Human Breast Milk microRNAs, Potential Players in the Regulation of Nervous System
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk Sample Collection
2.2. Exosome Extraction
2.3. Transmission Electron Microscopy (TEM)
2.4. RNA Extraction and Quality Control
2.5. microRNA-Sequencing
2.6. miRNA Functional Assays and Target Prediction
3. Results
3.1. RNA Extraction and Quality Control
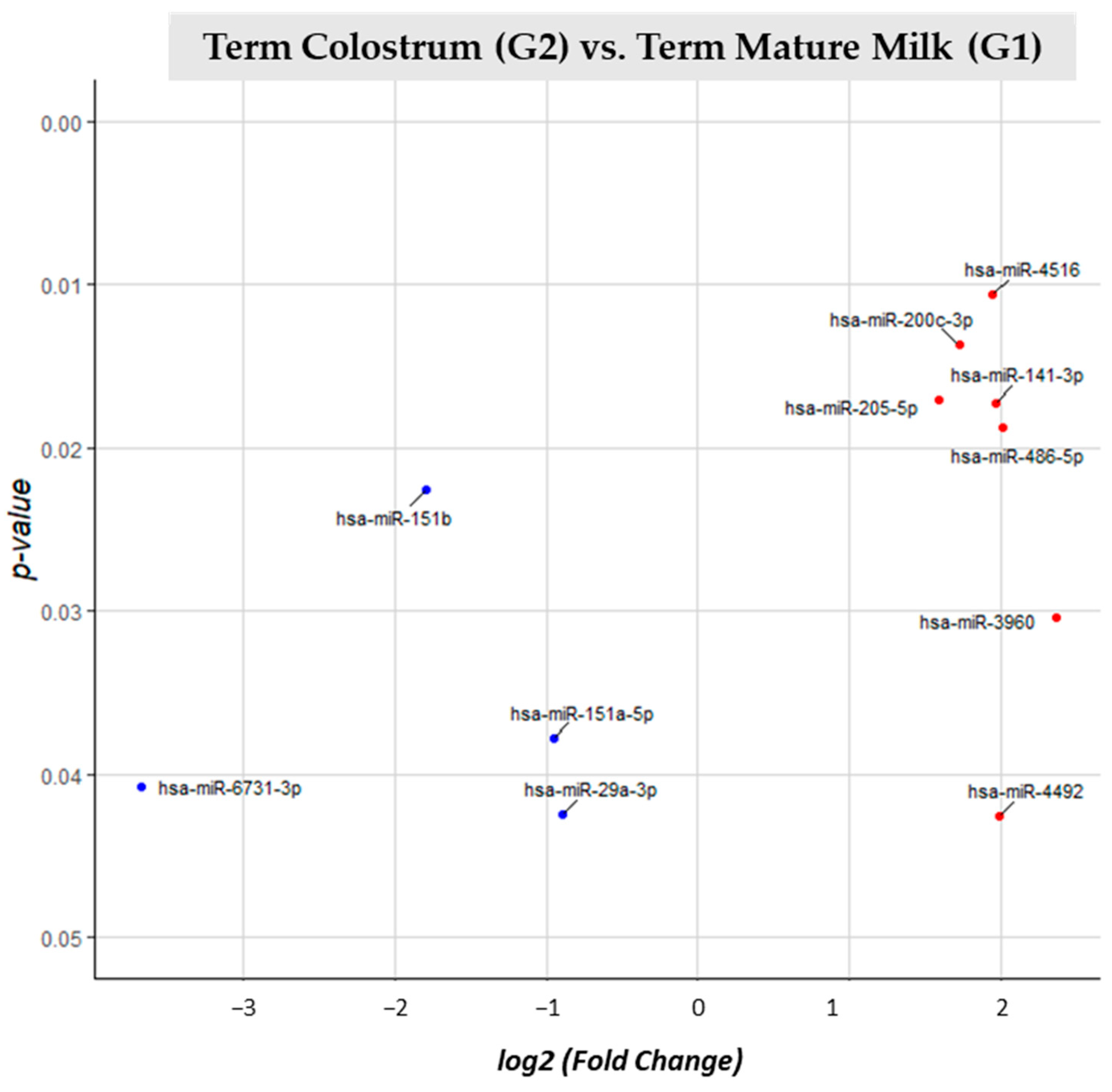
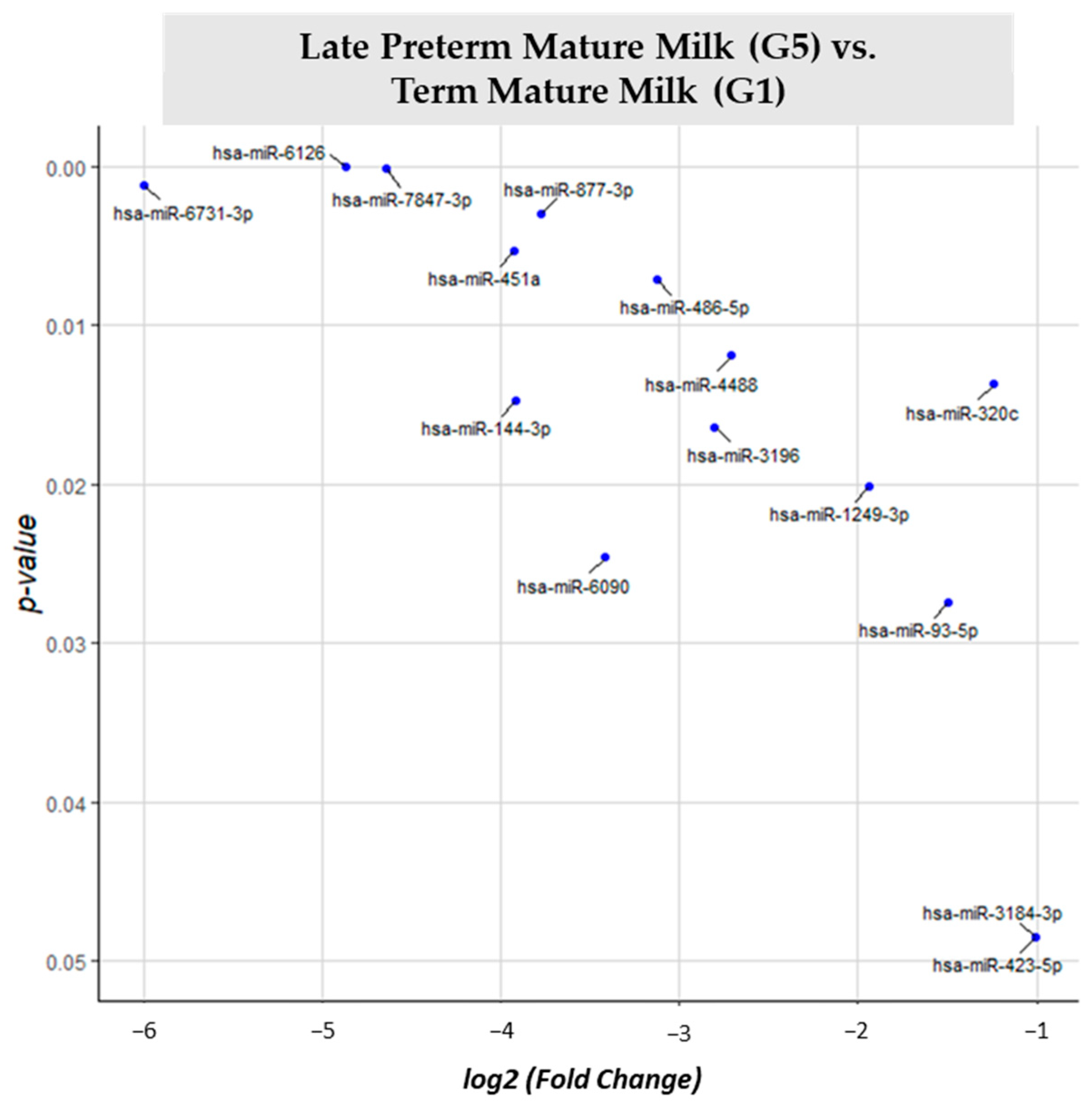
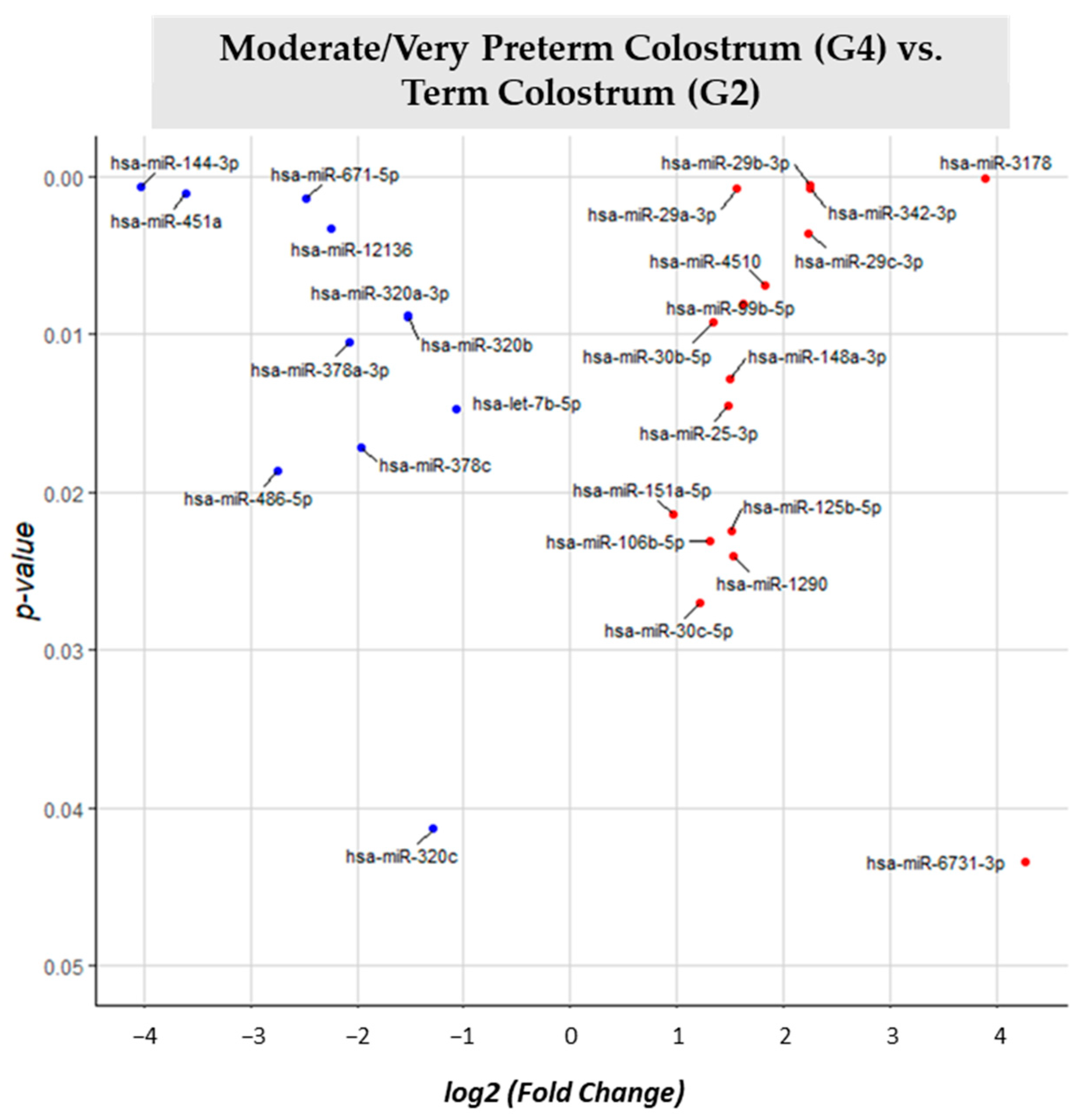
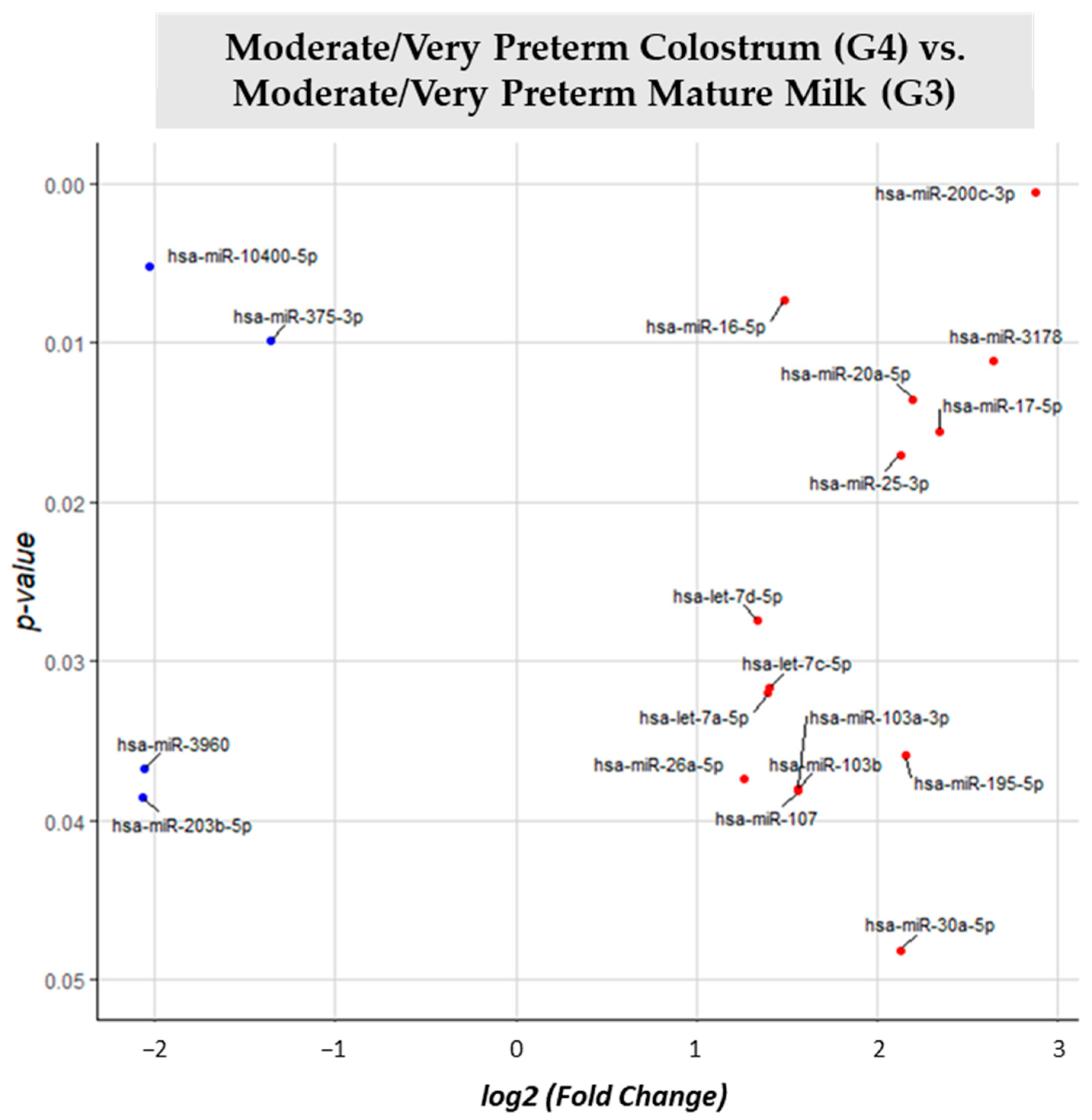
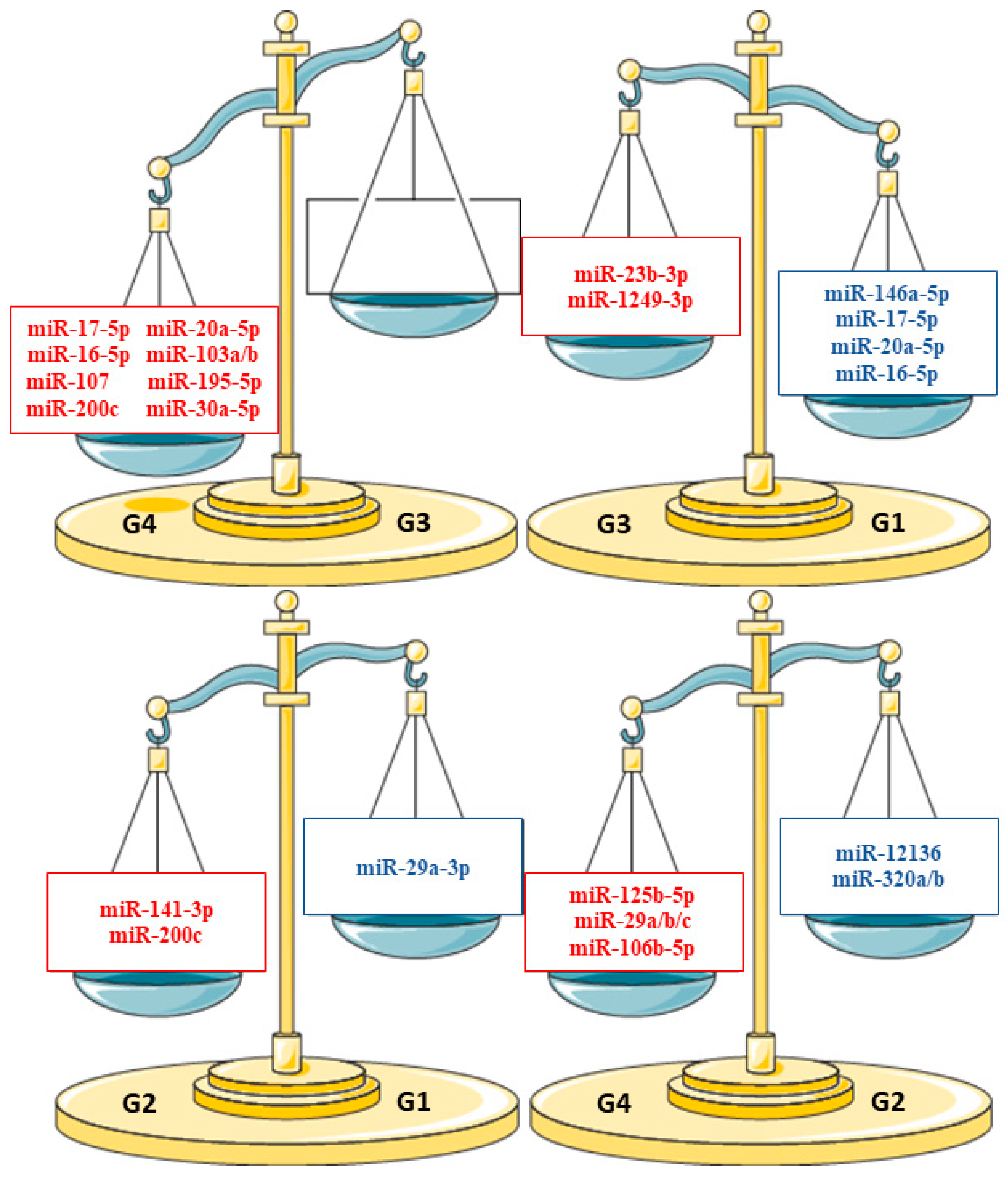
3.2. miRNA Differential Expression Analysis
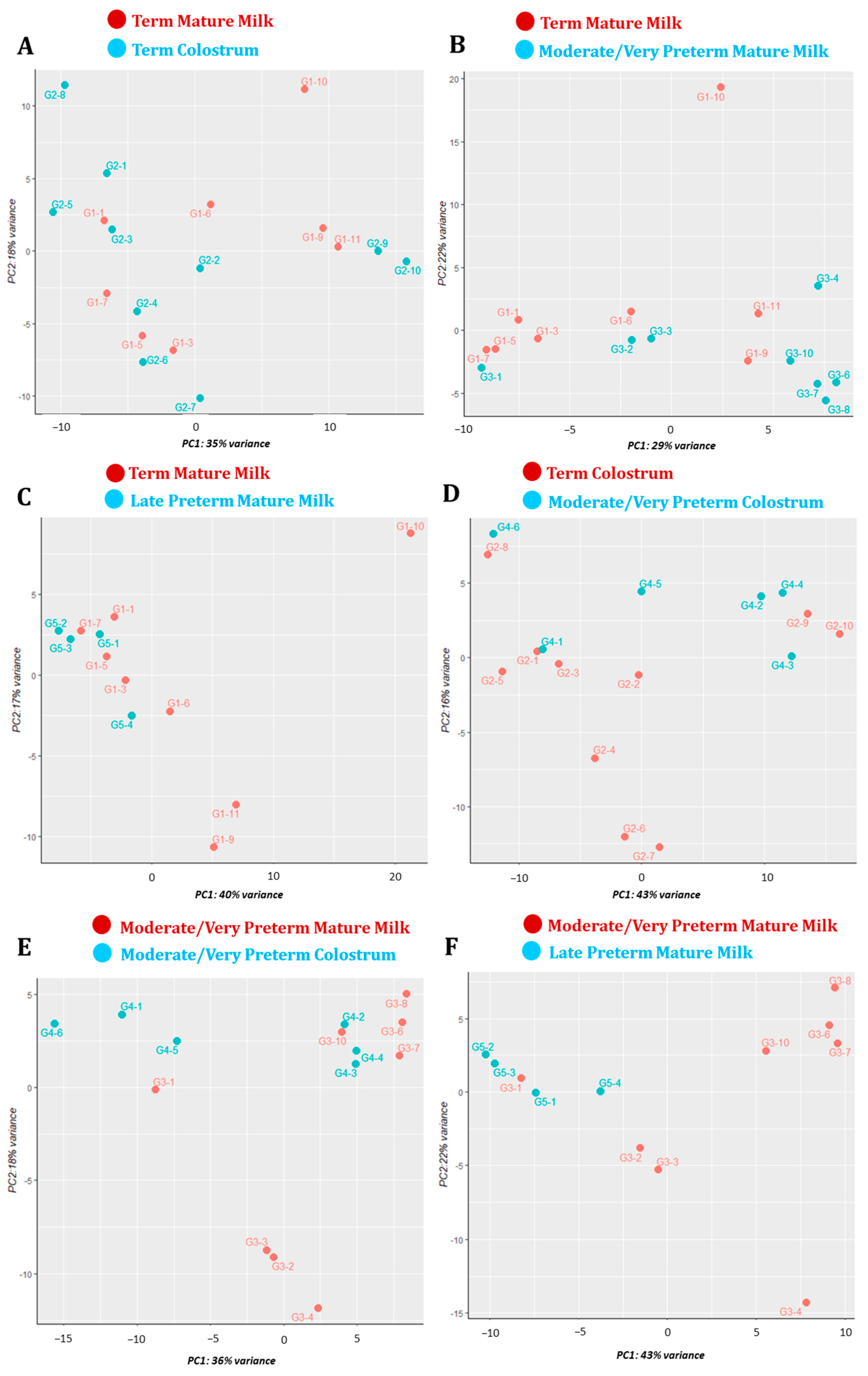
3.3. Principal Component Analysis
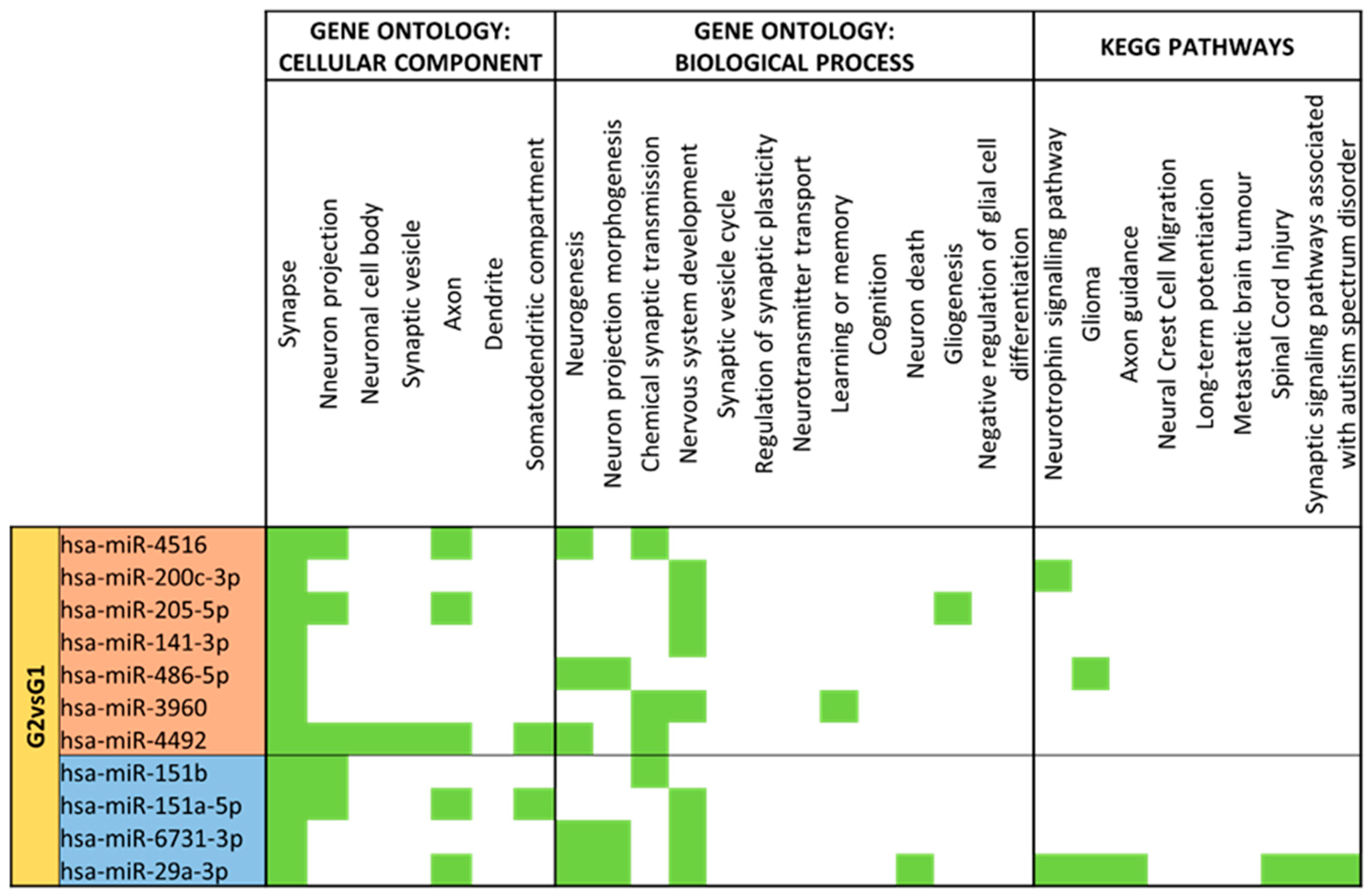
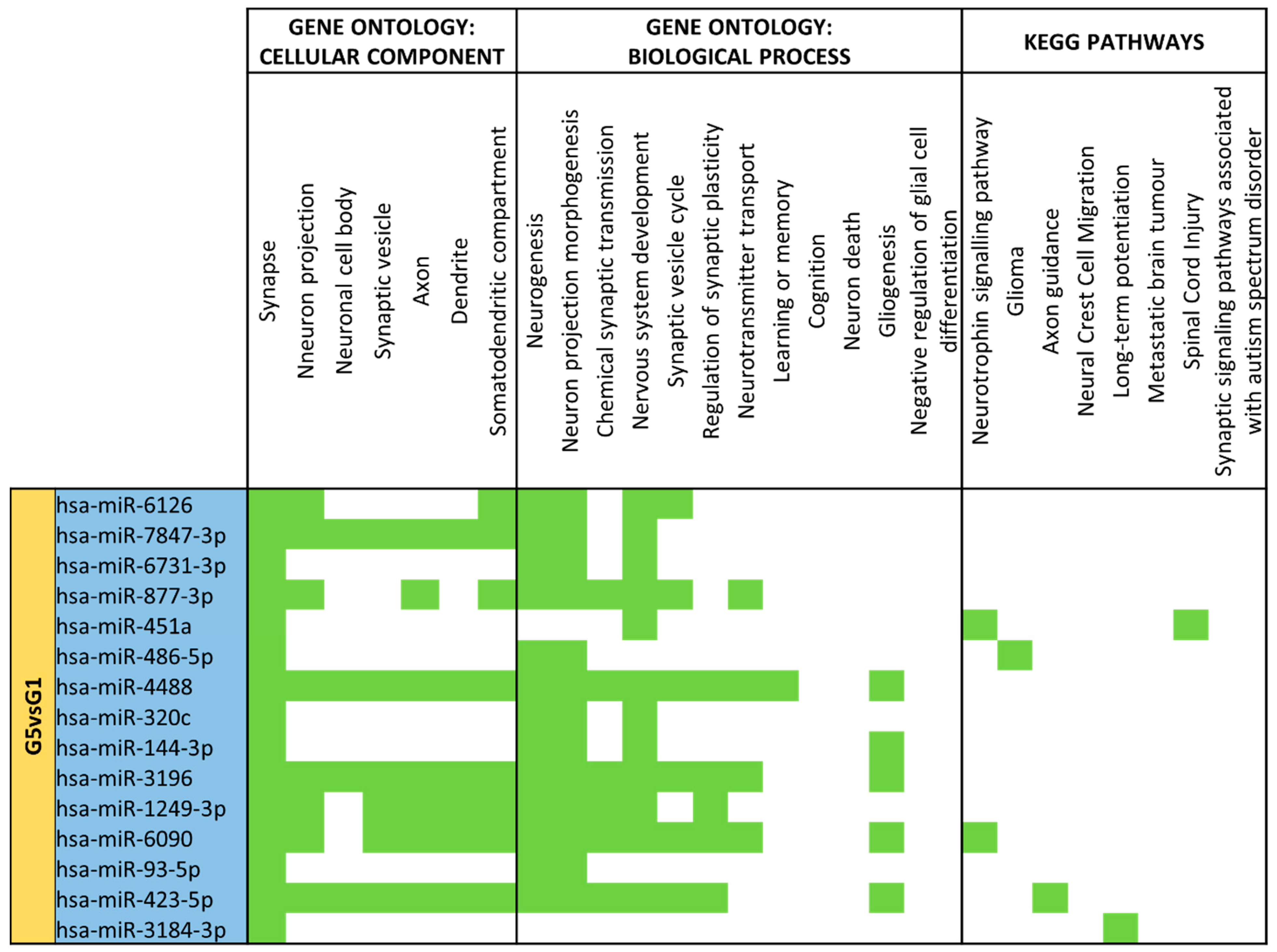
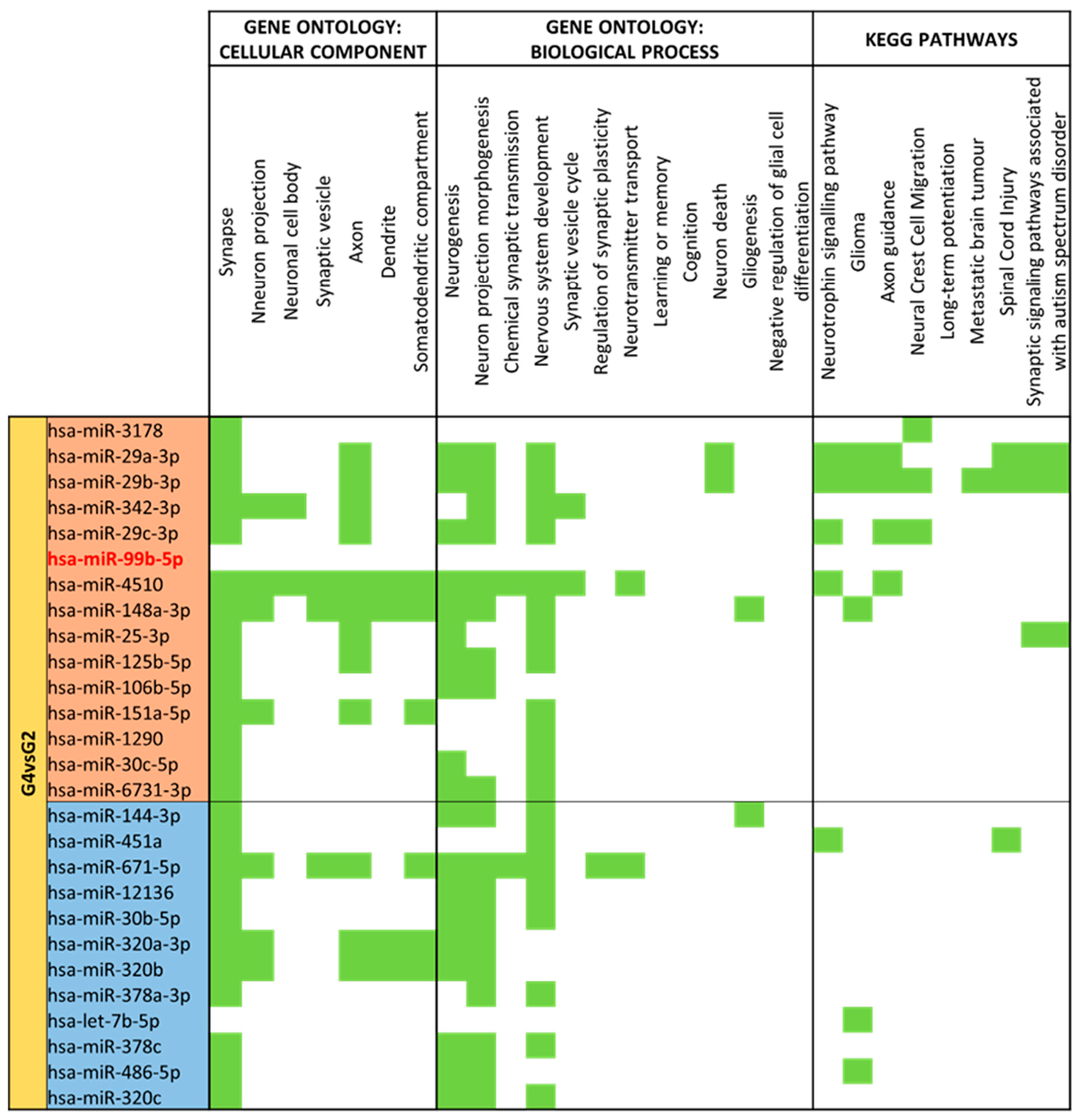
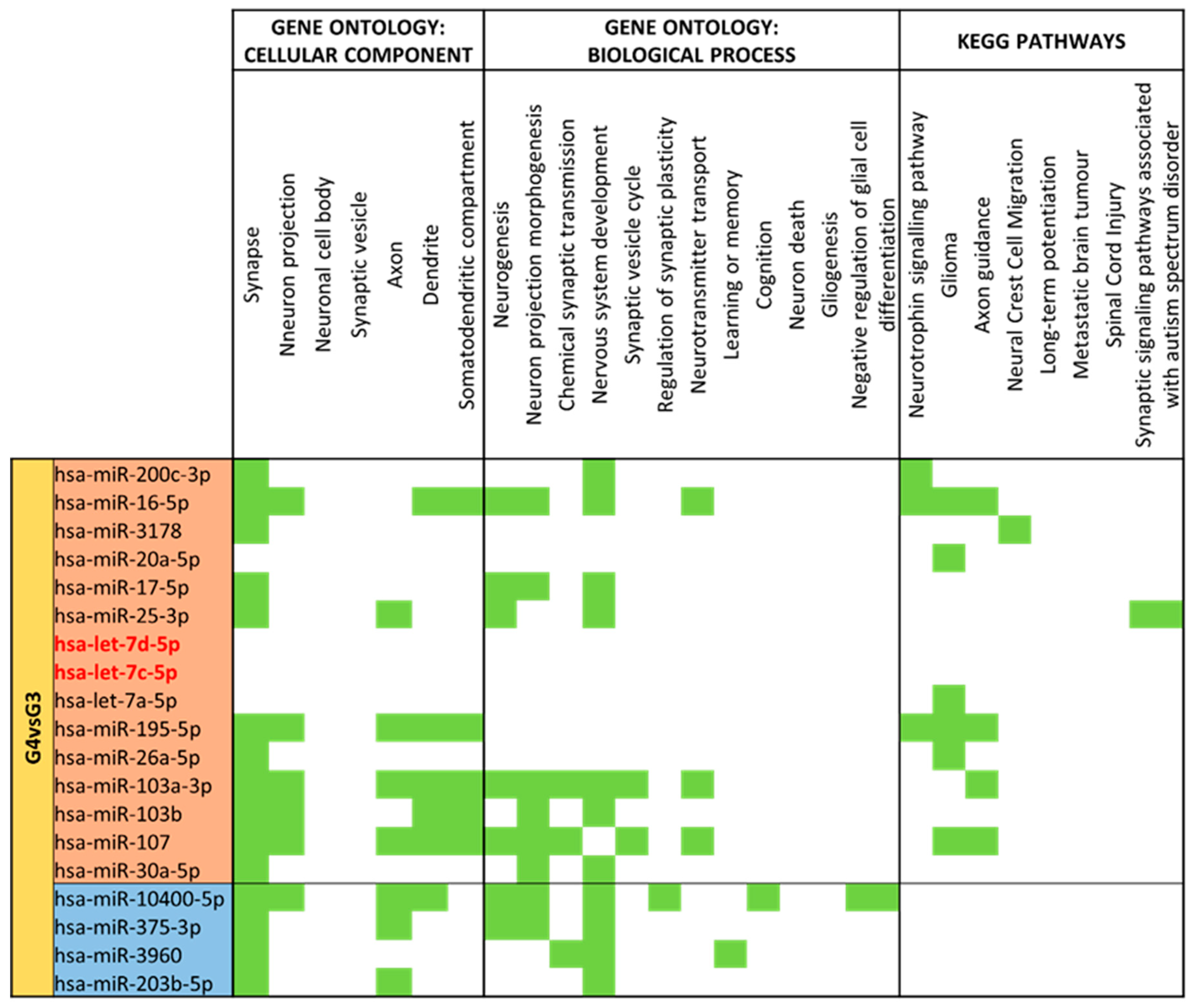
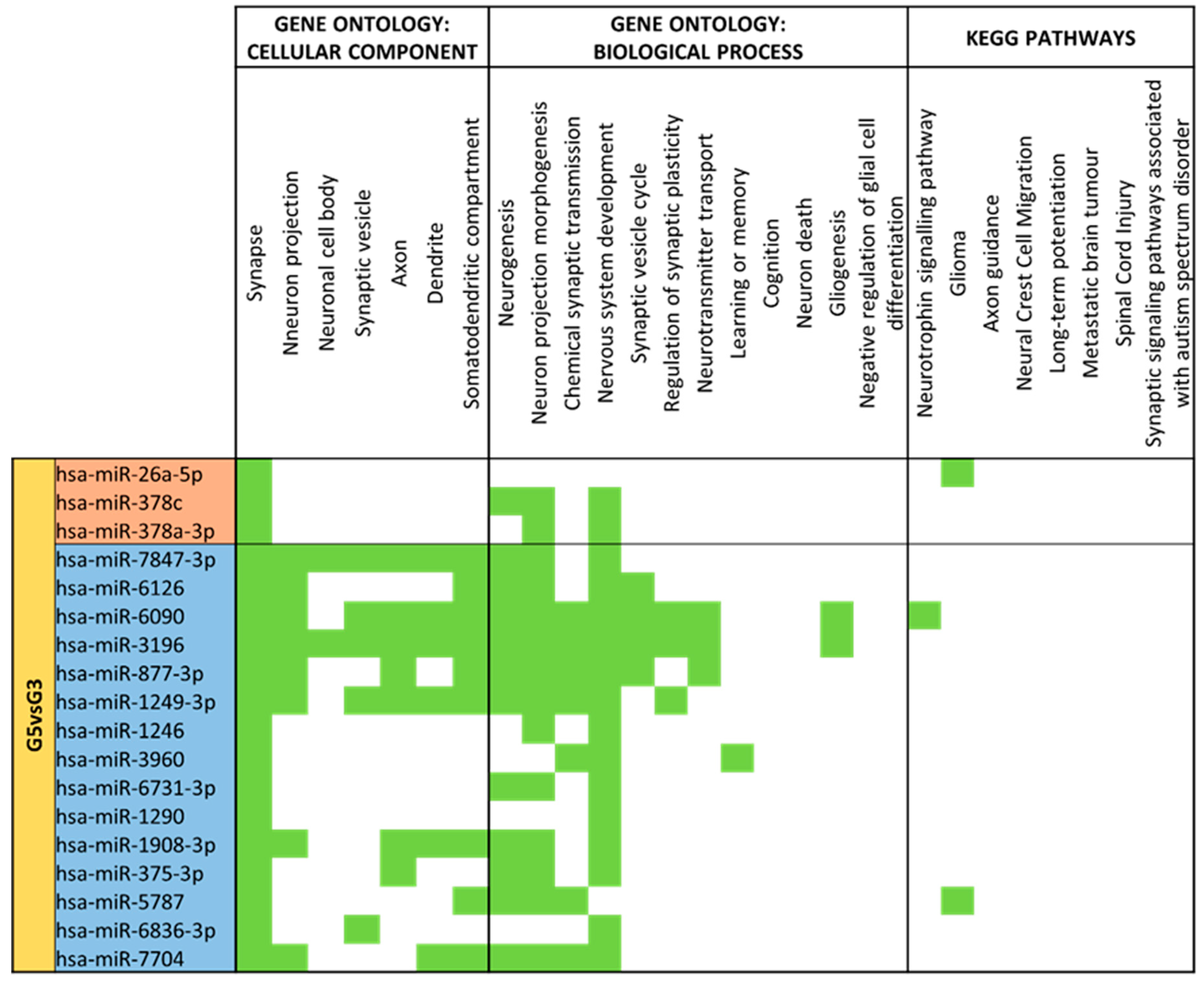
3.4. miRNA GO and KEGG Pathway Analysis
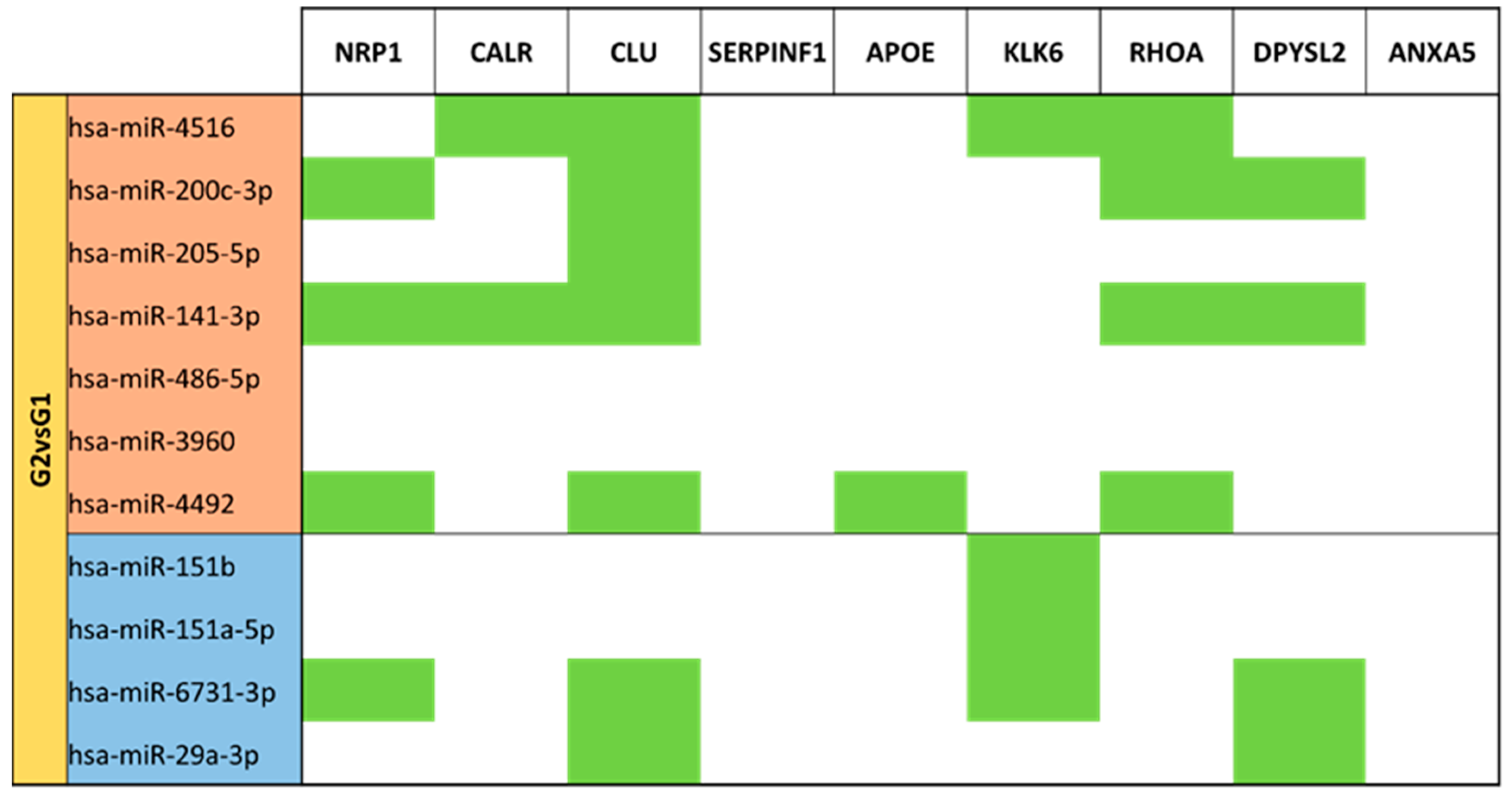
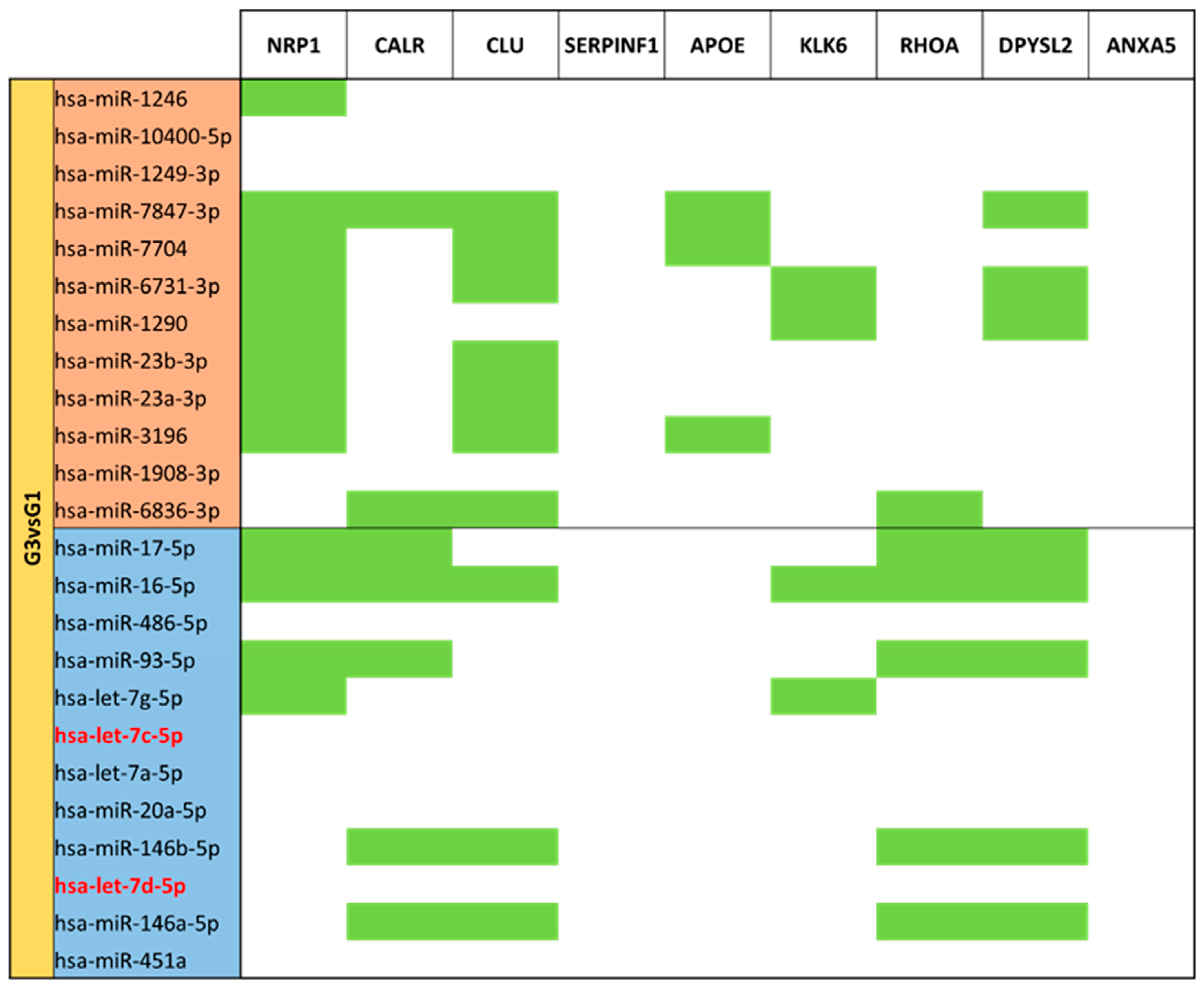
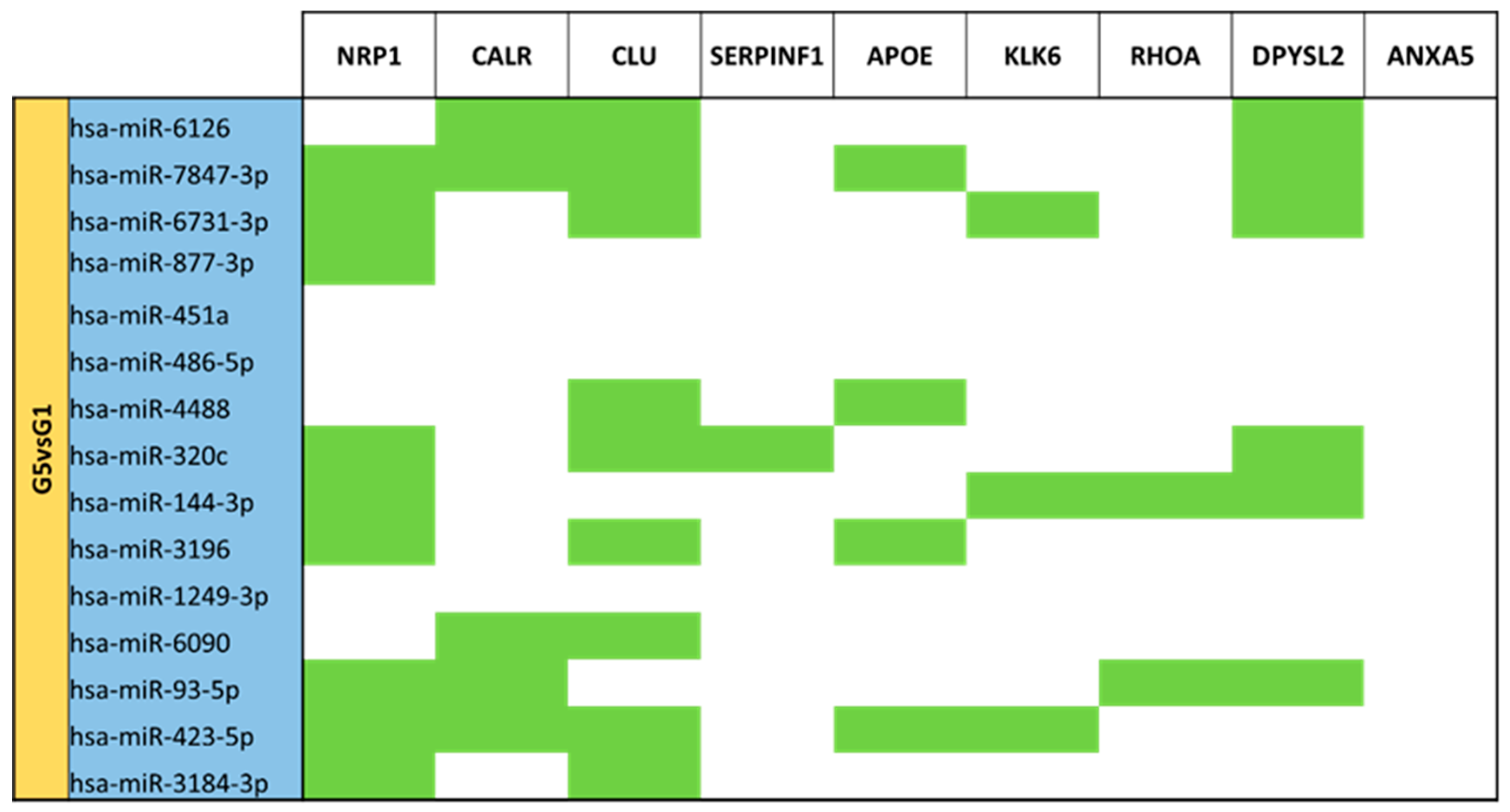
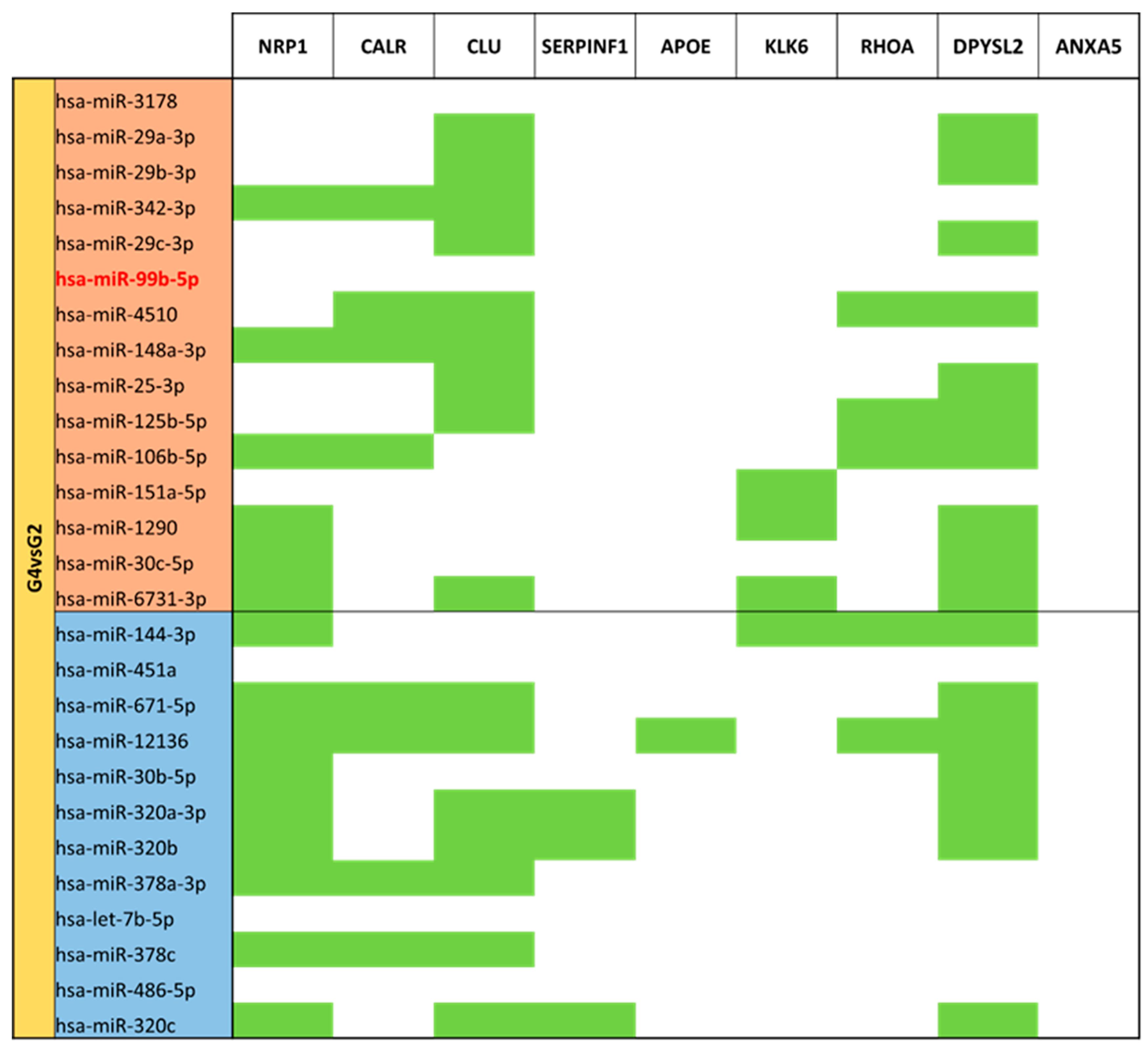
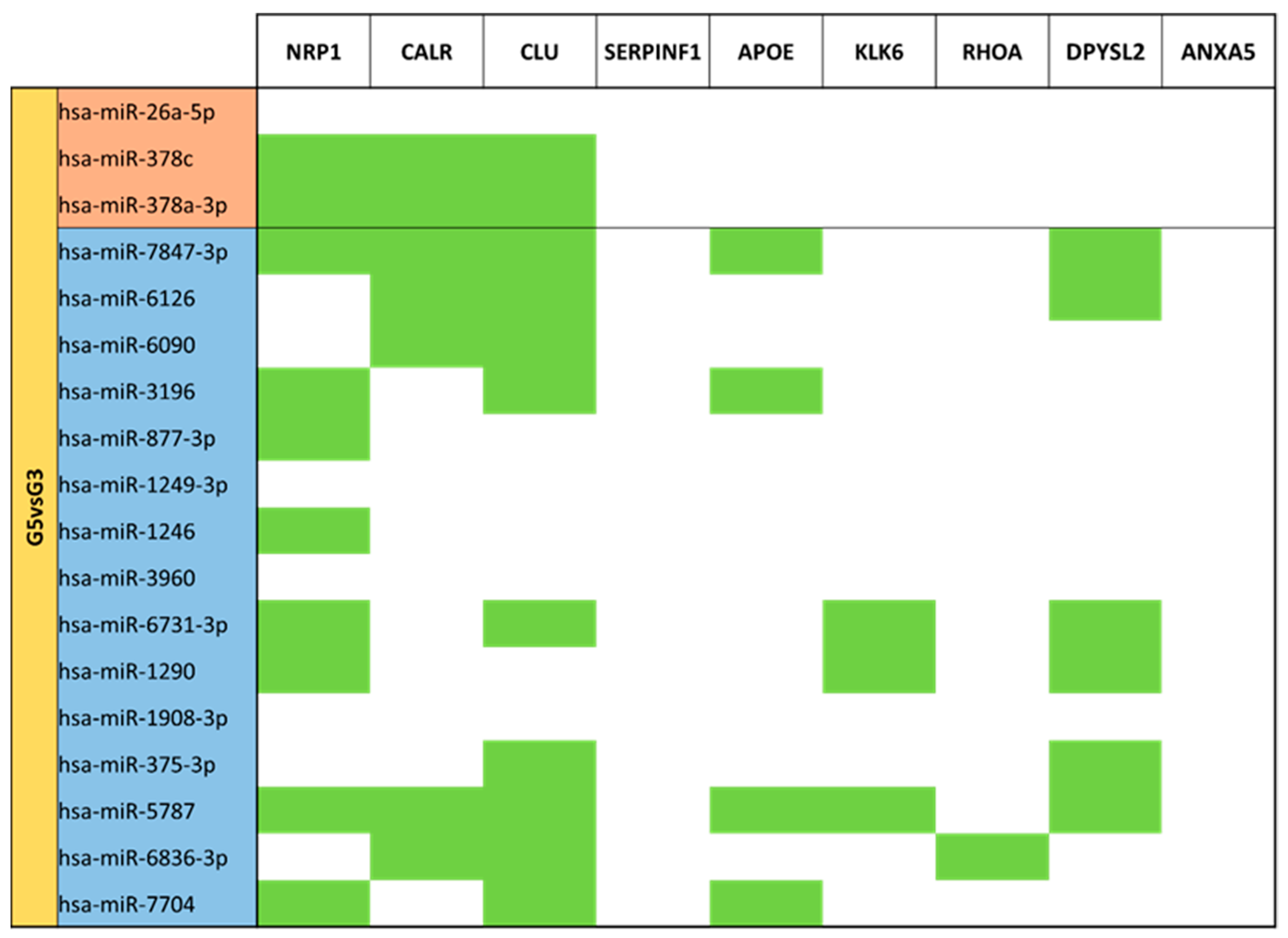
3.5. miRNA Targeting Neuro-Related Protein Selection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bzikowska-Jura, A.; Sobieraj, P.; Szostak-Węgierek, D.; Wesołowska, A. Impact of infant and maternal factors on energy and macronutrient composition of human milk. Nutrients 2020, 12, 2591. [Google Scholar] [CrossRef] [PubMed]
- Alsaweed, M.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Micrornas in breastmilk and the lactating breast: Potential immunoprotectors and developmental regulators for the infant and the mother. Int. J. Environ. Res. Public Health 2015, 12, 13981–14020. [Google Scholar] [CrossRef]
- Munch, E.M.; Harris, R.A.; Mohammad, M.; Benham, A.L.; Pejerrey, S.M.; Showalter, L.; Hu, M.; Shope, C.D.; Maningat, P.D.; Gunaratne, P.H.; et al. Transcriptome Profiling of microRNA by Next-Gen Deep Sequencing Reveals Known and Novel miRNA Species in the Lipid Fraction of Human Breast Milk. PLoS ONE 2013, 8, e50564. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.P.A.; Scheynius, A.; Gabrielsson, S. Exosomes with Immune Modulatory Features Are Present in Human Breast Milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef]
- Liu, C.G.; Calin, G.A.; Meloon, B.; Gamliel, N.; Sevignani, C.; Ferracin, M.; Dumitru, C.D.; Shimizu, M.; Zupo, S.; Dono, M.; et al. An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 9740–9744. [Google Scholar] [CrossRef]
- Carney, M.C.; Tarasiuk, A.; Diangelo, S.L.; Silveyra, P.; Podany, A.; Birch, L.L.; Paul, I.M.; Kelleher, S.; Hicks, S.D. Metabolism-related microRNAs in maternal breast milk are influenced by premature delivery. Pediatr. Res. 2017, 82, 226–236. [Google Scholar] [CrossRef]
- Jiang, M.; Sang, X.; Hong, Z. Beyond nutrients: Food-derived microRNAs provide cross-kingdom regulation. BioEssays 2012, 34, 280–284. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA profiling: Approaches and considerations. Nat. Rev. Genet. 2015, 13, 358–369. [Google Scholar] [CrossRef]
- Bredy, T.W.; Lin, Q.; Wei, W.; Baker-Andresen, D.; Mattick, J.S. MicroRNA regulation of neural plasticity and memory. Neurobiol. Learn. Mem. 2011, 96, 89–94. [Google Scholar] [CrossRef]
- Sempere, L.F.; Freemantle, S.; Pitha-Rowe, I.; Moss, E.; Dmitrovsky, E.; Ambros, V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004, 5, R13. [Google Scholar] [CrossRef] [PubMed]
- Giraldez, A.J.; Cinalli, R.M.; Glasner, M.E.; Enright, A.J.; Thomson, J.M.; Baskerville, S.; Hammond, S.M.; Bartel, D.P.; Schier, A.F. MicroRNAs regulate brain morphogenesis in zebrafish. Science 2005, 308, 833–838. [Google Scholar] [CrossRef]
