Eicosapentaenoic Acid and Medium-Chain Triacylglycerol Structured Lipids Improve Endurance Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
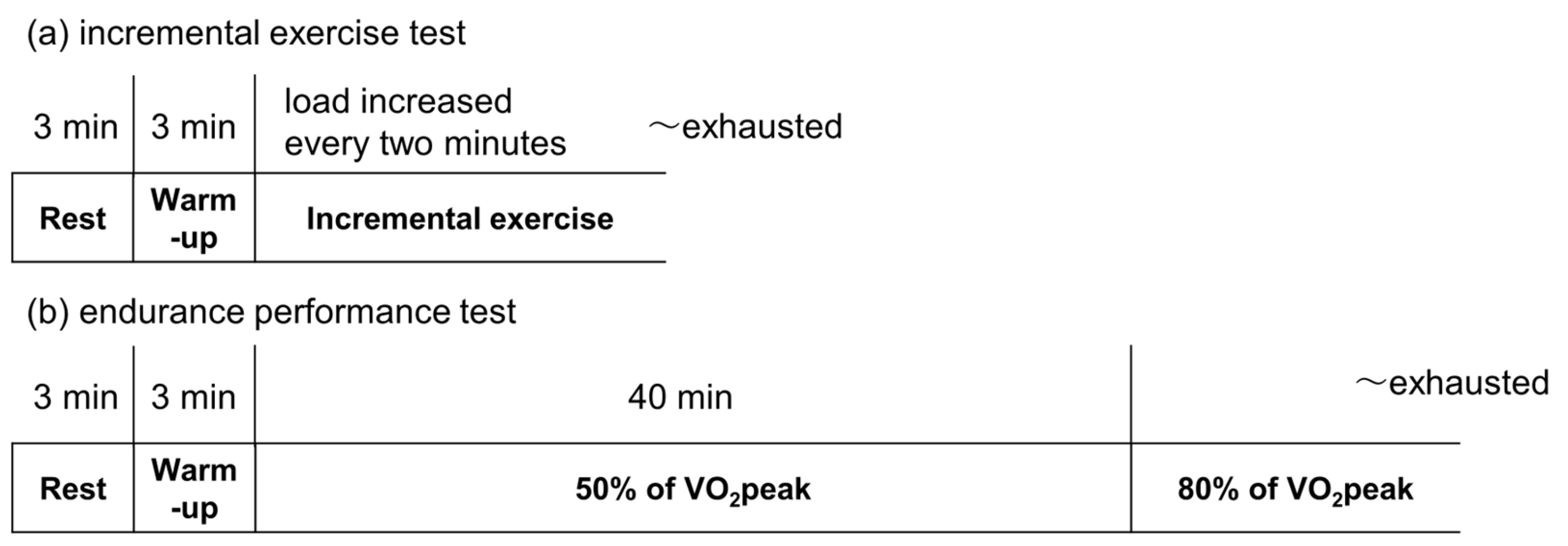
2.2. Study Design
2.3. Supplements
2.4. Measurements of Peak Oxygen Uptake (VO2peak)
2.5. Measurements of Anaerobic Threshold (AT)
2.6. Measurements of Endurance Test
2.7. Statistical Analyses
3. Results
Experimental Trial
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Paschos, G.K.; Magkos, F.; Panagiotakos, D.B.; Votteas, V.; Zampelas, A. Dietary supplementation with flaxseed oil lowers blood pressure in dyslipidaemic patients. Eur. J. Clin. Nutr. 2007, 61, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Li, Q.; Chu, J.; Zeng, W.; Yang, M.; Zhu, S. Effect of n-3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1422–1436. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J. Parenter. Enteral. Nutr. 2015, 39, 18s–32s. [Google Scholar] [CrossRef]
- Johnson, E.J.; McDonald, K.; Caldarella, S.M.; Chung, H.Y.; Troen, A.M.; Snodderly, D.M. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr. Neurosci. 2008, 11, 75–83. [Google Scholar] [CrossRef]
- Albert, C.M.; Campos, H.; Stampfer, M.J.; Ridker, P.M.; Manson, J.E.; Willett, W.C.; Ma, J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N. Engl. J. Med. 2002, 346, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Oomen, C.M.; Feskens, E.J.; Räsänen, L.; Fidanza, F.; Nissinen, A.M.; Menotti, A.; Kok, F.J.; Kromhout, D. Fish consumption and coronary heart disease mortality in Finland, Italy, and The Netherlands. Am. J. Epidemiol. 2000, 151, 999–1006. [Google Scholar] [CrossRef]
- Charnock, J.S.; Abeywardena, M.Y.; Poletti, V.M.; McLennan, P.L. Differences in fatty acid composition of various tissues of the marmoset monkey (Callithrix jacchus) after different lipid supplemented diets. Comp. Biochem. Physiol. Comp. Physiol. 1992, 101, 387–393. [Google Scholar] [CrossRef]
- Pepe, S.; McLennan, P.L. Dietary fish oil confers direct antiarrhythmic properties on the myocardium of rats. J. Nutr. 1996, 126, 34–42. [Google Scholar] [CrossRef]
- Andersson, A.; Nälsén, C.; Tengblad, S.; Vessby, B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am. J. Clin. Nutr. 2002, 76, 1222–1229. [Google Scholar] [CrossRef]
- Fletcher, G.; Eves, F.F.; Glover, E.I.; Robinson, S.L.; Vernooij, C.A.; Thompson, J.L.; Wallis, G.A. Dietary intake is independently associated with the maximal capacity for fat oxidation during exercise. Am. J. Clin. Nutr. 2017, 105, 864–872. [Google Scholar] [CrossRef]
