Enhancing Supplemental Effects of Acute Natural Antioxidant Derived from Yeast Fermentation and Vitamin C on Sports Performance in Triathlon Athletes: A Randomized, Double-Blinded, Placebo-Controlled, Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Dietary Supplementation
2.4. Measurements
2.4.1. Anthropometry and Body Composition
2.4.2. Metabolic Function
2.4.3. Skeletal Muscle Oxygenation
2.4.4. Cardiac Function
2.4.5. Blood Samples
2.4.6. RPE
2.5. Statistical Analysis
3. Results
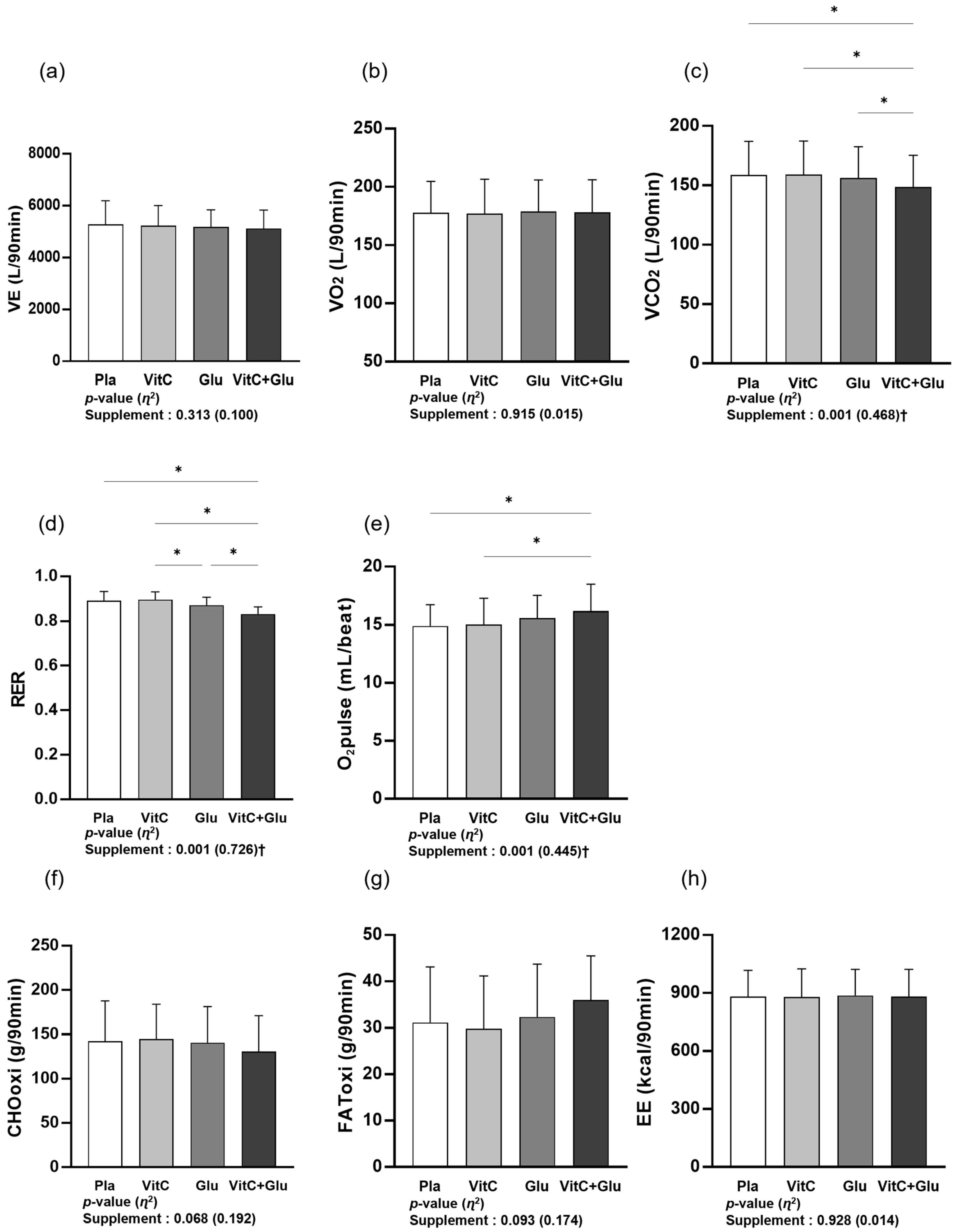
3.1. Metabolic Function
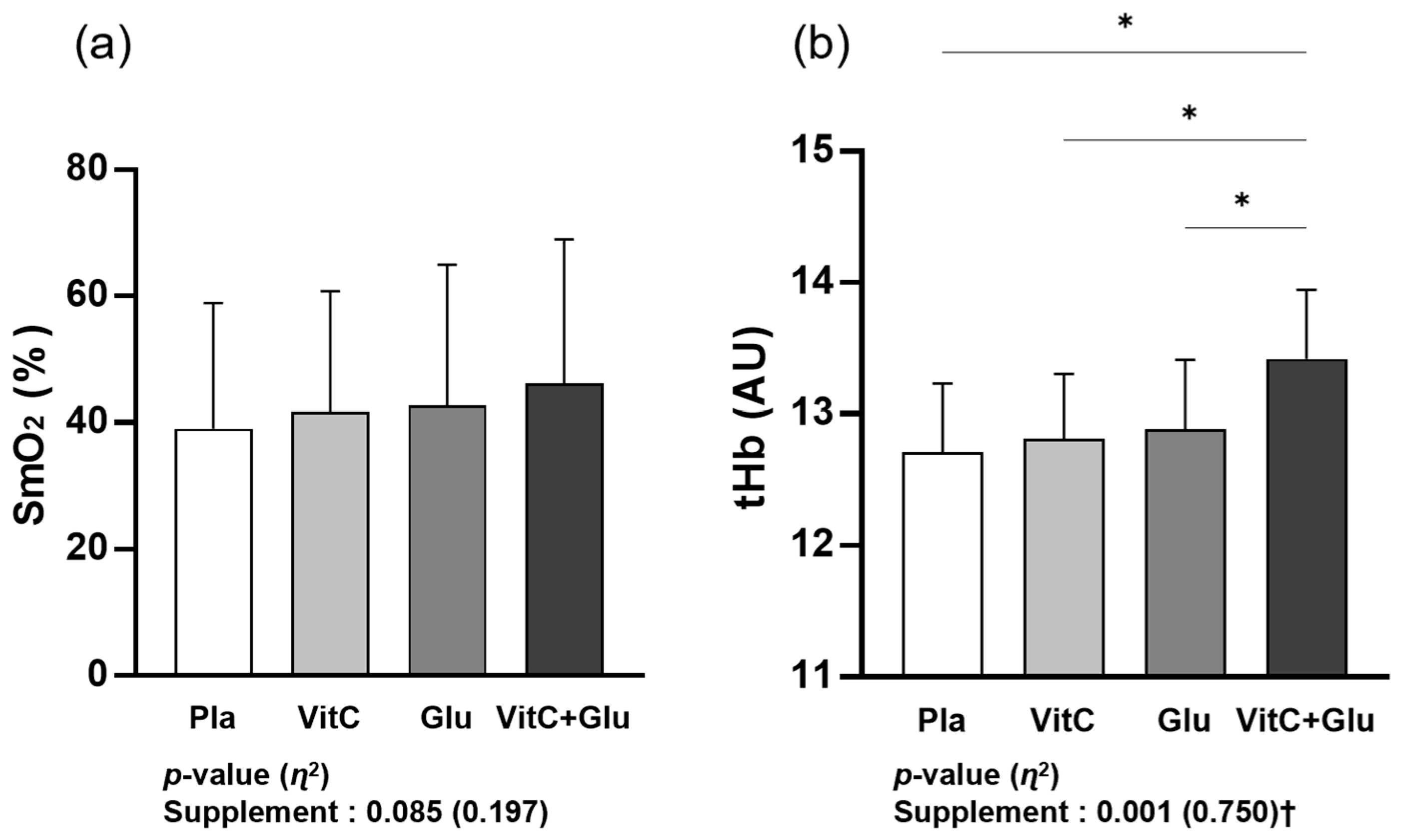
3.2. Skeletal Muscle Oxygenation
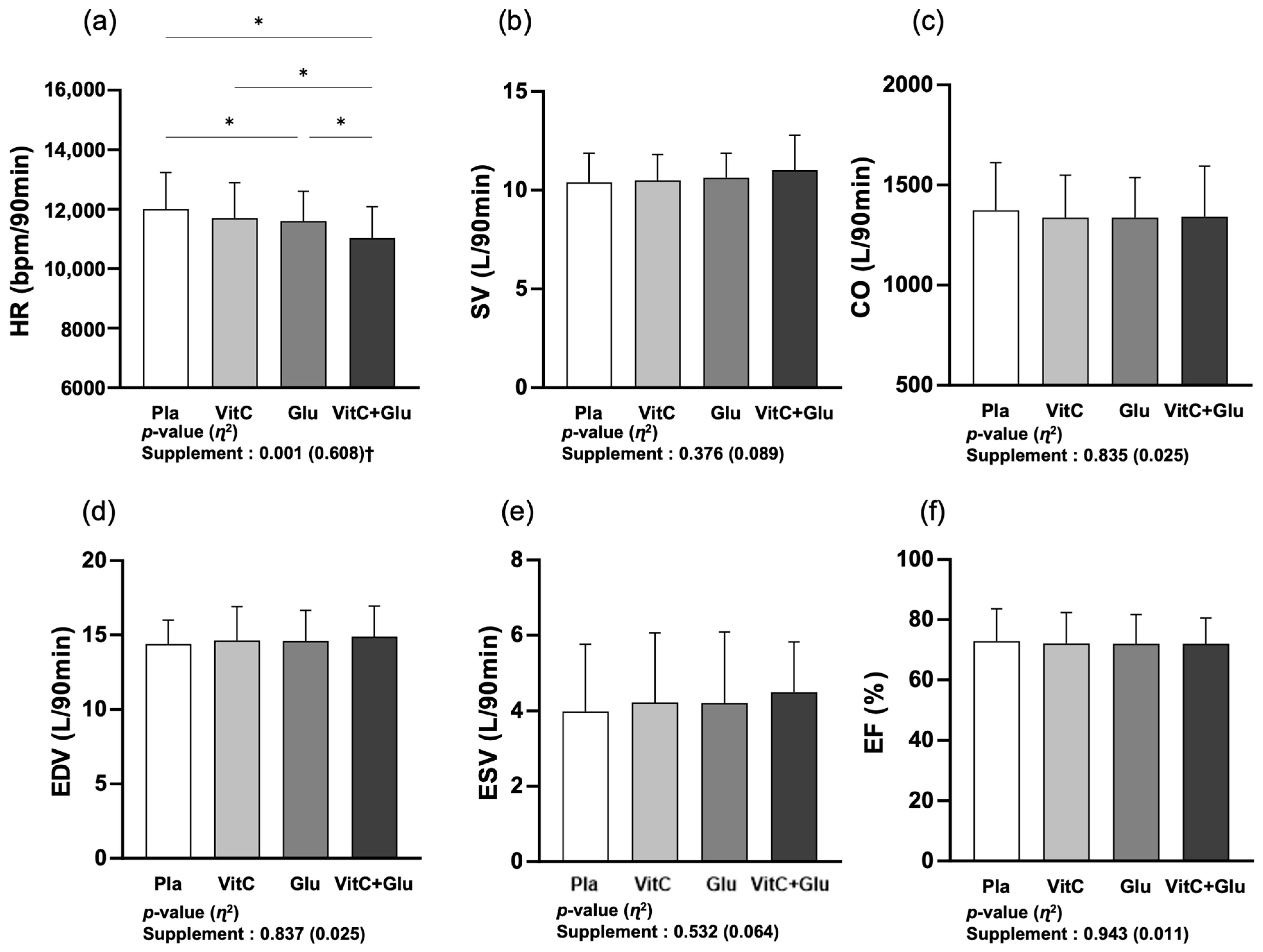
3.3. Cardiac Function
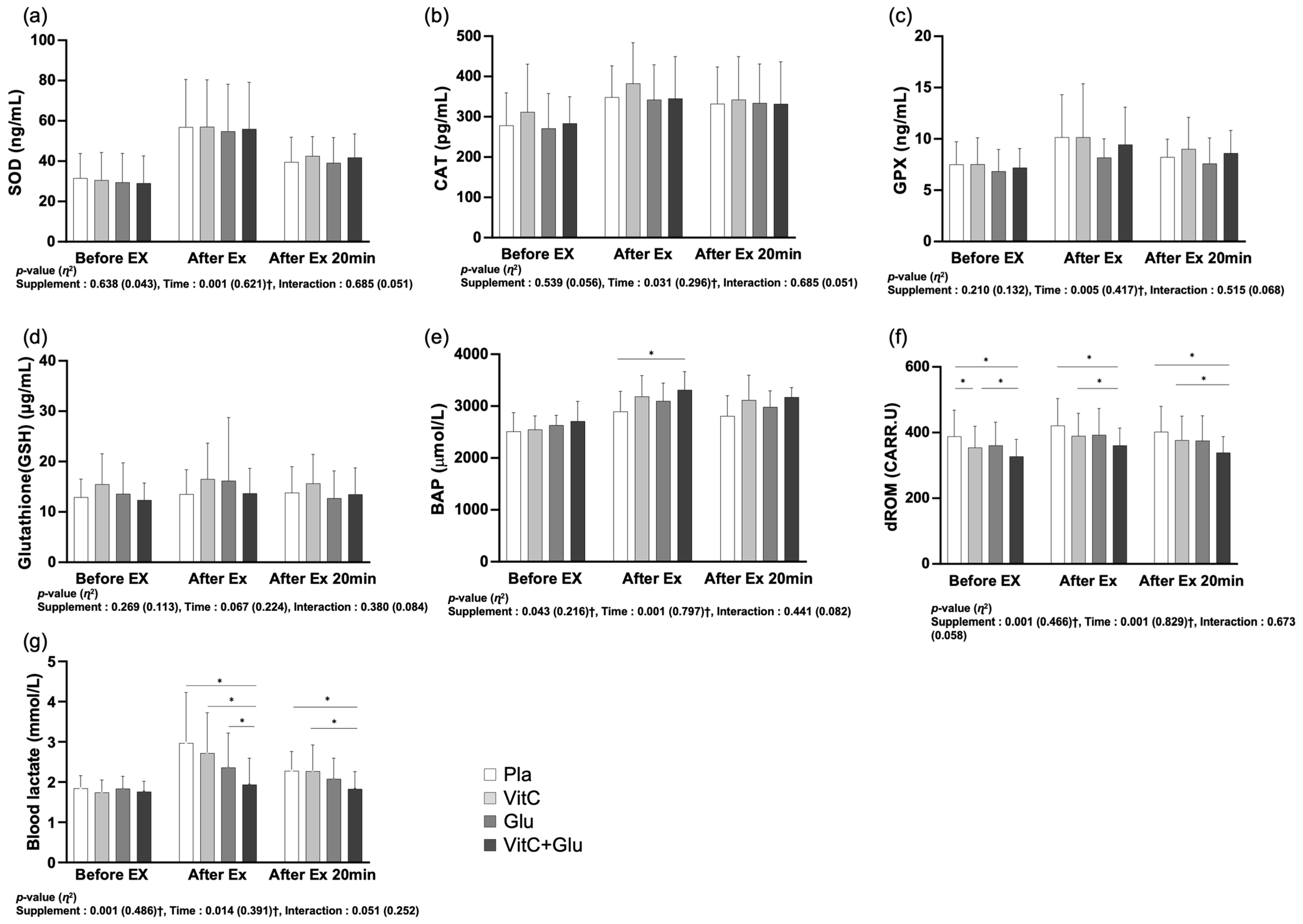
3.4. Blood Samples
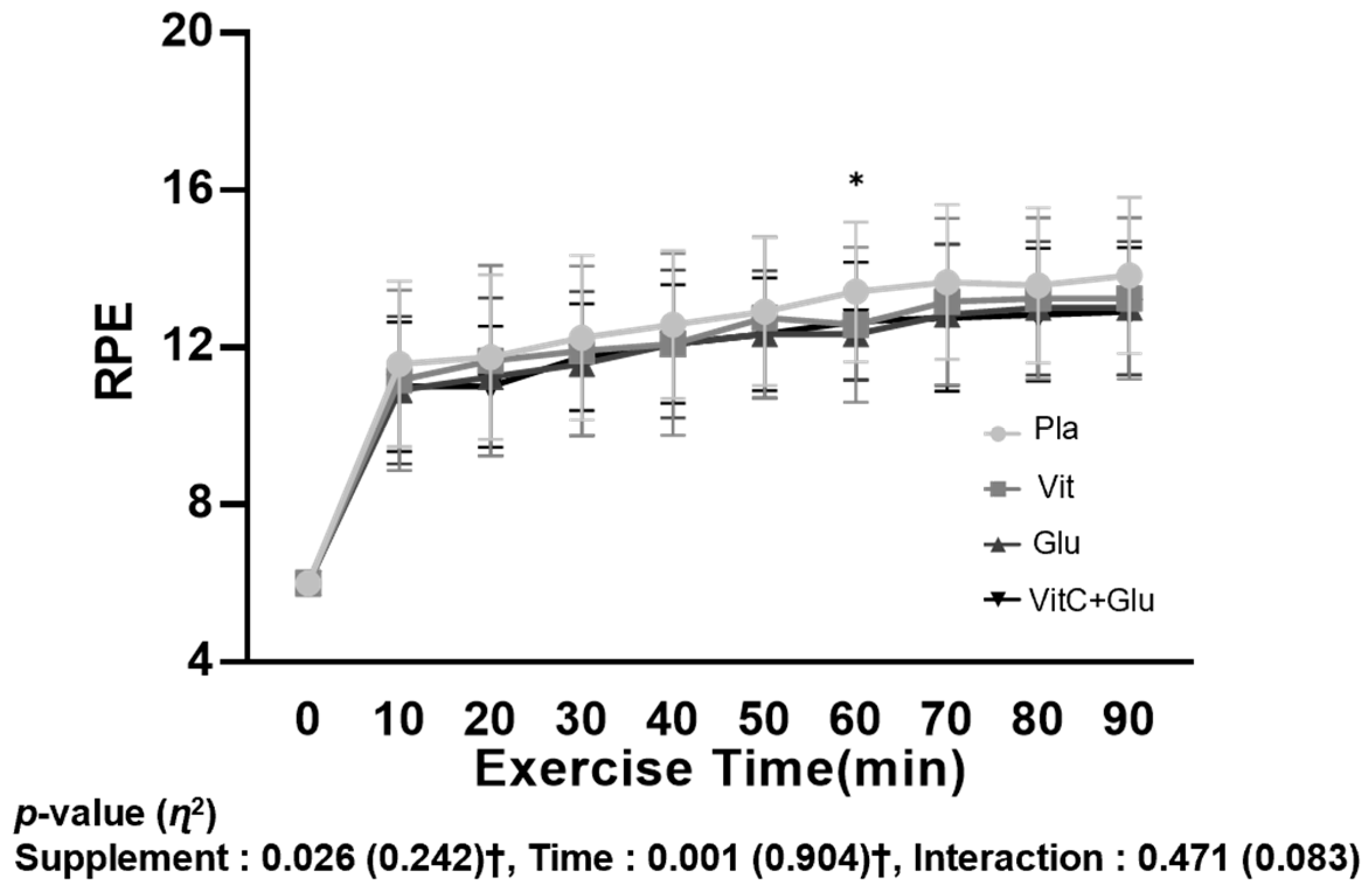
3.5. RPE
4. Discussion
4.1. Metabolic Function
4.2. Skeletal Muscle Oxygenation
4.3. Cardiac Function
4.4. Antioxidant Function and Blood Lactate
4.5. RPE
4.6. Strength and Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, A.N.; Lima, L.C.F. The Association between Physical Exercise and Reactive Oxygen Species (ROS) Production. J. Sport. Med. Stud. Doping. 2015, 5, 1. [Google Scholar] [CrossRef]
- Goldfarb, A.H.; Bloomer, R.J.; McKenzie, M.J. Combined Antioxidant Treatment Effects on Blood Oxidative Stress after Eccentric Exercise. Med. Sci. Sports Exerc. 2005, 37, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Urso, M.L.; Clarkson, P.M. Oxidative Stress, Exercise, and Antioxidant Supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef]
- Muñoz, M.E.; Galan, A.I.; Palacios, E.; Diez, M.A.; Muguerza, B.; Cobaleda, C.; Calvo, J.I.; Aruoma, O.I.; Sanchez-Garcia, I.; Jimenez, R. Effect of an Antioxidant Functional Food Beverage on Exercise-Induced Oxidative Stress: A Long-Term and Large-Scale Clinical Intervention Study. Toxicology 2010, 278, 101–111. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.; Bloomer, R.J. Acute Exercise and Oxidative Stress: A 30 Year History. Dyn. Med. 2009, 8, 1. [Google Scholar] [CrossRef]
- Vassalle, C.; Pingitore, A.; Giuseppe, R.D.; Vigna, L.; Bamonti, F. Biomarkers Part II: Biomarkers to Estimate Bioefficacy of Dietary/Supplemental Antioxidants in Sport. In Antioxidants in Sport Nutrition. Boca Raton (FL); Lamprecht, M., Ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Clarkson, P.M.; Thompson, H.S. Antioxidants: What Role Do They Play in Physical Activity and Health? Am. J. Clin. Nutr. 2000, 72, 637S–646S. [Google Scholar] [CrossRef]
- Chan, K.M.; Decker, E.A.; Feustman, C. Endogenous Skeletal Muscle Antioxidants. Crit. Rev. Food Sci. 1994, 34, 403–426. [Google Scholar] [CrossRef]
