Bioactivity of Macronutrients from Chlorella in Physical Exercise
Abstract
1. Introduction
2. Methods
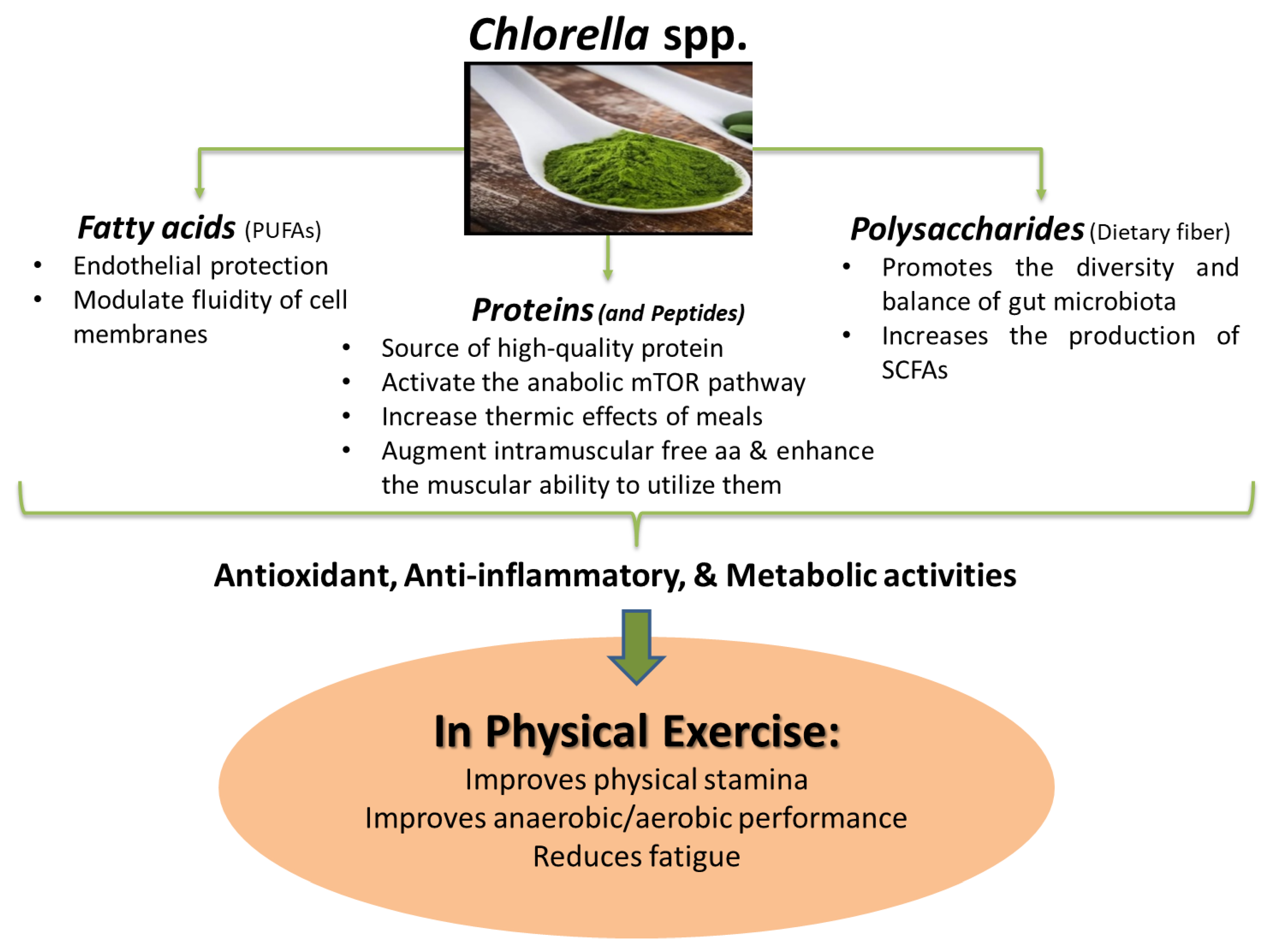
3. Chlorella spp. and Physical Exercise
4. Bioactive Macronutrients from Chlorella and Physical Exercise
4.1. Benefits of Proteins and Peptides from Chlorella for Physical Exercise
4.2. Benefits of Polysaccharides from Chlorella for Physical Exercise
4.3. Benefits of Fatty Acids from Chlorella for Physical Exercise
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Dinh-Toi, C.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; Miranda Bernardo de Morais, A.M.; Santos Costa de Morais, R.M. Emergent sources of prebiotics: Seaweeds and microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals from algae and cyanobacteria. In Algal Green Chemistry: Recent Progress in Biotechnology; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–89. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pages, T.; Viscor, G.; Torres, J.L. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Hseu, R.S.; Lin, L.P. Identification of Chlorella spp. isolates using ribosomal DNA sequences. Bot. Bull. Acad. Sin. 2001, 42, 115–121. [Google Scholar]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture: Applied Phycology and Biotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a dietary supplement to promote human health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef]
- Schulze, C.; Wetzel, M.; Reinhardt, J.; Schmidt, M.; Felten, L.; Mundt, S. Screening of microalgae for primary metabolites including β-glucans and the influence of nitrate starvation and irradiance on β-glucan production. J. Appl. Phycol. 2016, 28, 2719–2725. [Google Scholar] [CrossRef]
- Pieper, S.; Unterieser, I.; Mann, F.; Mischnick, P. A new arabinomannan from the cell wall of the chlorococcal algae Chlorella vulgaris. Carbohydr. Res. 2012, 352, 166–176. [Google Scholar] [CrossRef]