- Cao, D.D.; Li, L.; Chan, W.Y. MicroRNAs: Key regulators in the central nervous system and their implication in neurological diseases. Int. J. Mol. Sci. 2016, 17, 842. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qin, C. General hallmarks of microRNAs in brain evolution and development. RNA Biol. 2015, 12, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, W.Y.; Mao, Y.W.; Gräff, J.; Guan, J.S.; Pan, L.; Mak, G.; Kim, D.; Su, S.C.; Tsai, L.H. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 2010, 466, 1105–1109. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhu, Q.; Zhou, X.; Wang, X.; Gao, X.; Li, X. Immune-related microRNAs are abundant in breast milk exosomes. Int. J. Biol. Sci. 2012, 8, 118–123. [Google Scholar] [CrossRef]
- Liao, Y.; Du, X.; Li, J.; Lönnerdal, B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol. Nutr. Food Res. 2017, 61, 1700082. [Google Scholar] [CrossRef]
- Kahn, S.; Liao, Y.; Du, X.; Xu, W.; Li, J.; Lönnerdal, B. Exosomal MicroRNAs in Milk from Mothers Delivering Preterm Infants Survive in Vitro Digestion and Are Taken up by Human Intestinal Cells. Mol. Nutr. Food Res. 2018, 62, 1701050. [Google Scholar] [CrossRef]
- Hata, T.; Murakami, K.; Nakatani, H.; Yamamoto, Y.; Matsuda, T.; Aoki, N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem. Biophys. Res. Commun. 2010, 396, 528–533. [Google Scholar] [CrossRef]
- Izumi, H.; Kosaka, N.; Shimizu, T.; Sekine, K.; Ochiya, T.; Takase, M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J. Dairy Sci. 2012, 95, 4831–4841. [Google Scholar] [CrossRef]
- Gu, Y.; Li, M.; Wang, T.; Liang, Y.; Zhong, Z.; Wang, X.; Zhou, Q.; Chen, L.; Lang, Q.; He, Z.; et al. Lactation-related microRNA expression profiles of porcine breast milk exosomes. PLoS ONE 2012, 7, e43691. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xi, Q.Y.; Ye, R.S.; Cheng, X.; Qi, Q.E.; Wang, S.B.; Shu, G.; Wang, L.N.; Zhu, X.T.; Jiang, Q.Y.; et al. Exploration of microRNAs in porcine milk exosomes. BMC Genom. 2014, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xi, Q.Y.; Sun, J.J.; Ye, R.S.; Cheng, X.; Sun, R.P.; Wang, S.B.; Shu, G.; Wang, L.N.; Zhu, X.T.; et al. Revelation of mRNAs and proteins in porcine milk exosomes by transcriptomic and proteomic analysis. BMC Vet. Res. 2017, 13, 101. [Google Scholar] [CrossRef]
- Golan-Gerstl, R.; Elbaum Shiff, Y.; Moshayoff, V.; Schecter, D.; Leshkowitz, D.; Reif, S. Characterization and biological function of milk-derived miRNAs. Mol. Nutr. Food Res. 2017, 61, 1700009. [Google Scholar] [CrossRef]
- Lukasik, A.; Brzozowska, I.; Zielenkiewicz, U.; Zielenkiewicz, P. Detection of plant miRNAs abundance in human breast milk. Int. J. Mol. Sci. 2018, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci. Rep. 2016, 6, 20680. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, Z.; Sun, L.; Li, P. Fermentation Results in Quantitative Changes in Milk-Derived Exosomes and Different Effects on Cell Growth and Survival. J. Agric. Food Chem. 2017, 65, 1220–1228. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk cells contain numerous miRNAs that may change with milk removal and regulate multiple physiological processes. Int. J. Mol. Sci. 2016, 17, 956. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Wolf, T.; Baier, S.R.; Zempleni, J. The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells1-3. J. Nutr. 2015, 145, 2201–2206. [Google Scholar] [CrossRef]
- Lönnerdal, B. Human Milk MicroRNAs/Exosomes: Composition and Biological Effects. Nestle Nutr. Inst. Workshop Ser. 2019, 90, 83–92. [Google Scholar] [CrossRef]
- Kim, K.U.; Kim, W.H.; Jeong, C.H.; Yi, D.Y.; Min, H. More than nutrition: Therapeutic potential of breast milk-derived exosomes in cancer. Int. J. Mol. Sci. 2020, 21, 7327. [Google Scholar] [CrossRef]
