Yacon (Smallanthus sonchifolius) Flour Reduces Inflammation and Had No Effects on Oxidative Stress and Endotoxemia in Wistar Rats with Induced Colorectal Carcinogenesis
Abstract
1. Introduction
2. Methods and Materials
2.1. Animals and Experimental Diet
2.2. Yacon Flour Preparation and Experimental Diet
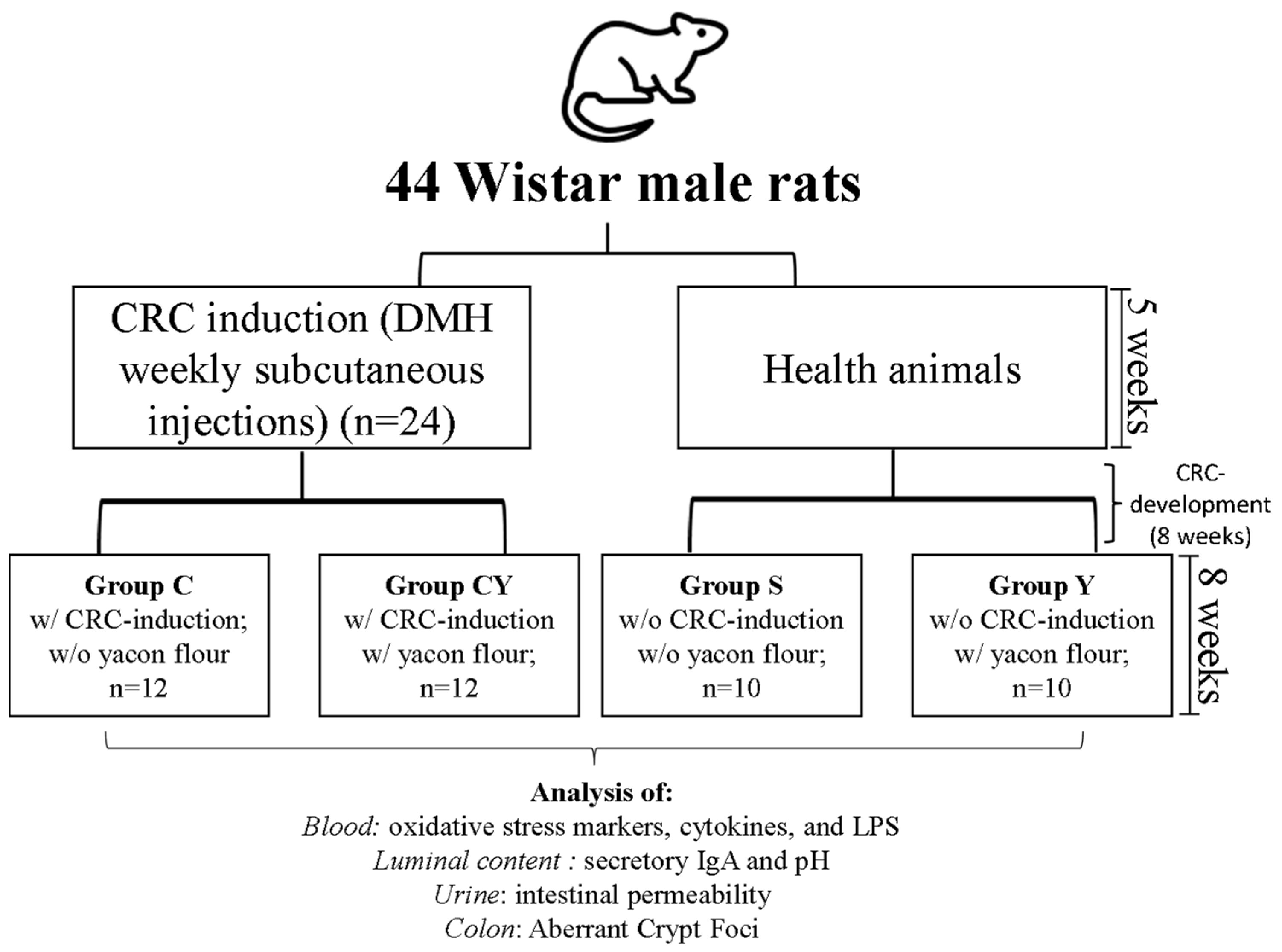
2.3. Experimental Design
- -
- Group S: animals without colorectal cancer induction and without yacon flour; n = 10
- -
- Group Y: animals without colorectal cancer induction and with yacon flour; n = 10
- -
- Group C: animals with colorectal cancer induction and without yacon flour; n = 12
- -
- Group CY: animals with colorectal cancer induction and with yacon flour; n = 12
2.4. Oxidative Stress Markers
2.5. Secretory IgA, Endotoxemia and Total Antioxidant Capacity of Plasma
2.6. Cytokines Release
2.7. Aberrant Crypt Foci (ACF) Analysis
2.8. Intestinal Permeability and Fecal Short-Chain Fatty Acids (SCFAs) Analysis
2.9. Intraluminal pH of the Colon
2.10. In Silico Analysis
2.11. Statistical Analysis
3. Results
3.1. Yacon Composition
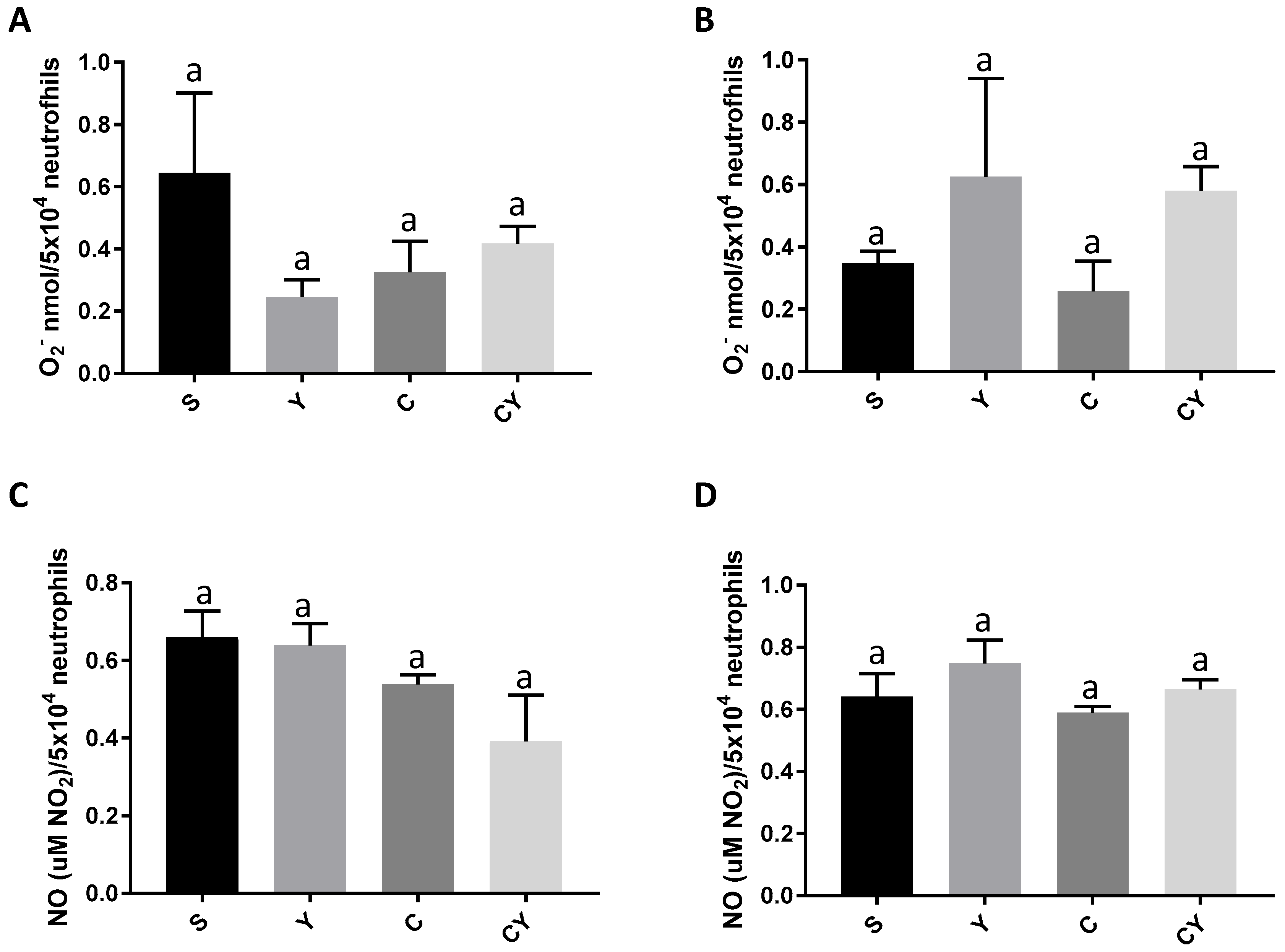
3.2. Superoxide Anion and Nitric Oxide Release
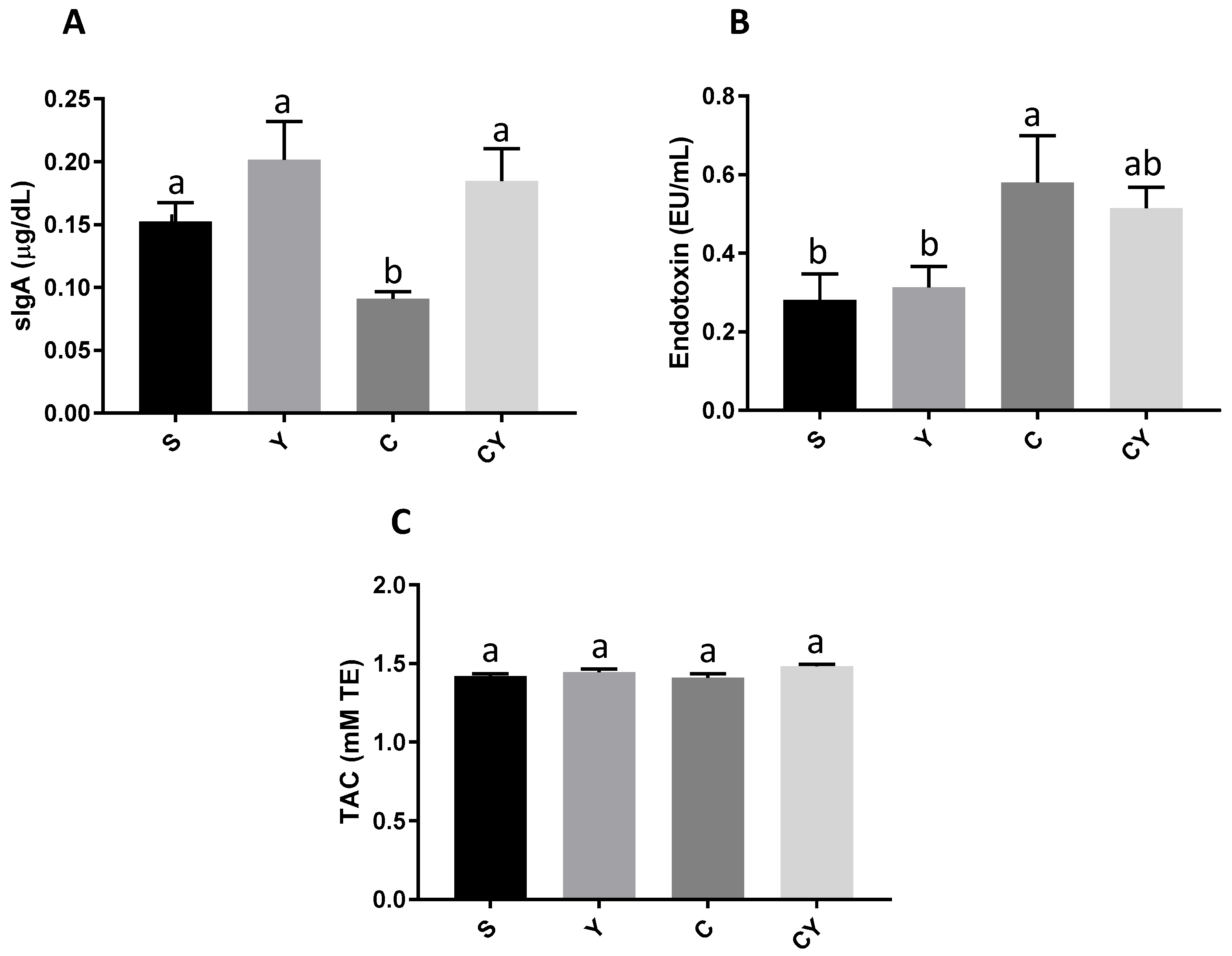
3.3. Secretory IgA Production, Endotoxemia and Total Antioxidant Capacity
3.4. Cytokines Release
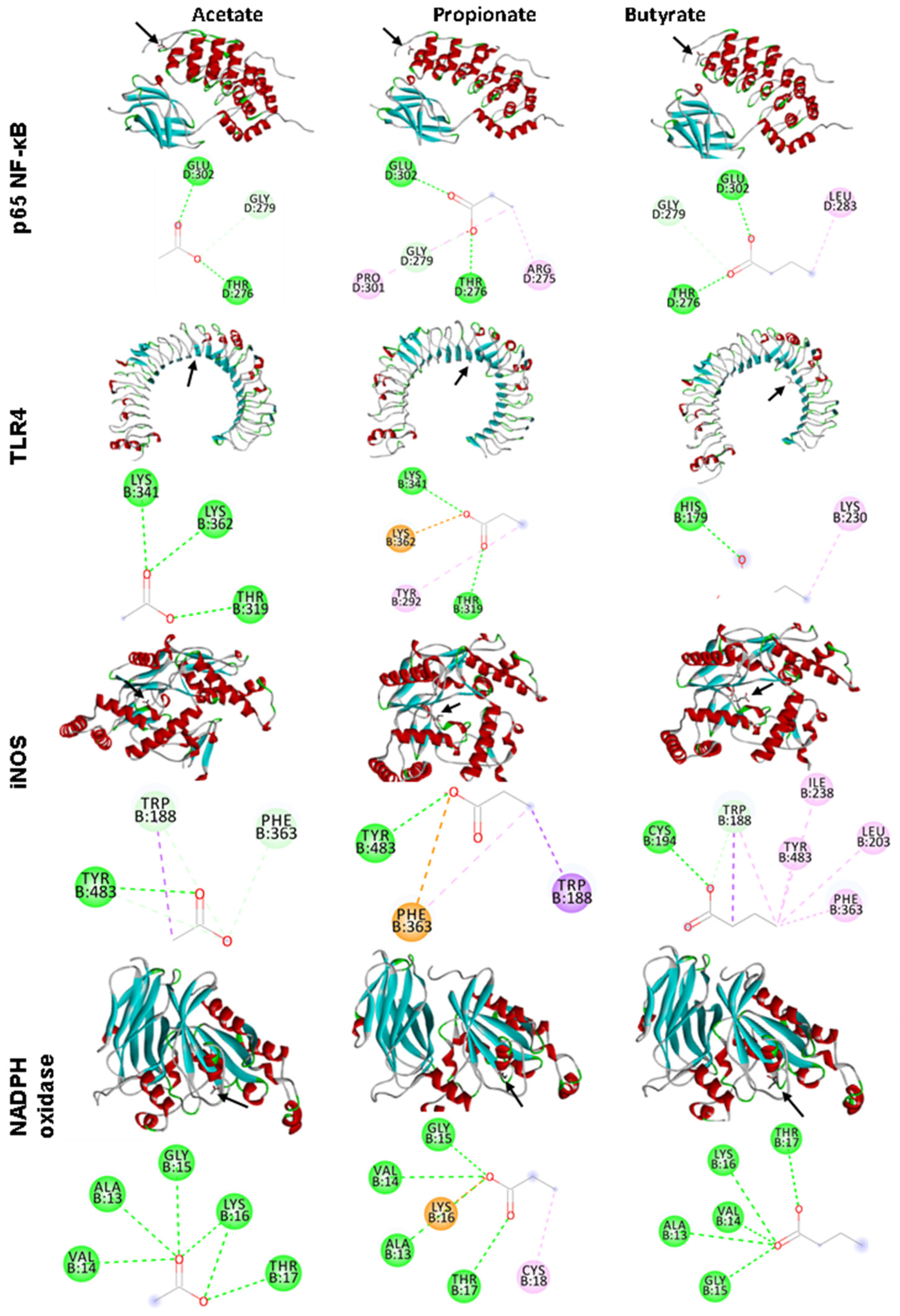
3.5. In Silico Analyses
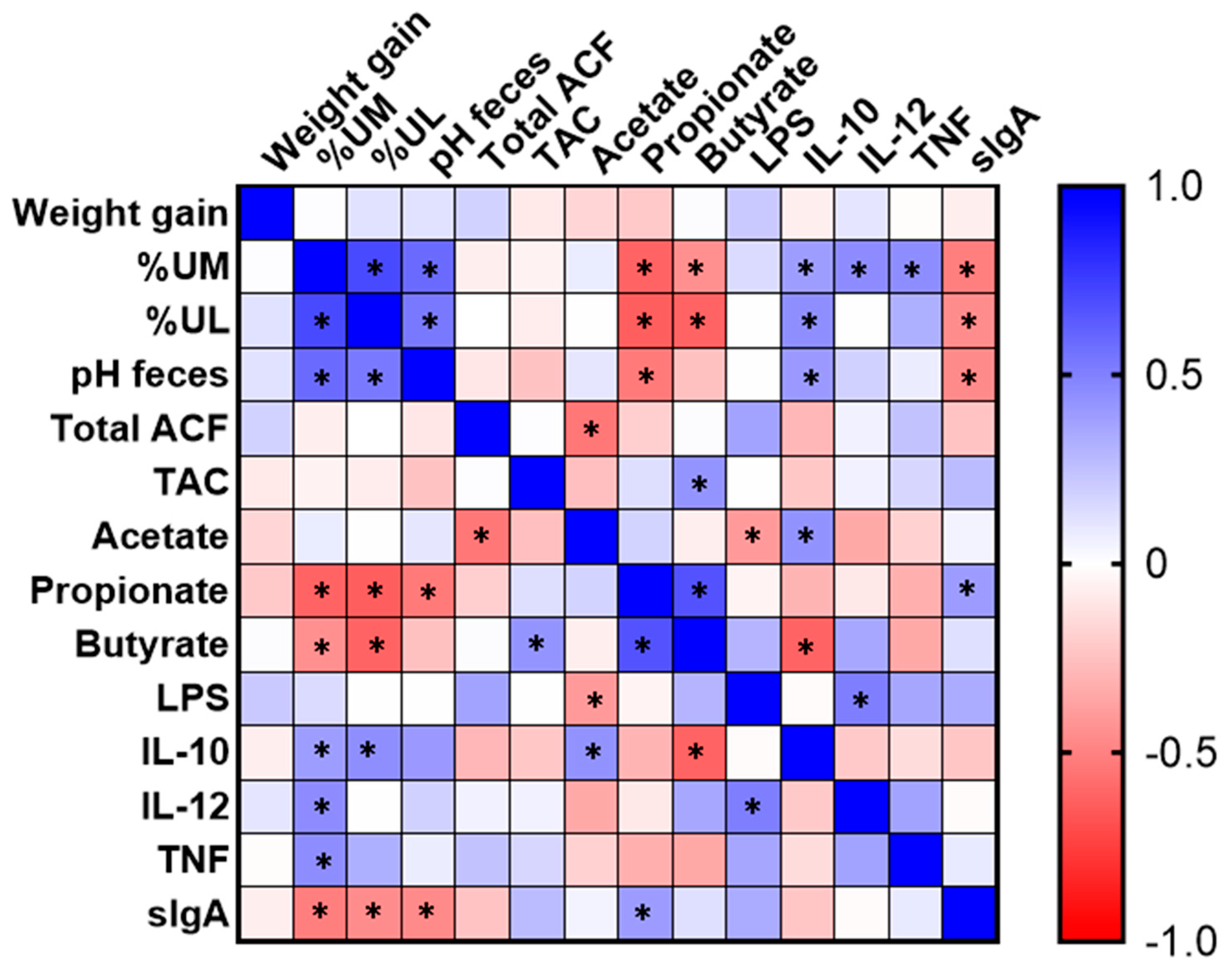
3.6. Correlation Analysis
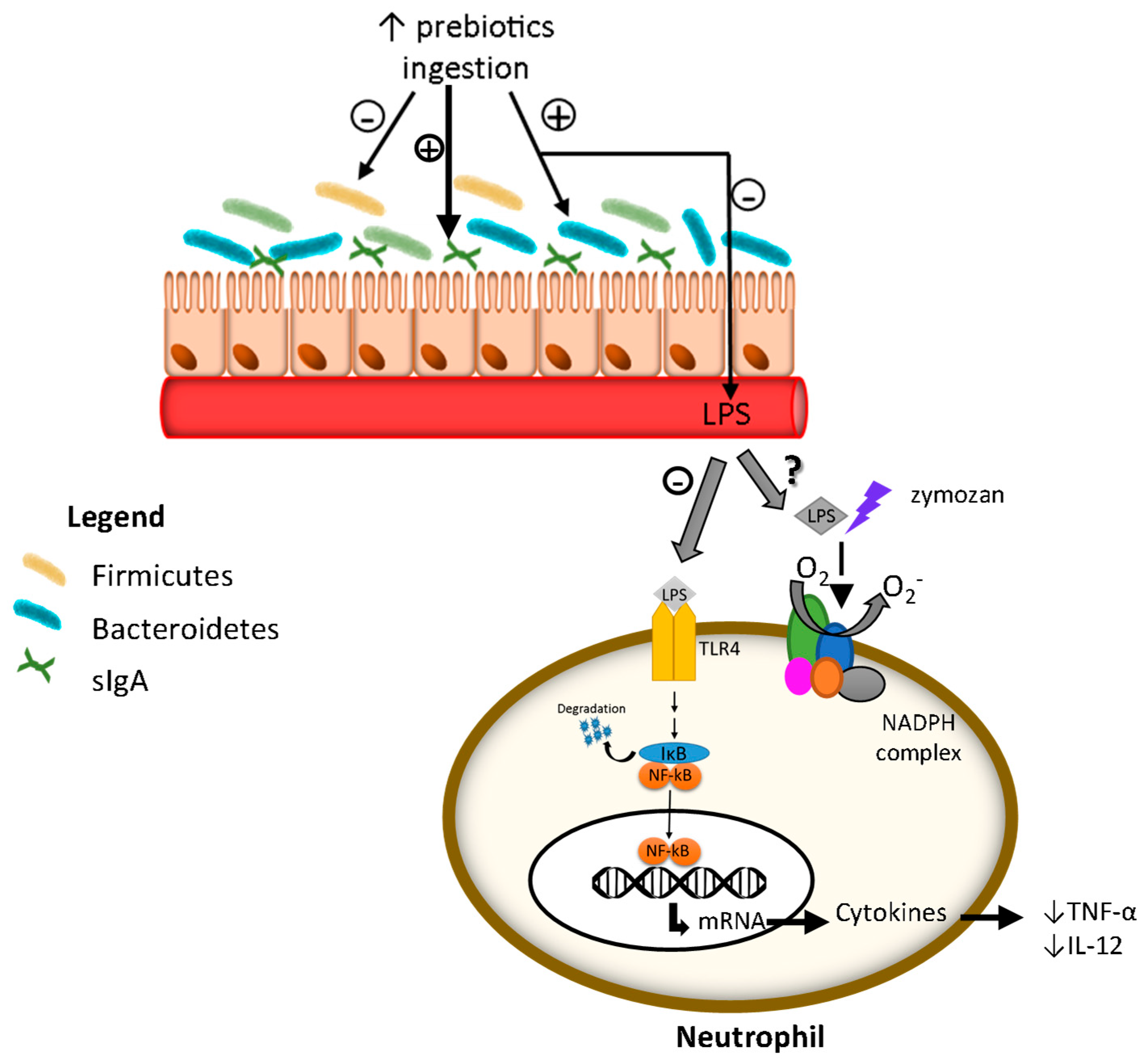
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bardelcíková, A.; Šoltys, J.; Mojžiš, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Katsaounou, K.; Nicolaou, E.; Vogazianos, P.; Brown, C.; Stavrou, M.; Teloni, S.; Hatzis, P.; Agapiou, A.; Fragkou, E.; Tsiaoussis, G.; et al. Colon Cancer: From Epidemiology to Prevention. Metabolites 2022, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.H.; Li, J.; Shu, H.J.; Li, Z.L.; Qian, J.M. Serum Immunoinflammation-Related Protein Complexes Discriminate between Inflammatory Bowel Disease and Colorectal Cancer. Clin. Transl. Oncol. 