- Sen, C.K.; Packer, L. Thiol Homeostasis and Supplements in Physical Exercise 1, 2, 3, 4. Am. J. Clin. Nutr. 2000, 72, S653–S669. [Google Scholar] [CrossRef] [PubMed]
- Ylikoski, T.; Piirainen, J.; Hanninen, O.; Penttinen, J. The Effect of Coenzyme Q10 on the Exercise Performance of Cross-Country Skiers. Mol. Asp. Med. 1997, 18, 283–290. [Google Scholar] [CrossRef]
- Mizuno, K.; Tanaka, M.; Nozaki, S.; Mizuma, H.; Ataka, S.; Tahara, T.; Sugino, T.; Shirai, T.; Kajimoto, Y.; Kuratsune, H.; et al. Antifatigue Effects of Coenzyme Q10 during Physical Fatigue. Nutrition 2008, 24, 293–299. [Google Scholar] [CrossRef]
- Cooke, M.; Iosia, M.; Buford, T.; Shelmadine, B.; Hudson, G.; Kerksick, C.; Rasmussen, C.; Greenwood, M.; Leutholtz, B.; Willoughby, D.; et al. Effects of Acute and 14-Day Coenzyme Q10 Supplementation on Exercise Performance in Both Trained and Untrained Individuals. J. Int. Soc. Sport Nutr. 2008, 5, 8. [Google Scholar] [CrossRef]
- Gökbel, H.; Gül, B.; Belviranl, M.; Okudan, N. The Effects of Coenzyme Q10 Supplementation on Performance During Repeated Bouts of Supramaximal Exercise in Sedentary Men. J. Strength Cond. Res. 2010, 24, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Davis, B. Quercetin Increases Brain and Muscle Mitochondrial Biogenesis and Exercise Tolerance. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R1071–R1077. [Google Scholar] [CrossRef]
- Davis, J.M.; Carlstedt, C.J.; Chen, S.; Carmichael, M.D.; Murphy, E.A. The Dietary Flavonoid Quercetin Increases VO2max and Endurance Capacity. Int. J. Sport Nutr. Exerc. 2010, 20, 56–62. [Google Scholar] [CrossRef]
- Lafay, S.; Jan, C.; Nardon, K.; Lemaire, B.; Ibarra, A.; Roller, M.; Houvenaeghel, M.; Juhel, C.; Cara, L. Grape Extract Improves Antioxidant Status and Physical Performance in Elite Male Athletes. J. Sports Sci. Med. 2008, 8, 468–480. [Google Scholar]
- Bloomer, R.J.; Goldfarb, A.H.; McKenzie, M.J. Oxidative Stress Response to Aerobic Exercise. Med. Sci. Sports Exerc. 2006, 38, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Bryer, S.C.; Goldfarb, A.H. Effect of High Dose Vitamin C Supplementation on Muscle Soreness, Damage, Function, and Oxidative Stress to Eccentric Exercise. Int. J. Sport Nutr. Exerc. 2006, 16, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, A.H.; Patrick, S.W.; Bryer, S.; You, T. Vitamin C Supplementation Affects Oxidative-Stress Blood Markers in Response to a 30-Minute Run at 75% VO2max. Int. J. Sport Nutr. Exerc. 2005, 15, 279–290. [Google Scholar] [CrossRef]
