 2 | 4.06 | 0.00 | 1.72, 6.39 | 4 | ||||
| stevia–control | −1.93 | 0.00 | −2.66, −1.19 | 10 | −1.56 | 0.00 | −2.43, −0.70 | 4 | −3.82 | 0.02 | −7.07, −0.55 | 2 | −1.59 | 0.00 | −2.51, −0.66 | 4 | ||||
| SOD, CAT, GSH, TAC | SOD | CAT | GSH | TAC | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference | Coef | p-value | 95% CI | # Studies | Coef | p-value | 95% CI | # Studies | Coef. | p-value | 95% CI | # Studies | Coef. | p-value | 95% CI | # Studies | Coef. | p-value | 95% CI | # Studies |
| Overall | ||||||||||||||||||||
| control–case | 6.13 | 0.00 | 4.42, 7.84 | 50 | 4.72 | 0.00 | 3.08, 6.37 | 15 | 7.67 | 0.00 | 3.01, 12.33 | 13 | 7.67 | 0.00 | 3.72, 11.61 | 20 | 1.24 | 0.00 | 0.46, 2.03 | 2 |
| stevia–case | 4.39 | 0.00 | 3.03, 5.74 | 50 | 2.77 | 0.00 | 1.39, 4.15 | 15 | 5.73 | 0.00 | 1.97, 9.5 | 13 | 5.89 | 0.00 | 2.79, 8.99 | 20 | 1.44 | 0.12 | −0.39, 3.27 | 2 |
| stevia–control | −1.74 | 0.00 | −2.46, −1.02 | 50 | −1.95 | 0.00 | −3.18, −0.72 | 15 | −1.93 | 0.07 a | −4.07, 0.19 | 13 | −1.77 | 0.00 | −2.92, −0.62 | 20 | 0.19 | 0.79 a | −1.24, 1.62 | 2 |
| Liver | ||||||||||||||||||||
| control–case | −5.34 | 0.00 | 3.73, 6.95 | 31 | 2.93 | 0.00 | 1.59, 4.28 | 6 | 4.73 | 0.00 | 1.85, 7.61 | 5 | 4.56 | 0.00 | 2.04, 7.07 | 9 | ||||
| stevia–case | 2.90 | 0.00 | 2.12, 3.67 | 31 | 2.62 | 0.00 | 0.79, 4.45 | 6 | 3.04 | 0.01 | 0.61, 5.47 | 5 | 3.49 | 0.01 | 0.97, 6.00 | 9 | ||||
| stevia–control | −2.45 | 0.00 | −3.99, −0.9 | 31 | −0.32 | 0.67 a | −1.79, 1.16 | 6 | −1.69 | 0.00 | −2.34, −1.04 | 5 | −1.07 | 0.04 | −2.09, −0.05 | 9 | ||||
| Kidney | ||||||||||||||||||||
| control–case | 5.38 | 0.00 | 2.96, 7.79 | 8 | 3.85 | 0.00 | 2.13, 5.57 | 2 | 4.85 | 0.01 | 1.36, 8.34 | 2 | 6.72 | 0.01 | 1.84, 11.60 | 4 | ||||
| stevia–case | 2.78 | 0.01 | 0.58, 4.99 | 8 | −0.12 | 0.94 | −3.28, 3.05 | 2 | 2.44 | 0.20 | −1.33, 6.21 | 2 | 4.53 | 0.01 | 1.00, 8.05 | 4 | ||||
| stevia–control | −2.60 | 0.00 | −3.46, −1.73 | 8 | −3.96 | 0.00 | −6.12, −1.81 | 2 | −2.41 | 0.00 | −3.35, −1.48 | 2 | −2.19 | 0.00 | −3.67, −0.71 | 4 | ||||
| Intestine (duodenum–Jejunum–ileum) | ||||||||||||||||||||
| control–case | 4.96 | 0.00 | 3.36, 6.56 | 6 | 4.28 | 0.00 | 1.96, 6.61 | 3 | ||||||||||||
| stevia–case | 3.44 | 0.00 | 1.96, 4.91 | 6 | 3.08 | 0.00 | 1.18, 4.98 | 3 | ||||||||||||
| stevia–control | −1.52 | 0.00 | −2.00, −1.05 | 6 | −1.20 | 0.00 | −1.92, −0.48 | 3 | ||||||||||||
| Difference | Coef. | p-Value | 95% CI | # of Studies | |
|---|---|---|---|---|---|
| Leaf Extract | control–case | −5.49 | 0.00 | −7.15, 0.83 | 25 |
| stevia–case | −4.78 | 0.00 | −6.37, −3.19 | 25 | |
| stevia–control | 0.71 | 0.00 | 0.24, 1.17 | 25 | |
| Aqueous | control–case | −3.85 | 0.00 | −5.57, −2.14 | 8 |
| stevia–case | −3.3 | 0.00 | −4.94, −1.65 | 8 | |
| stevia–control | 0.56 | 0.01 | 0.15, 0.96 | 8 | |
| Organic | control–case | −5.68 | 0.00 | −8.97, −2.39 | 6 |