- Martin, C.; Patel, M.; Williams, S.; Arora, H.; Sims, B. Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun. 2018, 24, 278–284. [Google Scholar] [CrossRef]
- Stremmel, W.; Weiskirchen, R.; Melnik, B.C. Milk Exosomes Prevent Intestinal Inflammation in a Genetic Mouse Model of Ulcerative Colitis: A Pilot Experiment. Inflamm. Intest. Dis. 2020, 5, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, X.; Yan, X.; Yu, Z.; Zhang, J.; Han, S. The emerging role of exosomes in the pathogenesis, prognosis and treatment of necrotizing enterocolitis. Am. J. Transl. Res. 2020, 12, 7020–7033. [Google Scholar] [PubMed]
- Miyake, H.; Lee, C.; Chusilp, S.; Bhalla, M.; Li, B.; Pitino, M.; Seo, S.; O’Connor, D.L.; Pierro, A. Human breast milk exosomes attenuate intestinal damage. Pediatr. Surg. Int. 2020, 36, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Mun, J.Y. Sample Preparation and Imaging of Exosomes by Transmission Electron Microscopy. J. Vis. Exp. 2018, 131, 56482. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences From High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W. Package ggplot2: An Implementation of the Grammar of Graphics. Creat. Elegant Data Vis. Using Gramm. Graph. 2014, 2, 1–189. [Google Scholar]
- Knuesel, I.; Chicha, L.; Britschgi, M.; Schobel, S.A.; Bodmer, M.; Hellings, J.A.; Toovey, S.; Prinssen, E.P. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014, 10, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003, 27, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Ziats, M.N.; Rennert, O.M. Identification of Differentially Expressed MicroRNAs Across the Developing Human Brain Mark. Mol. Psychiatry 2014, 19, 848–852. [Google Scholar] [CrossRef]
- Aksoy-Aksel, A.; Zampa, F.; Schratt, G. MicroRNAs and synaptic plasticity-a mutual relationship. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130515. [Google Scholar] [CrossRef]
- Nishimura, T.; Fukata, Y.; Kato, K.; Yamaguchi, T.; Matsuura, Y.; Kamiguchi, H.; Kaibuchi, K. CRMP-2 regulates polarized Numb-mediated endocytosis for axon growth. Nat. Cell Biol. 2003, 5, 819–826. [Google Scholar] [CrossRef]
- Goshima, Y.; Yamashita, N.; Nakamura, F.; Sasaki, Y. Regulation of dendritic development by semaphorin 3A through novel intracellular remote signaling. Cell Adhes. Migr. 2016, 10, 627–640. [Google Scholar] [CrossRef]
- Lanfranco, M.F.; Ng, C.A.; Rebeck, G.W. ApoE lipidation as a therapeutic target in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 6336. [Google Scholar] [CrossRef]
- Kosaka, N.; Izumi, H.; Sekine, K.; Ochiya, T. MicroRNA as a new immune-regulatory agent in breast milk. Silence 2010, 1, 7. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk cells and lipids conserve numerous known and novel miRNAs, some of which are differentially expressed during lactation. PLoS ONE 2016, 11, e0152610. [Google Scholar] [CrossRef]
- Batalle, D.; Hughes, E.J.; Zhang, H.; Tournier, J.D.; Tusor, N.; Aljabar, P.; Wali, L.; Alexander, D.C.; Hajnal, J.V.; Nosarti, C.; et al. Early development of structural networks and the impact of prematurity on brain connectivity. Neuroimage 2017, 149, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.R.; Spoelstra, N.S.; Richer, J.K. The role of miRNAs in progesterone action. Mol. Cell. Endocrinol. 2012, 357, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, L.; Ravo, M.; Nassa, G.; Tarallo, R.; de Filippo, M.R.; Giurato, G.; Cirillo, F.; Stellato, C.; Silvestro, S.; Cantarella, C.; et al. Effects of oestrogen on microRNA expression in hormone-responsive breast cancer cells. Horm. Cancer 2012, 3, 65–78. [Google Scholar] [CrossRef]