- Popovic, L.M.; Mitic, N.R.; Miric, D.; Bisevac, B.; Miric, M.; Popovic, B. Influence of Vitamin C Supplementation on Oxidative Stress and Neutrophil Inflammatory Response in Acute and Regular Exercise. Oxid. Med. Cell. Longev. 2015, 2015, 295497. [Google Scholar] [CrossRef]
- Aguiló, A.; Tauler, P.; Sureda, A.; Cases, N.; Tur, J.; Pons, A. Antioxidant Diet Supplementation Enhances Aerobic Performance in Amateur Sportsmen. J. Sport Sci. 2007, 25, 1203–1210. [Google Scholar] [CrossRef]
- Gauche, E.; Lepers, R.; Rabita, G.; Leveque, J.-M.; Bishop, D.; Brisswalter, J.; Hausswirth, C. Vitamin and Mineral Supplementation and Neuromuscular Recovery after a Running Race. Med. Sci. Sports Exerc. 2006, 38, 2110–2117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jourkesh, M.; Ostojic, S.M.; Azarbayjani, M.A. The Effects of Vitamin E and Vitamin C Supplementation on Bioenergetics Index. Res. Sports Med. 2007, 15, 249–256. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.C.X.; Rosa, F.T.; Simões-Ambrósio, L.; Jordao, A.A.; Deminice, R. Antioxidant Vitamin Supplementation Prevents Oxidative Stress but Does Not Enhance Performance in Young Football Athletes. Nutrition 2019, 63, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Falvo, M.J.; Schilling, B.K.; Smith, W.A. Prior Exercise and Antioxidant Supplementation: Effect on Oxidative Stress and Muscle Injury. J. Int. Soc. Sport Nutr. 2007, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.-C.; Domenech, E.; Viña, J. Moderate Exercise Is an Antioxidant: Upregulation of Antioxidant Genes by Training. Free Radic. Bio. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants Prevent Health-Promoting Effects of Physical Exercise in Humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]
- Yfanti, C.; Nielsen, A.R.; Åkerström, T.; Nielsen, S.; Rose, A.J.; Richter, E.A.; Lykkesfeldt, J.; Fischer, C.P.; Pedersen, B.K. Effect of Antioxidant Supplementation on Insulin Sensitivity in Response to Endurance Exercise Training. Am. J. Physiol.-Endoc. Metab. 2011, 300, E761–E770. [Google Scholar] [CrossRef]
- Peternelj, T.-T.; Coombes, J.S. Antioxidant Supplementation during Exercise Training. Sports Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Pizzorno, J. Glutathione! Integr. Med. Encinitas Calif. 2014, 13, 8–12. [Google Scholar]
- Gould, R.L.; Pazdro, R. Impact of Supplementary Amino Acids, Micronutrients, and Overall Diet on Glutathione Homeostasis. Nutrients 2019, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Pyke, S.; Lew, H.; Quintanilha, A. Severe Depletion in Liver Glutathione during Physical Exercise. Biochem. Biophys. Res. Co. 1986, 139, 926–931. [Google Scholar] [CrossRef]