- Sahlin, K.; Mogensen, M.; Bagger, M.; Fernström, M.; Pedersen, P.K. The potential for mitochondrial fat oxidation in human skeletal muscle influences whole body fat oxidation during low-intensity exercise. Am. J. Physiol. -Endocrinol. Metab. 2007, 292, E223–E230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawabata, F.; Neya, M.; Hamazaki, K.; Watanabe, Y.; Kobayashi, S.; Tsuji, T. Supplementation with eicosapentaenoic acid-rich fish oil improves exercise economy and reduces perceived exertion during submaximal steady-state exercise in normal healthy untrained men. Biosci. Biotechnol. Biochem. 2014, 78, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Żebrowska, A.; Mizia-Stec, K.; Mizia, M.; Gąsior, Z.; Poprzęcki, S. Omega-3 fatty acids supplementation improves endothelial function and maximal oxygen uptake in endurance-trained athletes. Eur. J. Sport Sci. 2015, 15, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Hingley, L.; Macartney, M.J.; Brown, M.A.; McLennan, P.L.; Peoples, G.E. DHA-rich Fish Oil Increases the Omega-3 Index and Lowers the Oxygen Cost of Physiologically Stressful Cycling in Trained Individuals. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 335–343. [Google Scholar] [CrossRef]
- Raastad, T.; Høstmark, A.T.; Strømme, S.B. Omega-3 fatty acid supplementation does not improve maximal aerobic power, anaerobic threshold and running performance in well-trained soccer players. Scand. J. Med. Sci. Sports 1997, 7, 25–31. [Google Scholar] [CrossRef]
- Thielecke, F.; Blannin, A. Omega-3 Fatty Acids for Sport Performance-Are They Equally Beneficial for Athletes and Amateurs? A Narrative Review. Nutrients 2020, 12, 3712. [Google Scholar] [CrossRef]
- Metges, C.C.; Wolfram, G. Medium- and long-chain triglycerides labeled with 13C: A comparison of oxidation after oral or parenteral administration in humans. J. Nutr. 1991, 121, 31–36. [Google Scholar] [CrossRef]
- Poppitt, S.D.; Strik, C.M.; MacGibbon, A.K.; McArdle, B.H.; Budgett, S.C.; McGill, A.T. Fatty acid chain length, postprandial satiety and food intake in lean men. Physiol. Behav. 2010, 101, 161–167. [Google Scholar] [CrossRef]
- Mumme, K.; Stonehouse, W. Effects of medium-chain triglycerides on weight loss and body composition: A meta-analysis of randomized controlled trials. J. Acad. Nutr. Diet. 2015, 115, 249–263. [Google Scholar] [CrossRef]
- Nosaka, N.; Suzuki, Y.; Nagatoishi, A.; Kasai, M.; Wu, J.; Taguchi, M. Effect of ingestion of medium-chain triacylglycerols on moderate- and high-intensity exercise in recreational athletes. J. Nutr. Sci. Vitaminol. 2009, 55, 120–125. [Google Scholar] [CrossRef]
- Nosaka, N.; Suzuki, Y.; Suemitsu, H.; Kasai, M.; Kato, K.; Taguchi, M. Medium-chain Triglycerides with Maltodextrin Increase Fat Oxidation during Moderate-intensity Exercise and Extend the Duration of Subsequent High-intensity Exercise. J. Oleo Sci. 2018, 67, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Høy, C.E. Intestinal absorption of specific structured triacylglycerols. J. Lipid Res. 2001, 42, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Tomari, Y.; Sugano, M.; Watanabe, S.; Nagata, J. Lymphatic absorption of structured glycerolipids containing medium-chain fatty acids and linoleic acid, and their effect on cholesterol absorption in rats. Lipids 1991, 26, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Vandenbogaerde, T.J.; Hopkins, W.G. Effects of acute carbohydrate supplementation on endurance performance: A meta-analysis. Sports Med. 2011, 41, 773–792. [Google Scholar] [CrossRef]