- Blesa, M.; Sullivan, G.; Anblagan, D.; Telford, E.J.; Quigley, A.J.; Sparrow, S.A.; Serag, A.; Semple, S.I.; Bastin, M.E.; Boardman, J.P. Early breast milk exposure modifies brain connectivity in preterm infants. Neuroimage 2019, 184, 431–439. [Google Scholar] [CrossRef]
- Garg, P.; Jamal, F.; Srivastava, P. Deciphering the role of precursor miR-12136 and miR-8485 in the progression of intellectual disability (ID). IBRO Neurosci. Rep. 2022, 13, 393–401. [Google Scholar] [CrossRef]
- Salloum-Asfar, S.; Elsayed, A.K.; Elhag, S.F.; Abdulla, S.A. Circulating non-coding rnas as a signature of autism spectrum disorder symptomatology. Int. J. Mol. Sci. 2021, 22, 6549. [Google Scholar] [CrossRef]
- Somel, M.; Liu, X.; Tang, L.; Yan, Z.; Hu, H.; Guo, S.; Jiang, X.; Zhang, X.; Xu, G.; Xie, G.; et al. MicroRNA-driven developmental remodeling in the brain distinguishes humans from other primates. PLoS Biol. 2011, 9, e1001214. [Google Scholar] [CrossRef]
- Denk, J.; Oberhauser, F.; Kornhuber, J.; Wiltfang, J.; Fassbender, K.; Schroeter, M.L.; Volk, A.E.; Diehl-Schmid, J.; Prudlo, J.; Danek, A.; et al. Specific serum and CSF microRNA profiles distinguish sporadic behavioural variant of frontotemporal dementia compared with Alzheimer patients and cognitively healthy controls. PLoS ONE 2018, 13, e0197329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Guo, T.; Peng, Y.; Wang, K.; Bai, K.; Huang, Y. Screening of schizophrenia associated miRNAs and the regulation of miR-320a-3p on integrin β1. Medicine 2019, 98, e14332. [Google Scholar] [CrossRef]
- Le, M.T.N.; Xie, H.; Zhou, B.; Chia, P.H.; Rizk, P.; Um, M.; Udolph, G.; Yang, H.; Lim, B.; Lodish, H.F. MicroRNA-125b Promotes Neuronal Differentiation in Human Cells by Repressing Multiple Targets. Mol. Cell. Biol. 2009, 29, 5290–5305. [Google Scholar] [CrossRef]
- Boissart, C.; Nissan, X.; Giraud-Triboult, K.; Peschanski, M.; Benchoua, A. MiR-125 potentiates early neural specification of human embryonic stem cells. Development 2012, 139, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Roese-Koerner, B.; Stappert, L.; Koch, P.; Brustle, O.; Borghese, L. Pluripotent Stem Cell-Derived Somatic Stem Cells as Tool to Study the Role of MicroRNAs in Early Human Neural Development. Curr. Mol. Med. 2013, 13, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J. Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. Neuroreport 2007, 18, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, L.; Meng, J. MicroRNA-125b promotes neurons cell apoptosis and Tau phosphorylation in Alzheimer’s disease. Neurosci. Lett. 2017, 661, 57–62. [Google Scholar] [CrossRef]
- McKeever, P.M.; Schneider, R.; Taghdiri, F.; Weichert, A.; Multani, N.; Brown, R.A.; Boxer, A.L.; Karydas, A.; Miller, B.; Robertson, J.; et al. MicroRNA Expression Levels Are Altered in the Cerebrospinal Fluid of Patients with Young-Onset Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 8826–8841. [Google Scholar] [CrossRef]
- Alexandrov, P.N.; Dua, P.; Hill, J.M.; Bhattacharjee, S.; Zhao, Y.; Lukiw, W.J. MicroRNA (miRNA) speciation in Alzheimer’s disease (AD) cerebrospinal fluid (CSF) and extracellular fluid (ECF). Int. J. Biochem. Mol. Biol. 2012, 3, 365–373. [Google Scholar]
- Hébert, S.S.; Horré, K.; Nicolaï, L.; Papadopoulou, A.S.; Mandemakers, W.; Silahtaroglu, A.N.; Kauppinen, S.; Delacourte, A.; De Strooper, B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/β-secretase expression. Proc. Natl. Acad. Sci. USA 2008, 105, 6415–6420. [Google Scholar] [CrossRef]
- Wang, W.X.; Huang, Q.; Hu, Y.; Stromberg, A.J.; Nelson, P.T. Patterns of microRNA expression in normal and early Alzheimer’s disease human temporal cortex: White matter versus gray matter. Acta Neuropathol. 2011, 121, 193–205. [Google Scholar] [CrossRef]