- Lew, H.; Pyke, S.; Quintanilha, A. Changes in the Glutathione Status of Plasma, Liver and Muscle Following Exhaustive Exercise in Rats. FEBS Lett. 1985, 185, 262–266. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Ji, L.L. Glutathone and Glutathione Ethyl Ester Supplementation of Mice Alter Glutathione Homeostasis during Exercise 1, 2. J. Nutr. 1998, 128, 2420–2426. [Google Scholar] [CrossRef]
- Novelli, G.P.; Falsini, S.; Bracciotti, G. Exogenous Glutathione Increases Endurance to Muscle Effort in Mice. Pharmacol. Res. 1991, 23, 149–155. [Google Scholar] [CrossRef]
- Aoi, W.; Ogaya, Y.; Takami, M.; Konishi, T.; Sauchi, Y.; Park, E.Y.; Wada, S.; Sato, K.; Higashi, A. Glutathione Supplementation Suppresses Muscle Fatigue Induced by Prolonged Exercise via Improved Aerobic Metabolism. J. Int. Soc. Sport Nutr. 2015, 12, 7. [Google Scholar] [CrossRef]
- Winkler, B.S.; Orselli, S.M.; Rex, T.S. The Redox Couple between Glutathione and Ascorbic Acid: A Chemical and Physiological Perspective. Free Radic. Bio. Med. 1994, 17, 333–349. [Google Scholar] [CrossRef]
- Meister, A. The Antioxidant Effects of Glutathione and Ascorbic Acid. In Oxidative Stress, Cell Activation and Viral Infection; Pasquier, C., Olivier, R.Y., Auclair, C., Packer, L., Eds.; Birkhäuser: Basel, Switzerland, 1994; pp. 101–111. [Google Scholar] [CrossRef]
- Meister, A. Glutathione-Ascorbic Acid Antioxidant System in Animals. J. Biol. Chem. 1994, 269, 9397–9400. [Google Scholar] [CrossRef]
- Waly, M.I.; Al-Attabi, Z.; Guizani, N. Low Nourishment of Vitamin C Induces Glutathione Depletion and Oxidative Stress in Healthy Young Adults. Prev. Nutr. Food Sci. 2015, 20, 198–203. [Google Scholar] [CrossRef]
- Johnston, C.; Meyer, C.; Srilakshmi, J. Vitamin C Elevates Red Blood Cell Glutathione in Healthy Adults. Am. J. Clin. Nutr. 1993, 58, 103–105. [Google Scholar] [CrossRef]
- Lenton, K.J.; Sané, A.T.; Therriault, H.; Cantin, A.M.; Payette, H.; Wagner, J.R. Vitamin C Augments Lymphocyte Glutathione in Subjects with Ascorbate Deficiency 1, 2, 3. Am. J. Clin. Nutr. 2003, 77, 189–195. [Google Scholar] [CrossRef]
- Sastre, J.; Asensi, M.; Gasco, E.; Pallardo, F.V.; Ferrero, J.A.; Furukawa, T.; Vina, J. Exhaustive Physical Exercise Causes Oxidation of Glutathione Status in Blood: Prevention by Antioxidant Administration. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1992, 263, R992–R995. [Google Scholar] [CrossRef]
- Silva, A.F.; Aghidemand, M.H.; Kharatzadeh, M.; Ahmadi, V.K.; Oliveira, R.; Clemente, F.M.; Badicu, G.; Murawska-Ciałowicz, E. Effects of High-Intensity Resistance Training on Physical Fitness, Hormonal and Antioxidant Factors: A Randomized Controlled Study Conducted on Young Adult Male Soccer Players. Biology 2022, 11, 909. [Google Scholar] [CrossRef]