- Nosaka, N.; Tsujino, S.; Honda, K.; Suemitsu, H.; Kato, K.; Kondo, K. Effect of Ingestion of Medium-Chain Triglycerides on Substrate Oxidation during Aerobic Exercise Could Depend on Sex Difference in Middle-Aged Sedentary Persons. Nutrients 2020, 13, 36. [Google Scholar] [CrossRef]
- Oostenbrug, G.S.; Mensink, R.P.; Hardeman, M.R.; De Vries, T.; Brouns, F.; Hornstra, G. Exercise performance, red blood cell deformability, and lipid peroxidation: Effects of fish oil and vitamin E. J. Appl. Physiol. 1997, 83, 746–752. [Google Scholar] [CrossRef]
- Laiglesia, L.M.; Lorente-Cebrián, S.; Prieto-Hontoria, P.L.; Fernández-Galilea, M.; Ribeiro, S.M.; Sáinz, N.; Martínez, J.A.; Moreno-Aliaga, M.J. Eicosapentaenoic acid promotes mitochondrial biogenesis and beige-like features in subcutaneous adipocytes from overweight subjects. J. Nutr. Biochem. 2016, 37, 76–82. [Google Scholar] [CrossRef]
- Macaluso, F.; Barone, R.; Catanese, P.; Carini, F.; Rizzuto, L.; Farina, F.; Di Felice, V. Do fat supplements increase physical performance? Nutrients 2013, 5, 509–524. [Google Scholar] [CrossRef]
- Tiryaki-Sönmez, G.; Schoenfeld, B.; Vatansever-Ozen, S. Omega-3 fatty acids and exercise: A review of their combined effects on body composition and physical performance. Biomed. Hum. Kinet. 2011, 3, 23–29. [Google Scholar] [CrossRef]
- Haghravan, S.; Keshavarz, S.A.; Mazaheri, R.; Alizadeh, Z.; Mansournia, M.A. Effect of Omega-3 PUFAs Supplementation with Lifestyle Modification on Anthropometric Indices and Vo2 max in Overweight Women. Arch. Iran. Med. 2016, 19, 342–347. [Google Scholar]
- Logan, S.L.; Spriet, L.L. Omega-3 Fatty Acid Supplementation for 12 Weeks Increases Resting and Exercise Metabolic Rate in Healthy Community-Dwelling Older Females. PLoS ONE 2015, 10, e0144828. [Google Scholar] [CrossRef] [PubMed]
- Cleland, C.L.; Hunter, R.F.; Kee, F.; Cupples, M.E.; Sallis, J.F.; Tully, M.A. Validity of the global physical activity questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health 2014, 14, 1255. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.H.; Ng, S.H.; Koh, D.; Muller-Riemenschneider, F. Reliability and Validity of the Self- and Interviewer-Administered Versions of the Global Physical Activity Questionnaire (GPAQ). PLoS ONE 2015, 10, e0136944. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Maslin, T.S.; Armstrong, T. Global physical activity questionnaire (GPAQ): Nine country reliability and validity study. J. Phys. Act. Health 2009, 6, 790–804. [Google Scholar] [CrossRef]
- World Health Organization. Global Physical Activity Surveillance. Available online: http://www.who.int/ncds/surveillance/steps/GPAQ/en/ (accessed on 1 July 2021).