2019, 21, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, K.; Aleksandrova, K.; Fedirko, V.; Jenab, M.; Gunter, M.J.; Siersema, P.D.; Wu, K.; Katzke, V.; Kaaks, R.; Panico, S.; et al. Pre-Diagnostic C-Reactive Protein Concentrations, CRP Genetic Variation and Mortality among Individuals with Colorectal Cancer in Western European Populations. BMC Cancer 2022, 22, 695. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhang, J.; Wu, Q.; Chen, J.; Liu, J.; Wang, L.; Chen, C.; Xu, J.; Zhang, H.; Shi, C.; et al. The Role of Microbiota in the Development of Colorectal Cancer. Int. J. Cancer 2019, 148, 2032–2041. [Google Scholar] [CrossRef]
- Li, Q.; von Ehrlich-Treuenstätt, V.; Schardey, J.; Wirth, U.; Zimmermann, P.; Andrassy, J.; Bazhin, A.V.; Werner, J.; Kühn, F. Gut Barrier Dysfunction and Bacterial Lipopolysaccharides in Colorectal Cancer. J. Gastrointest. Surg. 2023; ahead of print. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Zamyatnin, A.A.; Werner, J.; Bazhin, A.V. Reactive Oxygen Species and Colorectal Cancer. J. Cell. Physiol. 2018, 233, 5119–5132. [Google Scholar] [CrossRef]
- De Almeida, C.V.; De Camargo, M.R.; Russo, E.; Amedei, A. Role of Diet and Gut Microbiota on Colorectal Cancer Immunomodulation. World J. Gastroenterol. 2019, 25, 151–162. [Google Scholar] [CrossRef]
- Wang, F.; Ugai, T.; Haruki, K.; Wan, Y.; Akimoto, N.; Arima, K.; Zhong, R.; Twombly, T.S.; Wu, K.; Yin, K.; et al. Healthy and Unhealthy Plant-based Diets in Relation to the Incidence of Colorectal Cancer Overall and by Molecular Subtypes. Clin. Transl. Med. 2022, 12, e893. [Google Scholar] [CrossRef]
- Nadai, L.D.; Fernando, L.; Moraes, D.S.; Cristina, B.; Dias, M.; Teixeira, D.O.; Calcagno, T.; Bruno, V.; Egídio, I.; Caroline, A.; et al. Yacon (Smallanthus sonchifolius)-Based Product Increases Fecal Short-Chain Fatty Acids and Enhances Regulatory T Cells by Downregulating ROR γ t in the Colon of BALB/c Mice. J. Funct. Foods 2019, 55, 333–342. [Google Scholar] [CrossRef]
- Grancieri, M.; Costa, N.M.; Vaz Tostes, M.D.; de Oliveira, D.; Nunes, L.D.; Marcon, L.D.; Veridiano, T.; Viana, M. Yacon Flour (Smallanthus sonchifolius) Attenuates Intestinal Morbidity in Rats with Colon Cancer. J. Funct. Foods 2017, 37, 666–675. [Google Scholar] [CrossRef]
- de Moura, N.A.; Caetano, B.F.R.; Sivieri, K.; Urbano, L.H.; Cabello, C.; Rodrigues, M.A.M.; Barbisan, L.F. Protective Effects of Yacon (Smallanthus sonchifolius) Intake on Experimental Colon Carcinogenesis. Food Chem. Toxicol. 2012, 50, 2902–2910. [Google Scholar] [CrossRef]