- Xia, B.; Gao, J.; Li, S.; Huang, L.; Zhu, L.; Ma, T.; Zhao, L.; Yang, Y.; Luo, K.; Shi, X.; et al. Mechanical stimulation of Schwann cells promote peripheral nerve regeneration via extracellular vesicle-mediated transfer of microRNA 23b-3p. Theranostics 2020, 10, 8974–8995. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, J.; Guo, S.; Zeng, L.; Cai, Z.; Zhang, J.; Wang, L.; Li, Z.; Liu, R. miR-23b-3p rescues cognition in Alzheimer’s disease by reducing tau phosphorylation and apoptosis via GSK-3β signaling pathways. Mol. Ther. Nucleic Acids 2022, 28, 539–557. [Google Scholar] [CrossRef]
- Matamala, J.M.; Arias-Carrasco, R.; Sanchez, C.; Uhrig, M.; Bargsted, L.; Matus, S.; Maracaja-Coutinho, V.; Abarzua, S.; van Zundert, B.; Verdugo, R.; et al. Genome-wide circulating microRNA expression profiling reveals potential biomarkers for amyotrophic lateral sclerosis. Neurobiol. Aging 2018, 64, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Moloney, E.B.; de Winter, F.; Verhaagen, J. ALS as a distal axonopathy: Molecular mechanisms affecting neuromuscular junction stability in the presymptomatic stages of the disease. Front. Neurosci. 2014, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.; Teuber-Hanselmann, S.; Soub, D.; Harnisch, K.; Mairinger, F.; Junker, A. MicroRNA profiles of MS gray matter lesions identify modulators of the synaptic protein synaptotagmin-7. Brain Pathol. 2020, 30, 524–540. [Google Scholar] [CrossRef]
- Ye, Y.; Feng, Z.; Tian, S.; Yang, Y.; Jia, Y.; Wang, G.; Wang, J.; Bai, W.; Li, J.; He, X. HBO Alleviates Neural Stem Cell Pyroptosis via lncRNA-H19/miR-423-5p/NLRP3 Axis and Improves Neurogenesis after Oxygen Glucose Deprivation. Oxidative Med. Cell. Longev. 2022, 2022, 9030771. [Google Scholar] [CrossRef]
- Yoshino, Y.; Roy, B.; Dwivedi, Y. Differential and unique patterns of synaptic miRNA expression in dorsolateral prefrontal cortex of depressed subjects. Neuropsychopharmacology 2021, 46, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Sierksma, A.; Lu, A.; Salta, E.; Vanden Eynden, E.; Callaerts-Vegh, Z.; D’Hooge, R.; Blum, D.; Buée, L.; Fiers, M.; De Strooper, B. Deregulation of neuronal miRNAs induced by amyloid-β or TAU pathology 11 Medical and Health Sciences 1109 Neurosciences. Mol. Neurodegener. 2018, 13, 54. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Zhao, Y.; Jian, G.C. An NF-κB-sensitive micro RNA-146a-mediated inflammatory circuit in alzheimer disease and in stressed human brain cells. J. Biol. Chem. 2008, 283, 31315–31322. [Google Scholar] [CrossRef]
- Cui, J.G.; Li, Y.Y.; Zhao, Y.; Bhattacharjee, S.; Lukiw, W.J. Differential regulation of Interleukin-1 Receptor-associated Kinase-1 (IRAK-1) and IRAK-2 by microRNA-146a and NF-κB in stressed human astroglial cells and in Alzheimer disease. J. Biol. Chem. 2010, 285, 38951–38960. [Google Scholar] [CrossRef]
- Beveridge, N.J.; Tooney, P.A.; Carroll, A.P.; Tran, N.; Cairns, M.J. Down-regulation of miR-17 family expression in response to retinoic acid induced neuronal differentiation. Cell. Signal. 2009, 21, 1837–1845. [Google Scholar] [CrossRef]
- Santarelli, D.M.; Beveridge, N.J.; Tooney, P.A.; Cairns, M.J. Upregulation of dicer and MicroRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol. Psychiatry 2011, 69, 180–187. [Google Scholar] [CrossRef]
- Tanzer, A.; Stadler, P.F. Molecular evolution of a microRNA cluster. J. Mol. Biol. 2004, 339, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.K. miR-106b as an emerging therapeutic target in cancer. Genes Dis. 2022, 9, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Hébert, S.S.; Horré, K.; Nicolaï, L.; Bergmans, B.; Papadopoulou, A.S.; Delacourte, A.; De Strooper, B. MicroRNA regulation of Alzheimer’s Amyloid precursor protein expression. Neurobiol. Dis. 2009, 33, 422–428. [Google Scholar] [CrossRef]