- Bortolotti, M.; Tappy, L.; Schneiter, P. Fish oil supplementation does not alter energy efficiency in healthy males. Clin. Nutr. 2007, 26, 225–230. [Google Scholar] [CrossRef]
- Buckley, J.D.; Burgess, S.; Murphy, K.J.; Howe, P.R. DHA-rich fish oil lowers heart rate during submaximal exercise in elite Australian Rules footballers. J. Sci. Med. Sport 2009, 12, 503–507. [Google Scholar] [CrossRef]
- Peoples, G.E.; McLennan, P.L.; Howe, P.R.; Groeller, H. Fish oil reduces heart rate and oxygen consumption during exercise. J. Cardiovasc. Pharmacol. 2008, 52, 540–547. [Google Scholar] [CrossRef]

| Overall (n = 19) Mean (SEM) | PM (n = 10) Mean (SEM) | STG (n = 9) Mean (SEM) | p | |
|---|---|---|---|---|
| Age (y) | 20 (1) | 20 (1) | 20 (1) | n.s |
| Height (cm) | 171.5 (1.4) | 171.3 (2.2) | 171.7 (2.2) | n.s |
| Weight (kg) | 67.1 (2.0) | 67.3 (3.3) | 66.9 (3.3) | n.s |
| Body mass index (kg/m2) | 22.8 (0.7) | 22.9 (1.0) | 22.8 (1.0) | n.s |
| STG (n = 9) | PM (n = 10) | Between-Group Difference | |||
|---|---|---|---|---|---|
| Mean (SEM) | Mean (SEM) | Mean (95% CI) | ES | p | |
| VO2peak (mL/kg/min) | |||||
| Before | 42.4 (1.6) | 44.3 (2.3) | |||
| After | 39.6 (1.0) | 39.5 (2.4) | |||
| Within-group difference | −2.8 (1.1) | −4.8 (1.1) | 2.0 (−1.2 to 5.3) | 0.61 | 0.20 |
| AT (mL/kg/min) | |||||
| Before | 25.0 (1.5) | 25.4 (2.2) | |||
| After | 25.7 (1.0) | 22.5 (1.7) | |||
| Within-group difference | 0.6 (0.8) | −2.9 (0.8) | 3.5 (1.1 to 6.0) | 1.42 | <0.01 |
| TTE in VO2peak test (s) | |||||
| Before | 839 (26) | 849 (38) | |||
| After | 892 (29) | 839 (44) | |||
| Within-group difference | 53 (18) | −10 (20) | 63 (6 to 120) | 1.1 | <0.05 |
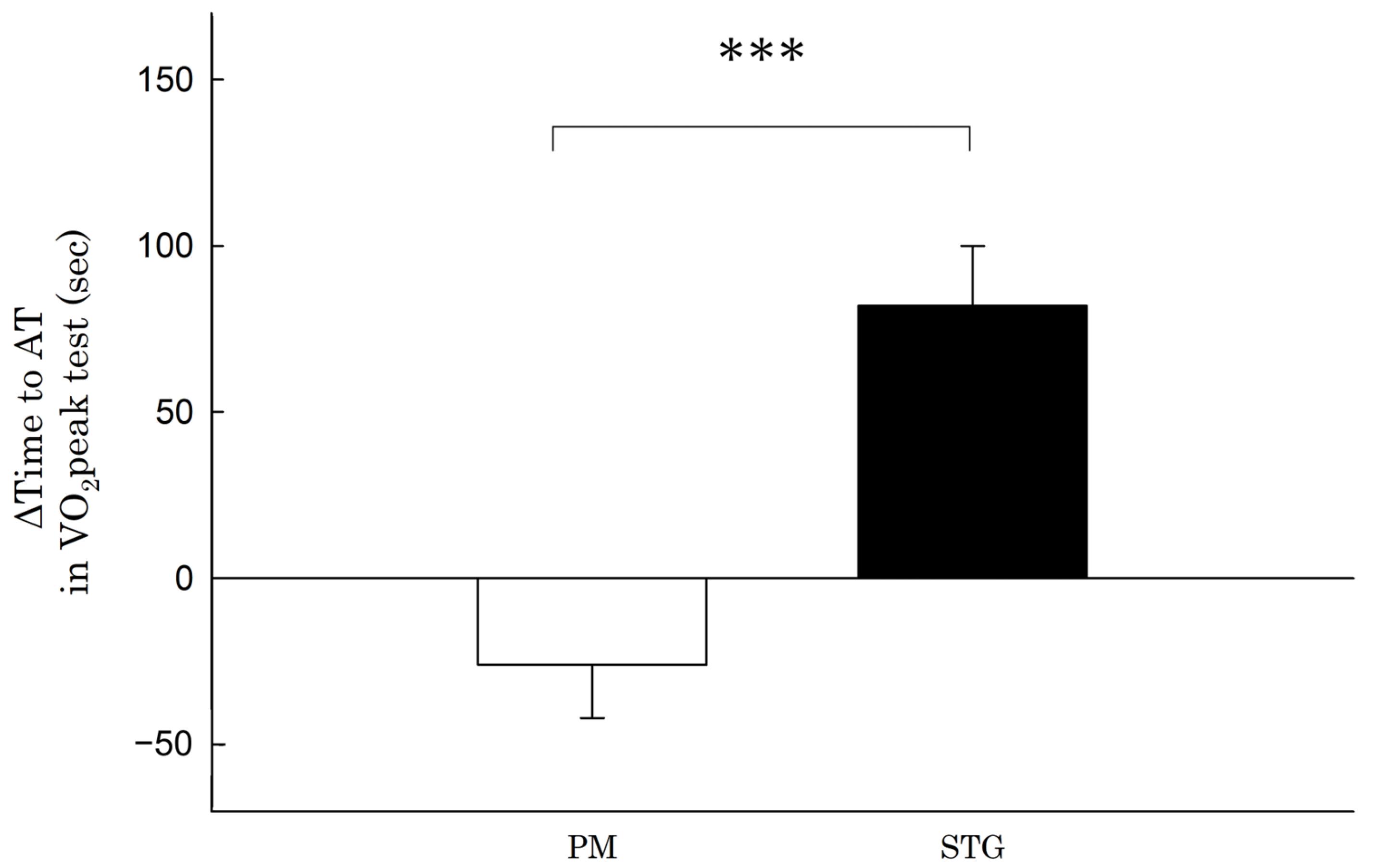
| Time to AT in VO2peak test (s) | |||||
| Before | 372 (27) | 371 (33) | |||
| After | 455 (30) | 344 (37) | |||
| Within-group difference | 82 (18) | −26 (16) | 109 (57 to 161) | 2.0 | <0.001 |
| TTE in endurance performance test at a workload to 80% VO2peak (s) | |||||