- Martino, H.S.D.; Kolba, N.; Tako, E. Yacon (Smallanthus sonchifolius) Flour Soluble Extract Improve Intestinal Bacterial Populations, Brush Border Membrane Functionality and Morphology In Vivo (Gallus Gallus). Food Res. Int. 2020, 137, 109705. [Google Scholar] [CrossRef]
- Verediano, T.A.; Viana, M.L.; das Graças Vaz Tostes, M.; de Oliveira, D.S.; de Carvalho Nunes, L.; Costa, N.M.B. Yacón (Smallanthus sonchifolius) Prevented Inflammation, Oxidative Stress, and Intestinal Alterations in an Animal Model of Colorectal Carcinogenesis. J. Sci. Food Agric. 2020, 100, 5442–5449. [Google Scholar] [CrossRef]
- Grancieri, M.; Machado, P.A.; Oliveira, D.F.; Marcon, L.N.; Vaz Tostes, M.G.; Costa, N.M.B.; Ignacchiti, M.D.C.; Nunes, L.C.; Viana, M.L. Efeito Da Farinha de Yacon (Smallanthus sonchifolius) Na Resposta Imunológica Intestinal No Câncer Colorretal. Braspen J. 2016, 31, 335–339. [Google Scholar]
- das Graças Vaz-Tostes, M.; Viana, M.L.; Grancieri, M.; dos Santos Luz, T.C.; de Paula, H.; Pedrosa, R.G.; Costa, N.M.B. Yacon Effects in Immune Response and Nutritional Status of Iron and Zinc in Preschool Children. Nutrition 2014, 30, 666–672. [Google Scholar] [CrossRef]
- Machado, A.M.; da Silva, N.B.M.; de Freitas, R.M.P.; de Freitas, M.B.D.; Chaves, J.B.P.; Oliveira, L.L.; Martino, H.S.D.; Alfenas, R.d.C.G. Effects of Yacon Flour Associated with an Energy Restricted Diet on Intestinal Permeability, Fecal Short Chain Fatty Acids, Oxidative Stress and Inflammation Markers Levels in Adults with Obesity or Overweight: A Randomized, Double Blind, Placebo Controll. Arch. Endocrinol. Metab. 2020, 64, 597–607. [Google Scholar] [CrossRef]
- Cocato, M.L.; Lobo, A.R.; Azevedo-Martins, A.K.; Mancini, J.; Sá, L.R.M.; Colli, C. Effects of a Moderate Iron Overload and Its Interaction with Yacon Flour, and/or Phytate, in the Diet on Liver Antioxidant Enzymes and Hepatocyte Apoptosis in Rats. Food Chem. 2019, 285, 171–179. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemistry Official Methods of Analysis; AOAC: Gaithersburg, MD, USA, 2012; Volume 19. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Commite on the Refomurlation of the AIN-76A Rodent Diet. Am. Inst. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Rodrigues, M.A.M.; Silva, L.A.G.; Salvadori, D.M.F.; De Camargo, J.L.V.; Montenegro, M.R. Aberrant Crypt Foci and Colon Cancer: Comparison between a Short- and Medium-Term Bioassay for Colon Carcinogenesis Using Dimethylhydrazine in Wistar Rats. Braz. J. Med. Biol. Res. 2002, 35, 351–355. [Google Scholar] [CrossRef]
- Bird, J.K.; Raederstorff, D.; Weber, P.; Steinert, R.E. Cardiovascular and Antiobesity Effects of Resveratrol Mediated through the Gut Microbiota. Adv. Nutr. Int. Rev. J. 2017, 8, 839–849. [Google Scholar] [CrossRef]
- Henson, P.M. The Immunologic Release of Constituents from Neutrophil Leukocytes. J. Immunol. 1971, 107, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.B.; Lehmeyer, J.E. Elaboration of Toxic Oxygen by Products by Neutrophils in a Model of Immune Complex Disease. J. Clin. Investig. 1976, 57, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite, and [15N] Nitrate in Biological Fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, R.; Wang, X.; He, P.; Tan, L.; Ma, X. Dietary Grape-Seed Procyanidins Decreased Postweaning Diarrhea by Modulating Intestinal Permeability and Suppressing Oxidative Stress in Rats. J. Agric. Food Chem. 2011, 59, 6227–6232. [Google Scholar] [CrossRef]
- Kotani, A.; Miyaguchi, Y.; Kohama, M.; Ohtsuka, T.; Shiratori, T.; Kusu, F. Determination of Short-Chain Fatty Acids in Rat and Human Feces by High-Performance Liquid Chromatography with Electrochemical Detection. Anal. Sci. 2009, 25, 1007–1011. [Google Scholar] [CrossRef]
- de Sá, L.R.V.; de Oliveira, M.A.L.; Cammarota, M.C.; Matos, A.; Ferreira-Leitão, V.S. Simultaneous Analysis of Carbohydrates and Volatile Fatty Acids by HPLC for Monitoring Fermentative Biohydrogen Production. Int. J. Hydrog. Energy 2011, 36, 15177–15186. [Google Scholar] [CrossRef]
- Morris, G.; Huey, R. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Grancieri, M.; Martino, H.S.D.; Gonzalez de Mejia, E. Digested Total Protein and Protein Fractions from Chia Seed (Salvia Hispanica L.) Had High Scavenging Capacity and Inhibited 5-LOX, COX-1-2, and INOS Enzymes. Food Chem. 2019, 289, 204–214. [Google Scholar] [CrossRef]