- Cohen, J. The Effect Size. In Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 1988; pp. 77–83. [Google Scholar]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use. Ann. Intern. Med. 2004, 140, 533. [Google Scholar] [CrossRef]
- Yimcharoen, M.; Kittikunnathum, S.; Suknikorn, C.; Nak-on, W.; Yeethong, P.; Anthony, T.G.; Bunpo, P. Effects of Ascorbic Acid Supplementation on Oxidative Stress Markers in Healthy Women Following a Single Bout of Exercise. J. Int. Soc. Sport Nutr. 2019, 16, 2. [Google Scholar] [CrossRef]
- Jagim, A.R.; Jones, M.T.; Wright, G.A.; Antoine, C.S.; Kovacs, A.; Oliver, J.M. The Acute Effects of Multi-Ingredient Pre-Workout Ingestion on Strength Performance, Lower Body Power, and Anaerobic Capacity. J. Int. Soc. Sports Nutr. 2016, 13, 11. [Google Scholar] [CrossRef]
- Rogers, J.M.; Gills, J.; Gray, M. Acute Effects of Nitrosigine® and Citrulline Malate on Vasodilation in Young Adults. J. Int. Soc. Sports Nutr. 2020, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Kerley, C.P.; Cahill, K.; Bolger, K.; McGowan, A.; Burke, C.; Faul, J.; Cormican, L. Dietary Nitrate Supplementation in COPD: An Acute, Double-Blind, Randomized, Placebo-Controlled, Crossover Trial. Nitric Oxide 2015, 44, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.J.; O’Brien, K.A.; Tanner, R.J.; Polkey, J.I.; Minnion, M.; Feelisch, M.; Polkey, M.I.; Edwards, L.M.; Hopkinson, N.S. Acute Dietary Nitrate Supplementation and Exercise Performance in COPD: A Double-Blind, Placebo-Controlled, Randomised Controlled Pilot Study. PLoS ONE 2015, 10, e0144504. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of Substrate Oxidation during Exercise by Means of Gas Exchange Measurements. Int. J. Sports Med. 2005, 26 (Suppl. 1), S28–S37. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Corte, C.; Swan, P.D. Marginal Vitamin C Status Is Associated with Reduced Fat Oxidation during Submaximal Exercise in Young Adults. Nutr. Metab. 2006, 3, 35. [Google Scholar] [CrossRef]
- Søndergård, S.D.; Cintin, I.; Kuhlman, A.B.; Morville, T.H.; Bergmann, M.L.; Kjær, L.K.; Poulsen, H.E.; Giustarini, D.; Rossi, R.; Dela, F.; et al. The Effects of 3 Weeks of Oral Glutathione Supplementation on Whole Body Insulin Sensitivity in Obese Males with and without Type 2 Diabetes: A Randomized Trial. Appl. Physiol. Nutr. Metab. 2021, 46, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.L.; Hattersley, J.; Frost, G.S.; Chambers, E.S.; Wallis, G.A. Maximal Fat Oxidation during Exercise Is Positively Associated with 24-Hour Fat Oxidation and Insulin Sensitivity in Young, Healthy Men. J. Appl. Physiol. 2015, 118, 1415–1422. [Google Scholar] [CrossRef]