- Annibali, D.; Gioia, U.; Savino, M.; Laneve, P.; Caffarelli, E.; Nasi, S. A new module in neural differentiation control: Two microRNAs upregulated by retinoic acid, miR-9 and -103, target the differentiation inhibitor ID2. PLoS ONE 2012, 7, e40269. [Google Scholar] [CrossRef]
- Chang, W.S.; Wang, Y.H.; Zhu, X.T.; Wu, C.J. Genome-wide profiling of miRNA and mRNA expression in alzheimer’s disease. Med. Sci. Monit. 2017, 23, 2721–2731. [Google Scholar] [CrossRef]
- Gallego, J.; Alsop, E.; Lencz, T.; Van Keuren-Jensen, K.; Malhotra, A. F10. Differential expression of miRNAs in cerebrospinal fluid and plasma samples in schizophrenia. Schizophr. Bull. 2018, 4 (Suppl. S1), S221–S222. [Google Scholar] [CrossRef]
- Nelson, P.T.; Wang, W.-X. MiR-107 is reduced in Alzheimer’s disease brain neocortex: Validation study. J. Alzheimers Dis. 2010, 21, 75–79. [Google Scholar] [CrossRef]
- Wang, W.X.; Rajeev, B.W.; Stromberg, A.J.; Ren, N.; Tang, G.; Huang, Q.; Rigoutsos, I.; Nelson, P.T. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of β-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008, 28, 1213–1223. [Google Scholar] [CrossRef]
- Zhong, Z.; Yuan, K.; Tong, X.; Hu, J.; Song, Z.; Zhang, G.; Fang, X.; Zhang, W. MiR-16 attenuates β-amyloid-induced neurotoxicity through targeting β-site amyloid precursor protein-cleaving enzyme 1 in an Alzheimer’s disease cell model. Neuroreport 2018, 29, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.W.; Ma, L.X.; Phillips, C.; Zhang, S.C. miR-200 and miR-96 families repress neural induction from human embryonic stem cells. Development 2013, 140, 2611–2618. [Google Scholar] [CrossRef]
- Mellios, N.; Huang, H.S.; Grigorenko, A.; Rogaev, E.; Akbarian, S. A set of differentially expressed miRNAs, including miR-30a-5p, act as post-transcriptional inhibitors of BDNF in prefrontal cortex. Hum. Mol. Genet. 2008, 17, 3030–3042. [Google Scholar] [CrossRef]
- Davis, G.M.; Haas, M.A.; Pocock, R. MicroRNAs: Not ‘Fine-Tuners’ but key regulators of neuronal development and function. Front. Neurol. 2015, 6, 245. [Google Scholar] [CrossRef]
- Monk, C.; Spicer, J.; Champagne, F.A. Linking prenatal maternal adversity to developmental outcomes in infants: The role of epigenetic pathways. Dev. Psychopathol. 2012, 24, 1361–1376. [Google Scholar] [CrossRef] [PubMed]
- Rajman, M.; Schratt, G. MicroRNAs in neural development: From master regulators to fine-tuners. Development 2017, 144, 2310–2322. [Google Scholar] [CrossRef] [PubMed]
- Yerbury, J.J.; Poon, S.; Meehan, S.; Thompson, B.; Kumita, J.R.; Dobson, C.M.; Wilson, M.R. The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J. 2007, 21, 2312–2322. [Google Scholar] [CrossRef]
- Tatebe, H.; Watanabe, Y.; Kasai, T.; Mizuno, T.; Nakagawa, M.; Tanaka, M.; Tokuda, T. Extracellular neurosin degrades α-synuclein in cultured cells. Neurosci. Res. 2010, 67, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Tokuda, T.; Yamaguchi, N.; Watanabe, Y.; Kametani, F.; Nakagawa, M.; Mizuno, T. Cleavage of normal and pathological forms of α-synuclein by neurosin in vitro. Neurosci. Lett. 2008, 436, 52–56. [Google Scholar] [CrossRef]
- Reid, K.M.; Kitchener, E.J.A.; Butler, C.A.; Cockram, T.O.J.; Brown, G.C. Brain Cells Release Calreticulin That Attracts and Activates Microglia, and Inhibits Amyloid Beta Aggregation and Neurotoxicity. Front. Immunol. 2022, 13, 859686. [Google Scholar] [CrossRef]
- Davidi, D.; Schechter, M.; Elhadi, S.A.; Matatov, A.; Nathanson, L.; Sharon, R. α-Synuclein Translocates to the Nucleus to Activate Retinoic-Acid-Dependent Gene Transcription. iScience 2020, 23, 100910. [Google Scholar] [CrossRef]