- Lwanga, S.K.; Lemeshow, S.; World Health Organization. Sample Size Determination in Health Studies: A Practical Manual; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- de Almeida Brasiel, P.G.; Luquetti, S.C.P.D.; Medeiros, J.D.; do Amaral Corrêa, J.O.; Machado, A.B.F.; Moreira, A.P.B.; Rocha, V.N.; de Souza, C.T.; Peluzio, M.d.C.G. Kefir Modulates Gut Microbiota and Reduces DMH-Associated Colorectal Cancer via Regulation of Intestinal Inflammation in Adulthood Offsprings Programmed by Neonatal Overfeeding. Food Res. Int. 2022, 152, 110708. [Google Scholar] [CrossRef]
- Alatorre-Santamaría, S.; Cruz-Guerrero, A.; Guzmán-Rodríguez, F. Fructooligosaccharides (FOS). In Handbook of Food Bioactive Ingredients; Springer: Berlin/Heidelberg, Germany, 2022; p. 1. [Google Scholar]
- Mahalak, K.K.; Firrman, J.; Narrowe, A.B.; Hu, W.; Jones, S.M.; Bittinger, K.; Moustafa, A.M.; Liu, L.S. Fructooligosaccharides (FOS) Differentially Modifies the in Vitro Gut Microbiota in an Age-Dependent Manner. Front. Nutr. 2023, 9, 1058910. [Google Scholar] [CrossRef]
- Silva-Reis, R.; Castro-Ribeiro, C.; Gonçalves, M.; Ferreira, T.; Pires, M.J.; Iglesias-Aguirre, C.E.; Cortés-Martín, A.; Selma, M.V.; Espín, J.C.; Nascimento-Gonçalves, E.; et al. An Integrative Approach to Characterize the Early Phases of Dimethylhydrazine-Induced Colorectal Carcinogenesis in the Rat. Biomedicines 2022, 10, 409. [Google Scholar] [CrossRef]
- Jangid, A.; Fukuda, S.; Kato, T.; Seki, M.; Suzuki, Y.; Taylor, T.D.; Ohno, H.; Prakash, T. Impact of Dietary Fructooligosaccharides (FOS) on Murine Gut Microbiota and Intestinal IgA Secretion. 3 Biotech 2022, 12, 56. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ichimura, M.; Tsuneyama, K.; Moritoki, Y.; Tsunashima, H.; Omagari, K.; Hara, M.; Yasuda, I.; Miyakawa, H.; Kikuchi, K. Fructo-Oligosaccharides and Intestinal Barrier Function in a Methionine–Choline-Deficient Mouse Model of Nonalcoholic Steatohepatitis. PLoS ONE 2017, 12, e0175406. [Google Scholar] [CrossRef]
- Xie, X.; He, Y.; Li, H.; Yu, D.; Na, L.; Sun, T.; Zhang, D.; Shi, X.; Xia, Y.; Jiang, T.; et al. Effects of Prebiotics on Immunologic Indicators and Intestinal Microbiota Structure in Perioperative Colorectal Cancer Patients. Nutrition 2019, 61, 132–142. [Google Scholar] [CrossRef]