- Chai, S.C.; Davis, K.; Zhang, Z.; Zha, L.; Kirschner, K.F. Effects of Tart Cherry Juice on Biomarkers of Inflammation and Oxidative Stress in Older Adults. Nutrients 2019, 11, 228. [Google Scholar] [CrossRef]
- Fitzhugh, D.J.; Shan, S.; Dewhirst, M.W.; Hale, L.P. Bromelain Treatment Decreases Neutrophil Migration to Sites of Inflammation. Clin. Immunol. 2008, 128, 66–74. [Google Scholar] [CrossRef]
- Ferrari, M.; Muthalib, M.; Quaresima, V. The Use of Near-Infrared Spectroscopy in Understanding Skeletal Muscle Physiology: Recent Developments. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2011, 369, 4577–4590. [Google Scholar] [CrossRef]
- Bailey, S.J.; Wilkerson, D.P.; DiMenna, F.J.; Jones, A.M. Influence of Repeated Sprint Training on Pulmonary O2 Uptake and Muscle Deoxygenation Kinetics in Humans. J. Appl. Physiol. 2009, 106, 1875–1887. [Google Scholar] [CrossRef]
- Fulford, J.; Winyard, P.G.; Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; Jones, A.M. Influence of Dietary Nitrate Supplementation on Human Skeletal Muscle Metabolism and Force Production during Maximum Voluntary Contractions. Pflügers Arch.—Eur. J. Physiol. 2013, 465, 517–528. [Google Scholar] [CrossRef]
- Conley, K.E.; Lindstedt, S.L.; Hoppeler, H.H. Mitochondria to Motion: Optimizing Oxidative Phosphorylation to Improve Exercise Performance. J. Exp. Biol. 2016, 219, 243–249. [Google Scholar] [CrossRef]
- Pekas, E.J.; Shin, J.; Headid, R.J.; Son, W.-M.; Layec, G.; Yadav, S.K.; Scott, S.D.; Park, S.-Y. Combined Anthocyanins and Bromelain Supplement Improves Endothelial Function and Skeletal Muscle Oxygenation Status in Adults: A Double-Blind Placebo-Controlled Randomised Crossover Clinical Trial. Brit. J. Nutr. 2021, 125, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.F.; Nylander, E.; Gad, P.; Areskog, N. Non-autonomic Component in Bradycardia of Endurance Trained Men at Rest and during Exercise. Acta Physiol. Scand. 1980, 109, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, R.L.; Bauer, T.A.; Harrall, K.; Moreau, K.; Ozemek, C.; Herlache, L.; McMillin, S.; Huebschmann, A.G.; Dorosz, J.; Reusch, J.E.B.; et al. Acute Vitamin C Improves Cardiac Function, Not Exercise Capacity, in Adults with Type 2 Diabetes. Diabetol. Metab. Syndr. 2018, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Csányi, G.; Miller, F.J., Jr. Oxidative Stress in Cardiovascular Disease. Int. J. Mol. Sci. 2014, 15, 6002–6008. [Google Scholar] [CrossRef] [PubMed]