| stevia–case | −5.06 | 0.00 | −6.82, −3.30 | 6 | |
| stevia–control | 0.62 | 0.48 a | −1.10, 2.34 | 6 | |
| Hydroalcoholic | control–case | −8.33 | 0.00 | −14.08, −2.59 | 6 |
| stevia–case | −7.35 | 0.01 | −13.01, −1.69 | 6 | |
| stevia–control | 0.98 | 0.01 | 0.25, 1.71 | 6 | |
| Diabetes melittus | control–case | −7.33 | 0.00 | −12.25, 2.42 | 13 |
| stevia–case | −6.93 | 0.00 | −11.73, −2.12 | 13 | |
| stevia–control | 0.41 | 0.12 a | −0.10, 0.92 | 13 | |
| Liver injury | control–case | −6.33 | 0.00 | −10.04, −2.61 | 6 |
| stevia–case | −4.37 | 0.00 | −6.61, −2.13 | 6 | |
| stevia–control | 1.95 | 0.02 | 0.35, 3.55 | 6 | |
| Renal disorder | control–case | −4.53 | 0.00 | −5.77, −3.29 | 5 |
| stevia–case | −3.82 | 0.00 | −5.11, −2.53 | 5 | |
| stevia–control | 0.71 | 0.01 | 0.17, 1.25 | 5 | |
| Blood | control–case | −1.81 | 0.15 | −4.30, 0.67 | 2 |
| stevia–case | −1.32 | 0.19 | −3.29, 0.64 | 2 | |
| stevia–control | 0.49 | 0.26 a | −0.36, 1.35 | 2 | |
| Liver | control–case | −6.96 | 0.00 | −9.72, −4.19 | 11 |
| stevia–case | −5.91 | 0.00 | −8.66, −3.16 | 11 | |
| stevia–control | 1.05 | 0.04 | 0.04, 2.05 | 11 | |
| Kidney | control–case | −3.13 | 0.00 | −4.59, −1.66 | 4 |
| stevia–case | −2.45 | 0.00 | −3.91, −0.99 | 4 | |
| stevia–control | 0.68 | 0.13 a | −0.21, 1.57 | 4 | |
| Intestine (duodenum–jejunum–ileum) | control–case | −4.99 | 0.00 | −7.00, −2.98 | 3 |
| stevia–case | −4.64 | 0.00 | −6.54, −2.74 | 3 | |
| stevia–control | 0.35 | 0.23 a | −0.23, 0.93 | 3 | |
| Pancreas | control–case | −3.91 | 0.02 | −7.11, −0.71 | 2 |
| stevia–case | −3.89 | 0.01 | −6.64, −1.13 | 2 | |
| stevia–control | 0.02 | 0.95 a | −0.81, 0.85 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaefthimiou, M.; Kontou, P.I.; Bagos, P.G.; Braliou, G.G. Antioxidant Activity of Leaf Extracts from Stevia rebaudiana Bertoni Exerts Attenuating Effect on Diseased Experimental Rats: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3325. https://doi.org/10.3390/nu15153325
Papaefthimiou M, Kontou PI, Bagos PG, Braliou GG. Antioxidant Activity of Leaf Extracts from Stevia rebaudiana Bertoni Exerts Attenuating Effect on Diseased Experimental Rats: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(15):3325. https://doi.org/10.3390/nu15153325
Chicago/Turabian StylePapaefthimiou, Maria, Panagiota I. Kontou, Pantelis G. Bagos, and Georgia G. Braliou. 2023. "Antioxidant Activity of Leaf Extracts from Stevia rebaudiana Bertoni Exerts Attenuating Effect on Diseased Experimental Rats: A Systematic Review and Meta-Analysis" Nutrients 15, no. 15: 3325. https://doi.org/10.3390/nu15153325
APA StylePapaefthimiou, M., Kontou, P. I., Bagos, P. G., & Braliou, G. G. (2023). Antioxidant Activity of Leaf Extracts from Stevia rebaudiana Bertoni Exerts Attenuating Effect on Diseased Experimental Rats: A Systematic Review and Meta-Analysis. Nutrients, 15(15), 3325. https://doi.org/10.3390/nu15153325