- Turula, H.; Wobus, C.E. The Role of the Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins during Mucosal Infection and Immunity. Viruses 2018, 10, 237. [Google Scholar] [CrossRef]
- Roche, L.D.; López, A.M.V.; Martínez-Sánchez, G. Procesos Moleculares Patogénicos de La Aterosclerosis y Alternativas Terapéuticas Para Su Control. Rev. Cuba. Farm. 2012, 46, 267–280. [Google Scholar]
- Page, M.J.; Bester, J.; Pretorius, E. The Inflammatory Effects of TNF-α and Complement Component 3 on Coagulation. Sci. Rep. 2018, 8, 1812. [Google Scholar] [CrossRef]
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Picu, A.; Petcu, L.; Cucu, N.; Chifiriuc, M.C. Gut Microbiota, Host Organism, and Diet Trialogue in Diabetes and Obesity. Front. Nutr. 2019, 6, 21. [Google Scholar] [CrossRef]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective Increases of Bifidobacteria in Gut Microflora Improve High-Fat-Diet-Induced Diabetes in Mice through a Mechanism Associated with Endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef]
- Bo Liu, D.; Tonkonogy, S.L.; Sartor, R.B. IL-10 Produced by Antigen Presenting Cells Inhibits Bacterial- Responsive TH1/TH17 Cells and Suppresses Colitis in Mice. Gastroenterology 2011, 141, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.; Morris, V.; Greenbaum, J.A.; Park, Y.; Bjoerheden, U.; Mikulski, Z.; Muffley, T.; Shui, J.W.; Kim, G.; Cheroutre, H.; et al. IL-10-Producing Intestinal Macrophages Prevent Excessive Antibacterial Innate Immunity by Limiting IL-23 Synthesis. Nat. Commun. 2015, 6, 7055. [Google Scholar] [CrossRef] [PubMed]
- Owoloye, A.J.; Ligali, F.C.; Enejoh, O.A.; Musa, A.Z.; Aina, O.; Idowu, E.T.; Oyebola, K.M. Molecular Docking, Simulation and Binding Free Energy Analysis of Small Molecules as Pf HT1 Inhibitors. PLoS ONE 2022, 17, e0268269. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights into Protein–Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Döppler, H.; Storz, P. Mitochondrial and Oxidative Stress- Mediated Activation of Protein Kinase D1 and Its Importance in Pancreatic Cancer. Front. Oncol. 2017, 7, 41. [Google Scholar] [CrossRef]
- Khosravi, M.; Poursaleh, A.; Ghasempour, G.; Farhad, S.; Najafi, M. The Effects of Oxidative Stress on the Development of Atherosclerosis. Biol. Chem. 2019, 400, 711–732. [Google Scholar] [CrossRef]