- Matuz-Mares, D.; Riveros-Rosas, H.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Glutathione Participation in the Prevention of Cardiovascular Diseases. Antioxidants 2021, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Atalay, M.; Hanninen, O. Exercise-Induced Oxidative Stress: Glutathione Supplementation and Deficiency. J. Appl. Physiol. 1994, 77, 2177–2187. [Google Scholar] [CrossRef]
- Sen, C.K.; Rankinen, T.; Vaisanen, S.; Rauramaa, R. Oxidative Stress after Human Exercise: Effect of N-Acetylcysteine Supplementation. J. Appl. Physiol. 1994, 76, 2570–2577. [Google Scholar] [CrossRef]
- Khassaf, M.; McArdle, A.; Esanu, C.; Vasilaki, A.; McArdle, F.; Griffiths, R.D.; Brodie, D.A.; Jackson, M.J. Effect of Vitamin C Supplements on Antioxidant Defence and Stress Proteins in Human Lymphocytes and Skeletal Muscle. J. Physiol. 2003, 549, 645–652. [Google Scholar] [CrossRef]
- Yfanti, C.; Fischer, C.P.; Nielsen, S.; Åkerström, T.; Nielsen, A.R.; Veskoukis, A.S.; Kouretas, D.; Lykkesfeldt, J.; Pilegaard, H.; Pedersen, B.K. Role of Vitamin C and E Supplementation on IL-6 in Response to Training. J. Appl. Physiol. 2012, 112, 990–1000. [Google Scholar] [CrossRef]
- Park, E.Y.; Shimura, N.; Konishi, T.; Sauchi, Y.; Wada, S.; Aoi, W.; Nakamura, Y.; Sato, K. Increase in the Protein-Bound Form of Glutathione in Human Blood after the Oral Administration of Glutathione. J. Agric. Food Chem. 2014, 62, 6183–6189. [Google Scholar] [CrossRef]
- Allen, J.; Bradley, R.D. Effects of Oral Glutathione Supplementation on Systemic Oxidative Stress Biomarkers in Human Volunteers. J. Altern. Complement. Med. 2011, 17, 827–833. [Google Scholar] [CrossRef]
- Witschi, A.; Reddy, S.; Stofer, B.; Lauterburg, B.H. The Systemic Availability of Oral Glutathione. Eur. J. Clin. Pharmacol. 1992, 43, 667–669. [Google Scholar] [CrossRef]
- Kelly, R.P.; Yeo, K.P.; Isaac, H.B.; Lee, C.-Y.J.; Huang, S.H.; Teng, L.; Halliwell, B.; Wise, S.D. Lack of Effect of Acute Oral Ingestion of Vitamin C on Oxidative Stress, Arterial Stiffness or Blood Pressure in Healthy Subjects. Free Radic. Res. 2008, 42, 514–522. [Google Scholar] [CrossRef]
- Wilkinson, I.B.; Megson, I.L.; MacCallum, H.; Sogo, N.; Cockcroft, J.R.; Webb, D.J. Oral Vitamin C Reduces Arterial Stiffness and Platelet Aggregation in Humans. J. Cardiovasc. Pharm. 1999, 34, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Robaczewska, J.; Kedziora-Kornatowska, K.; Kozakiewicz, M.; Zary-Sikorska, E.; Pawluk, H.; Pawliszak, W.; Kedziora, J. Role of Glutathione Metabolism and Glutathione-Related Antioxidant Defense Systems in Hypertension. J. Physiol. Pharmacol. 2012, 67, 331–337. [Google Scholar]