| Before | 726 (171) | 648 (106) | |||
| After | 883 (162) | 864 (97) | |||
| Within-group difference | 157 (160) | 216 (73) | −58 (−417 to 300) | −0.16 | 0.74 |
| Total workload in endurance performance test (J/kg) | |||||
| Before | 5383 (433) | 5397 (244) | |||
| After | 6140 (508) | 5716 (365) | |||
| Within-group difference | 757 (454) | 319 (234) | 439 (−605 to 1483) | 0.42 | 0.41 |
| Total workload at 50%VO2peak in endurance performance test (J/kg) | |||||
| Before | 3512 (154) | 3714 (223) | |||
| After | 3683 (111) | 3514 (282) | |||
| Within-group difference | 170 (100) | −200 (109) | 370 (56 to 684) | 1.15 | <0.05 |
| Total workload at 80%VO2peak in endurance performance test (J/kg) | |||||
| Before | 1870 (428) | 1684 (208) | |||
| After | 2457 (479) | 2202 (242) | |||
| Within-group difference | 587 (443) | 518 (189) | 69 (−911 to 1048) | 0.07 | 0.890 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuji, K.; Tsuchiya, Y.; Yokoi, K.; Yanagimoto, K.; Ueda, H.; Ochi, E. Eicosapentaenoic Acid and Medium-Chain Triacylglycerol Structured Lipids Improve Endurance Performance. Nutrients 2023, 15, 3692. https://doi.org/10.3390/nu15173692
Tsuji K, Tsuchiya Y, Yokoi K, Yanagimoto K, Ueda H, Ochi E. Eicosapentaenoic Acid and Medium-Chain Triacylglycerol Structured Lipids Improve Endurance Performance. Nutrients. 2023; 15(17):3692. https://doi.org/10.3390/nu15173692
Chicago/Turabian StyleTsuji, Katsunori, Yosuke Tsuchiya, Kaori Yokoi, Kenichi Yanagimoto, Hisashi Ueda, and Eisuke Ochi. 2023. "Eicosapentaenoic Acid and Medium-Chain Triacylglycerol Structured Lipids Improve Endurance Performance" Nutrients 15, no. 17: 3692. https://doi.org/10.3390/nu15173692
APA StyleTsuji, K., Tsuchiya, Y., Yokoi, K., Yanagimoto, K., Ueda, H., & Ochi, E. (2023). Eicosapentaenoic Acid and Medium-Chain Triacylglycerol Structured Lipids Improve Endurance Performance. Nutrients, 15(17), 3692. https://doi.org/10.3390/nu15173692