- Wyatt, A.R.; Yerbury, J.J.; Berghofer, P.; Greguric, I.; Katsifis, A.; Dobson, C.M.; Wilson, M.R. Clusterin facilitates in vivo clearance of extracellular misfolded proteins. Cell. Mol. Life Sci. 2011, 68, 3919–3931. [Google Scholar] [CrossRef]
- Herrera, E.; Erskine, L.; Morenilla-Palao, C. Guidance of retinal axons in mammals. Semin. Cell Dev. Biol. 2019, 85, 48–59. [Google Scholar] [CrossRef]
- Rebeck, G.W. The role of APOE on lipid homeostasis and inflammation in normal brains. J. Lipid Res. 2017, 58, 1493–1499. [Google Scholar] [CrossRef]
- Stankiewicz, T.R.; Linseman, D.A. Rho family GTPases: Key players in neuronal development, neuronal survival, and neurodegeneration. Front. Cell. Neurosci. 2014, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.I.; Blaabjerg, M.; Freude, K.; Meyer, M. RhoA Signaling in Neurodegenerative Diseases. Cells 2022, 11, 1520. [Google Scholar] [CrossRef] [PubMed]
- Niwa, S.; Nakamura, F.; Tomabechi, Y.; Aoki, M.; Shigematsu, H.; Matsumoto, T.; Yamagata, A.; Fukai, S.; Hirokawa, N.; Goshima, Y.; et al. Structural basis for CRMP2-induced axonal microtubule formation. Sci. Rep. 2017, 7, 10681. [Google Scholar] [CrossRef]
- Fukata, Y.; Itoh, T.J.; Kimura, T.; Ménager, C.; Nishimura, T.; Shiromizu, T.; Watanabe, H.; Inagaki, N.; Iwamatsu, A.; Hotani, H.; et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat. Cell Biol. 2002, 4, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Calero, M.; Rostagno, A.; Matsubara, E.; Zlokovic, B.; Frangione, B.; Ghiso, J. Apolipoprotein J (Clusterin) and Alzheimer’s Disease. Microsc. Res. Tech. 2000, 50, 305–315. [Google Scholar] [CrossRef]
- Imhof, A.; Charnay, Y.; Vallet, P.G.; Aronow, B.; Kovari, E.; French, L.E.; Bouras, C.; Giannakopoulos, P. Sustained astrocytic clusterin expression improves remodeling after brain ischemia. Neurobiol. Dis. 2006, 22, 274–283. [Google Scholar] [CrossRef]
- Klimaschewski, L.; Obermüller, N.; Witzgall, R. Regulation of clusterin expression following spinal cord injury. Cell Tissue Res. 2001, 306, 209–216. [Google Scholar] [CrossRef]
- Spencer, B.; Valera, E.; Rockenstein, E.; Trejo-Morales, M.; Adame, A.; Masliah, E. A brain-targeted, modified neurosin (kallikrein-6) reduces α-synuclein accumulation in a mouse model of multiple system atrophy. Mol. Neurodegener. 2015, 10, 48. [Google Scholar] [CrossRef]
- Qin, W.; Dasgupta, S.; Corradi, J.; Sauter, E.R. Human Milk and Matched Serum Demonstrate Concentration of Select miRNAs. Breastfeed. Med. 2016, 12, 63–66. [Google Scholar] [CrossRef] [PubMed]
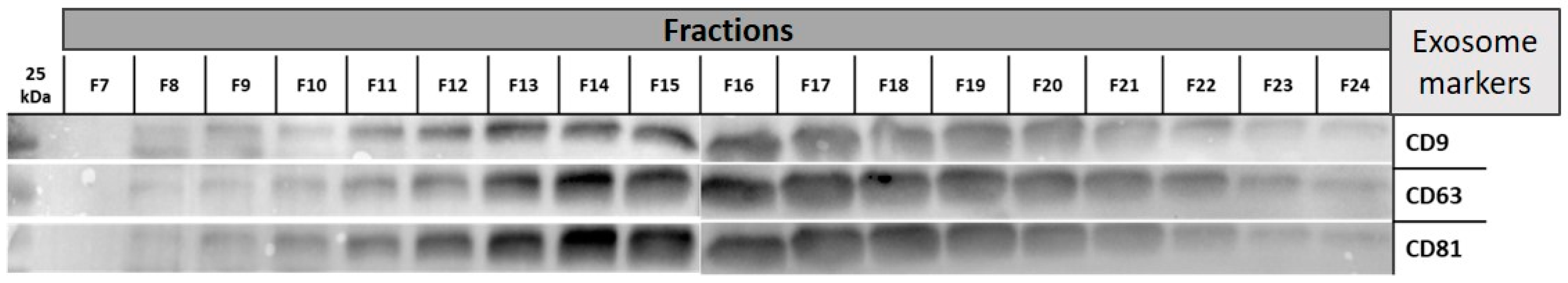
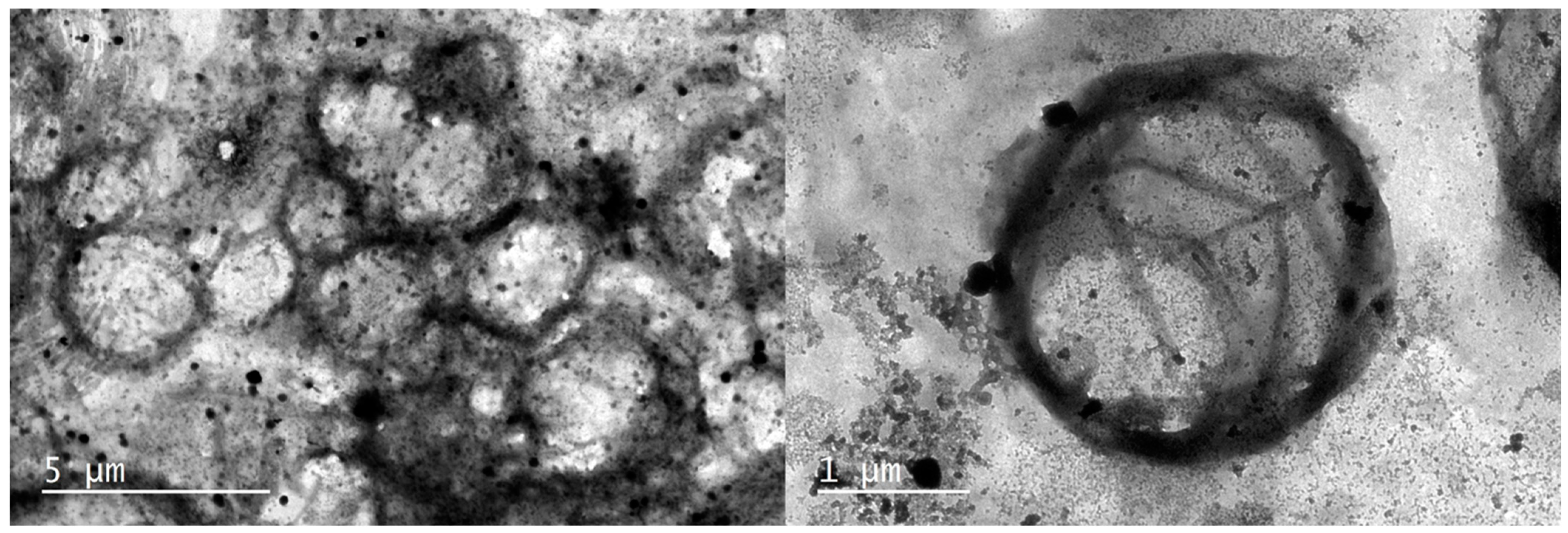






| Sample ID | Multiplexing Group | [RNA] (ng/µL) | R 260/280 | R 260/230 | RIN | miRNA Fraction |
|---|---|---|---|---|---|---|
| G1-1 | Term Mature Milk | 39.97 | 1.725 | 0.303 | 1.8 | 87% |
| G1-3 | Term Mature Milk | 20.69 | 1.631 | 0.455 | 1.1 | 90% |
| G1-5 | Term Mature Milk | 39.87 | 1.929 | 0.793 | 2.6 | 76% |
| G1-6 | Term Mature Milk | 16.95 | 1.606 | 0.105 | 1 | 85% |
| G1-7 | Term Mature Milk | 24.96 | 1.545 | 0.576 | 1.4 | 85% |
| G1-9 | Term Mature Milk | 10.601 | 1.75 | 0.412 | 1 | 93% |
| G1-10 | Term Mature Milk | 13.71 | 1.539 | 0.161 | 1 | 91% |
| G1-11 | Term Mature Milk | 19.62 | 1.484 | 1.043 | 2 | 93% |
| G2-1 | Term Colostrum | 19.72 | 1.806 | 0.349 | 1.6 | 86% |
| G2-2 | Term Colostrum | 14.63 | 1.62 | 0.289 | 5.4 | 83% |
| G2-3 | Term Colostrum | 22.32 | 1.476 | 0.231 | 1 | 87% |
| G2-4 | Term Colostrum | 20.53 | 1.639 | 0.616 | 1 | 80% |
| G2-5 | Term Colostrum | 16.31 | 1.963 | 0.629 | 2.2 | 80% |
| G2-6 | Term Colostrum | 28.32 | 1.924 | 0.797 | 2.4 | 89% |
| G2-7 | Term Colostrum | 31.35 | 1.85 | 0.737 | 2.6 | 89% |