- Delgado, G.T.C.; Thomé, R.; Gabriel, D.L.; Tamashiro, W.M.S.C.; Pastore, G.M. Yacon (Smallanthus sonchifolius)-Derived Fructooligosaccharides Improves the Immune Parameters in the Mouse. Nutr. Res. 2012, 32, 884–892. [Google Scholar] [CrossRef]
- Sousa, S.; Pinto, J.; Rodrigues, C.; Gião, M.; Pereira, C.; Tavaria, F.; Xavier Malcata, F.; Gomes, A.; Bertoldo Pacheco, M.T.; Pintado, M. Antioxidant Properties of Sterilized Yacon (Smallanthus sonchifolius) Tuber Flour. Food Chem. 2015, 188, 504–509. [Google Scholar] [CrossRef]
- Kampa, M.; Nistikaki, A.; Tsaousis, V.; Maliaraki, N.; Notas, G.; Castanas, E. A New Automated Method for the Determination of the Total Antioxidant Capacity (TAC) of Human Plasma, Based on the Crocin Bleaching Assay. BMC Clin. Pathol. 2002, 16, 3. [Google Scholar] [CrossRef]
- Viceconti, M.; Pappalardo, F.; Rodriguez, B.; Horner, M.; Bischoff, J.; Tshinanu, F.M. In silico trials: Verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods 2021, 185, 120–127. [Google Scholar] [CrossRef]

| Components | Amount (g) |
|---|---|
| Non-digestible carbohydrates | |
| Fructooligosaccharide | 52.20 ± 0.01 |
| Inulin | 6.61 ± 0.00 |
| Other Fibers | 10.68 ± 0.08 |
| Macronutrients | |
| Simple Carbohydrates | |
| Fructose | 8.16 ± 0.01 |
| Glucose | 3.76 ± 0.00 |
| Sucrose | 7.25 ± 0.01 |
| Proteins | 4.52 ± 0.25 |
| Moisture | 3.72 ± 0.51 |
| Ash | 2.94 ± 0.03 |
| Lipids | 0.33 ± 0.01 |
| Ingredients (per kg of Diet) | S and C Groups | Y and CY Groups |
|---|---|---|
| AIN-93M | AIN-93M + YF | |
| Casein (g) | 140.0 | 130.14 |
| Dextrinized starch (g) | 150.5 | 150.5 |
| Sucrose (g) | 100.0 | 70.24 |
| Soybean oil (mL) | 40.0 | 40.0 |
| Microcrystalline cellulose (g) | 50.0 | 0 |
| Minerals Mix (g) | 30.5 | 30.5 |
| Vitamin Mix (g) | 10.0 | 10.0 |
| L-cystine (g) | 1.80 | 1.80 |
| Choline bitartrate (g) | 2.50 | 2.50 |
| Corn starch (g) | 474.7 | 423.95 |
| Yacon flour (g) | 0 | 140.37 |
| Nutrition composition | ||
| Caloric Density (kcal/g) | 3.72 | 3.54 |
| Acetate | Propionate | Butyrate | ||||
|---|---|---|---|---|---|---|
| EFE | IAAR | EFE | IAAR | EFE | IAAR | |