- Juel, C.; Holten, M.K.; Dela, F. Effects of Strength Training on Muscle Lactate Release and MCT1 and MCT4 Content in Healthy and Type 2 Diabetic Humans. J. Physiol. 2004, 556, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; González-Alonso, J.; Graham, T.E.; Saltin, B. Hemodynamics and O2 Uptake during Maximal Knee Extensor Exercise in Untrained and Trained Human Quadriceps Muscle: Effects of Hyperoxia. J. Appl. Physiol. 2004, 97, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Vidal, K.; Robinson, N.; Ives, S.J. Exercise Performance and Physiological Responses: The Potential Role of Redox Imbalance. Physiol. Rep. 2017, 5, e13225. [Google Scholar] [CrossRef]
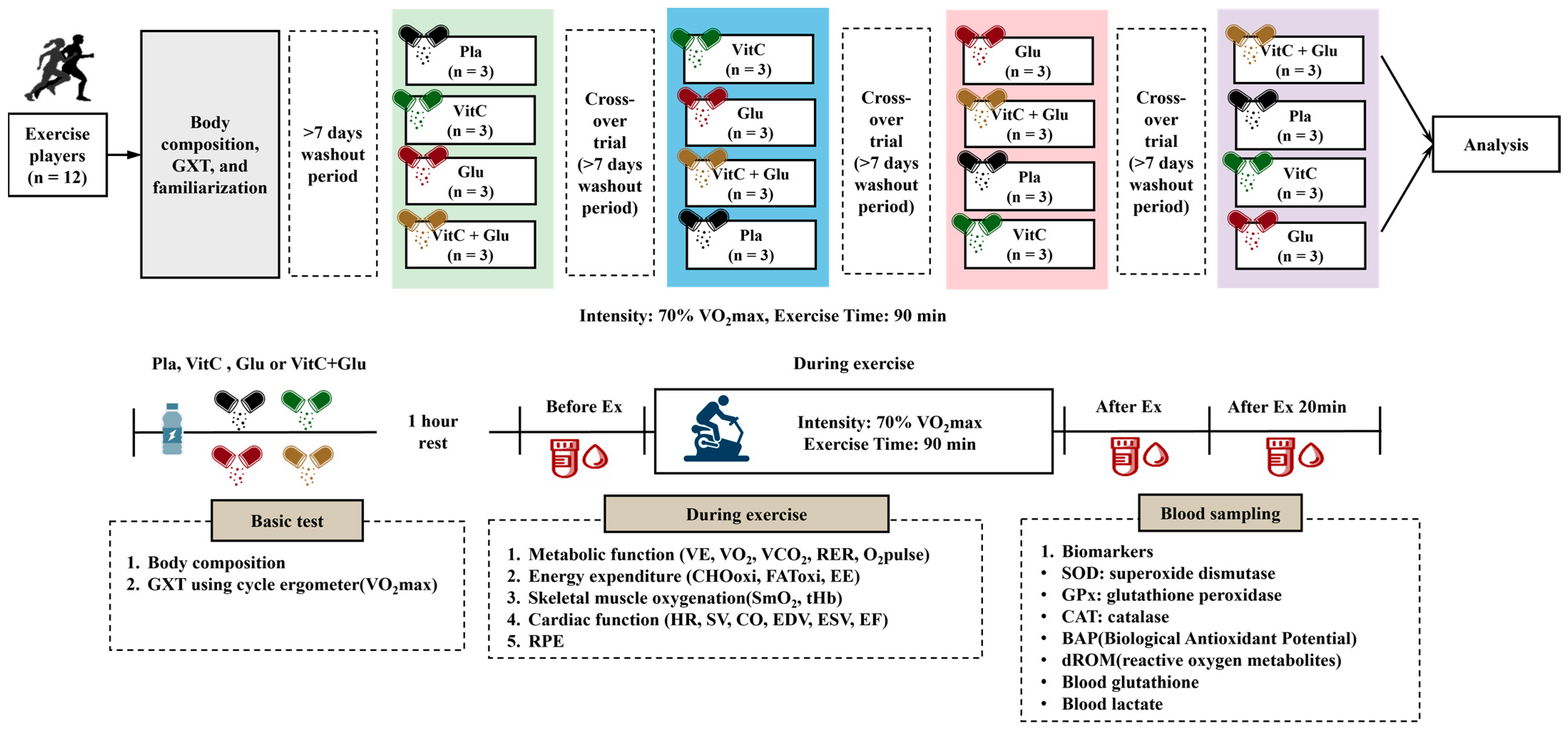
| Sequence | 1st Visit | 2nd Visit | 3rd Visit | 4th Visit | 5th Visit |
|---|---|---|---|---|---|
| A (n = 3) | - | Pla | VitC | Glu | VitC+Glu |
| B (n = 3) | - | VitC | Glu | VitC+Glu | Pla |
| C (n = 3) | - | Glu | VitC+Glu | Pla | VitC |
| D (n = 3) | - | VitC+Glu | VitC | Glu | Glu |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.; Park, H.-Y.; Kim, S.-W.; Sun, Y.; Choi, J.-H.; Seo, J.; Jung, Y.P.; Kim, A.-J.; Kim, J.; Lim, K. Enhancing Supplemental Effects of Acute Natural Antioxidant Derived from Yeast Fermentation and Vitamin C on Sports Performance in Triathlon Athletes: A Randomized, Double-Blinded, Placebo-Controlled, Crossover Trial. Nutrients 2023, 15, 3324. https://doi.org/10.3390/nu15153324
Lee E, Park H-Y, Kim S-W, Sun Y, Choi J-H, Seo J, Jung YP, Kim A-J, Kim J, Lim K. Enhancing Supplemental Effects of Acute Natural Antioxidant Derived from Yeast Fermentation and Vitamin C on Sports Performance in Triathlon Athletes: A Randomized, Double-Blinded, Placebo-Controlled, Crossover Trial. Nutrients. 2023; 15(15):3324. https://doi.org/10.3390/nu15153324
Chicago/Turabian StyleLee, Eunjoo, Hun-Young Park, Sung-Woo Kim, Yerin Sun, Jae-Ho Choi, Jisoo Seo, Yanghoon Peter Jung, Ah-Jin Kim, Jisu Kim, and Kiwon Lim. 2023. "Enhancing Supplemental Effects of Acute Natural Antioxidant Derived from Yeast Fermentation and Vitamin C on Sports Performance in Triathlon Athletes: A Randomized, Double-Blinded, Placebo-Controlled, Crossover Trial" Nutrients 15, no. 15: 3324. https://doi.org/10.3390/nu15153324
APA StyleLee, E., Park, H.-Y., Kim, S.-W., Sun, Y., Choi, J.-H., Seo, J., Jung, Y. P., Kim, A.-J., Kim, J., & Lim, K. (2023). Enhancing Supplemental Effects of Acute Natural Antioxidant Derived from Yeast Fermentation and Vitamin C on Sports Performance in Triathlon Athletes: A Randomized, Double-Blinded, Placebo-Controlled, Crossover Trial. Nutrients, 15(15), 3324. https://doi.org/10.3390/nu15153324