| G2-8 | Term Colostrum | 10.812 | 1.694 | 0.449 | 1 | 43% |
| G2-9 | Term Colostrum | 10.894 | 1.478 | 0.805 | 1 | 84% |
| G2-10 | Term Colostrum | 12.55 | 1.468 | 0.528 | 1 | 82% |
| G3-1 | Moderate/Very Preterm Mature Milk | 16.31 | 1.963 | 0.629 | 2.5 | 80% |
| G3-2 | Moderate/Very Preterm Mature Milk | 12.55 | 1.489 | 0.337 | 1 | 56% |
| G3-3 | Moderate/Very Preterm Mature Milk | 10.821 | 1.687 | 0.045 | 1 | 57% |
| G3-4 | Moderate/Very Preterm Mature Milk | 10.739 | 1.904 | 0.36 | 1 | 60% |
| G3-6 | Moderate/Very Preterm Mature Milk | 10.853 | 1.876 | 0.364 | 1 | 90% |
| G3-7 | Moderate/Very Preterm Mature Milk | 13.23 | 1.433 | 0.703 | 1 | 89% |
| G3-8 | Moderate/Very Preterm Mature Milk | 10.058 | 1.546 | 0.386 | 1 | 90% |
| G3-10 | Moderate/Very Preterm Mature Milk | 10.023 | 1.662 | 0.284 | 1 | 85% |
| G4-1 | Moderate/Very Preterm Colostrum | 16 | 1.818 | 0.426 | 1.7 | 84% |
| G4-2 | Moderate/Very Preterm Colostrum | 11.55 | 1.53 | 0.526 | 1 | 87% |
| G4-3 | Moderate/Very Preterm Colostrum | 14.43 | 1.634 | 0.401 | 1 | 88% |
| G4-4 | Moderate/Very Preterm Colostrum | 10.512 | 1.726 | 0.426 | 1 | 91% |
| G4-5 | Moderate/Very Preterm Colostrum | 10.91 | 1.786 | 0.264 | 1 | 83% |
| G4-6 | Moderate/Very Preterm Colostrum | 11 | 1.571 | 0.158 | 1 | 54% |
| G5-1 | Late Preterm Mature Milk | 26.61 | 1.641 | 0.636 | 1.3 | 86% |
| G5-2 | Late Preterm Mature Milk | 27.02 | 1.9 | 0.606 | 2.6 | 68% |
| G5-3 | Late Preterm Mature Milk | 19.71 | 1.806 | 0.387 | 1.2 | 53% |
| G5-4 | Late Preterm Mature Milk | 18.42 | 1.915 | 0.426 | 1.3 | 72% |
| Comparison | Reference Group | Significant Mirnas |
|---|---|---|
| T Colostrum (G2) vs. T Mature Milk (G1) | G1 | 11 |
| MVPT Mature Milk (G3) vs. T Mature Milk (G1) | G1 | 24 |
| LPT Mature Milk (G5) vs. T Mature Milk (G1) | G1 | 15 |
| MVPT Colostrum (G4) vs. T Colostrum (G2) | G2 | 27 |
| MVPT Colostrum (G4) vs. MVPT Mature Milk (G3) | G3 | 19 |
| LPT Mature Milk (G5) vs. MVPT Mature Milk (G3) | G3 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freiría-Martínez, L.; Iglesias-Martínez-Almeida, M.; Rodríguez-Jamardo, C.; Rivera-Baltanás, T.; Comís-Tuche, M.; Rodrígues-Amorím, D.; Fernández-Palleiro, P.; Blanco-Formoso, M.; Diz-Chaves, Y.; González-Freiria, N.; et al. Human Breast Milk microRNAs, Potential Players in the Regulation of Nervous System. Nutrients 2023, 15, 3284. https://doi.org/10.3390/nu15143284
Freiría-Martínez L, Iglesias-Martínez-Almeida M, Rodríguez-Jamardo C, Rivera-Baltanás T, Comís-Tuche M, Rodrígues-Amorím D, Fernández-Palleiro P, Blanco-Formoso M, Diz-Chaves Y, González-Freiria N, et al. Human Breast Milk microRNAs, Potential Players in the Regulation of Nervous System. Nutrients. 2023; 15(14):3284. https://doi.org/10.3390/nu15143284
Chicago/Turabian StyleFreiría-Martínez, Luis, Marta Iglesias-Martínez-Almeida, Cynthia Rodríguez-Jamardo, Tania Rivera-Baltanás, María Comís-Tuche, Daniela Rodrígues-Amorím, Patricia Fernández-Palleiro, María Blanco-Formoso, Yolanda Diz-Chaves, Natalia González-Freiria, and et al. 2023. "Human Breast Milk microRNAs, Potential Players in the Regulation of Nervous System" Nutrients 15, no. 14: 3284. https://doi.org/10.3390/nu15143284
APA StyleFreiría-Martínez, L., Iglesias-Martínez-Almeida, M., Rodríguez-Jamardo, C., Rivera-Baltanás, T., Comís-Tuche, M., Rodrígues-Amorím, D., Fernández-Palleiro, P., Blanco-Formoso, M., Diz-Chaves, Y., González-Freiria, N., Suárez-Albo, M., Martín-Forero-Maestre, M., Durán Fernández-Feijoo, C., Fernández-Lorenzo, J. R., Concheiro Guisán, A., Olivares, J. M., & Spuch, C. (2023). Human Breast Milk microRNAs, Potential Players in the Regulation of Nervous System. Nutrients, 15(14), 3284. https://doi.org/10.3390/nu15143284