| p65 NF-KB | −2.9 | GLU D: 302; GLY D: 279; THR D: 276 | −3.2 | GLU D: 302; GLY D: 279; PRO D: 301; THR D:276; ARD D: 275 | −3.4 | TRH D: 276; GLY D: 276; GLU D: 302; LEU D: 283 |
| TLR4 | −3.4 | LYS B: 341; LYS B: 362; THR B: 319 | −3.6 | LYS B: 341; LYS B: 362; THR B: 319; TYR B: 292 | −3.1 | HIS B: 179; LYS B: 230 |
| iNOS | −3.2 | TYR B: 483; TRP B: 188; PHE B: 363 | −3.9 | TYR B: 483; TRP B: 188; PHE B: 363 | −4.3 | CYS B: 194; TRP B: 188; ILE B: 238; TYR B: 483; LEU B: 203; PHE B: 363 |
| NADPH oxidase | −3.6 | VAL B: 14; ALA B: 13; GLY B: 15; LYS B: 16; THR B: 17 | −4 | GLY B: 15; VAL B: 14; LYS B: 16; ALA B: 13; THR B: 17; CYS B: 18 | −4.1 | GLY B: 15; ALA B: 13; VAL B: 14; LYS B: 16; THR B: 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grancieri, M.; Viana, M.L.; de Oliveira, D.F.; Vaz Tostes, M.d.G.; Costa Ignacchiti, M.D.; Costa, A.G.V.; Brunoro Costa, N.M. Yacon (Smallanthus sonchifolius) Flour Reduces Inflammation and Had No Effects on Oxidative Stress and Endotoxemia in Wistar Rats with Induced Colorectal Carcinogenesis. Nutrients 2023, 15, 3281. https://doi.org/10.3390/nu15143281
Grancieri M, Viana ML, de Oliveira DF, Vaz Tostes MdG, Costa Ignacchiti MD, Costa AGV, Brunoro Costa NM. Yacon (Smallanthus sonchifolius) Flour Reduces Inflammation and Had No Effects on Oxidative Stress and Endotoxemia in Wistar Rats with Induced Colorectal Carcinogenesis. Nutrients. 2023; 15(14):3281. https://doi.org/10.3390/nu15143281
Chicago/Turabian StyleGrancieri, Mariana, Mirelle Lomar Viana, Daniela Furtado de Oliveira, Maria das Graças Vaz Tostes, Mariana Drummond Costa Ignacchiti, André Gustavo Vasconcelos Costa, and Neuza Maria Brunoro Costa. 2023. "Yacon (Smallanthus sonchifolius) Flour Reduces Inflammation and Had No Effects on Oxidative Stress and Endotoxemia in Wistar Rats with Induced Colorectal Carcinogenesis" Nutrients 15, no. 14: 3281. https://doi.org/10.3390/nu15143281
APA StyleGrancieri, M., Viana, M. L., de Oliveira, D. F., Vaz Tostes, M. d. G., Costa Ignacchiti, M. D., Costa, A. G. V., & Brunoro Costa, N. M. (2023). Yacon (Smallanthus sonchifolius) Flour Reduces Inflammation and Had No Effects on Oxidative Stress and Endotoxemia in Wistar Rats with Induced Colorectal Carcinogenesis. Nutrients, 15(14), 3281. https://doi.org/10.3390/nu15143281