- Wan, X.-z.; Ai, C.; Chen, Y.-h.; Gao, X.-x.; Zhong, R.-t.; Liu, B.; Chen, X.-h.; Zhao, C. Physicochemical characterization of a polysaccharide from green microalga Chlorella pyrenoidosa and its hypolipidemic activity via gut microbiota regulation in rats. J. Agric. Food Chem. 2020, 68, 1186–1197. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae for human and animal nutrition. In Handbook of Microalgal Culture; Richmon, A., Ed.; John Wiley & Sons: Oxford, UK, 2013; pp. 461–503. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Ryu, B.; Ahn, G.; Yeo, I.-K.; Jeon, Y.-J. Therapeutic potential of algal natural products against metabolic syndrome: A review of recent developments. Trends Food Sci. Technol. 2020, 97, 286–299. [Google Scholar] [CrossRef]
- Suetsuna, K.; Chen, J.-R. Identification of antihypertensive peptides from peptic digest of two microalgae, Chlorella vulgaris and Spirulina platensis. Mar. Biotechnol. 2001, 3, 305–309. [Google Scholar] [CrossRef]
- Tiberg, E.; Dreborg, S.; Bjorksten, B. Allergy to green-algae (Chlorella) among children. J. Allergy Clin. Immunol. 1995, 96, 257–259. [Google Scholar] [CrossRef]
- Fallah, A.A.; Sarmast, E.; Habibian Dehkordi, S.; Engardeh, J.; Mahmoodnia, L.; Khaledifar, A.; Jafari, T. Effect of Chlorella supplementation on cardiovascular risk factors: A meta-analysis of randomized controlled trials. Clin. Nutr. 2018, 37, 1892–1901. [Google Scholar] [CrossRef]
- Ferreira de Oliveira, A.P.; Arisseto Bragotto, A.P. Microalgae-based products: Food and public health. Future Foods 2022, 6, 100157. [Google Scholar] [CrossRef]
- Woortman, D.V.; Fuchs, T.; Striegel, L.; Fuchs, M.; Weber, N.; Brück, T.B.; Rychlik, M. Microalgae a auperior source of folates: Quantification of folates in halophile microalgae by stable isotope dilution assay. Front. Bioeng. Biotechnol. 2020, 7, 481. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef]
- Gómez-Zavaglia, A.; Prieto Lage, M.A.; Jiménez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef]
- Zunner, B.E.M.; Wachsmuth, N.B.; Eckstein, M.L.; Scherl, L.; Schierbauer, J.R.; Haupt, S.; Stumpf, C.; Reusch, L.; Moser, O. Myokines and Resistance Training: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 3501. [Google Scholar] [CrossRef]
- Louzada, R.A.; Bouviere, J.; Matta, L.P.; Werneck-de-Castro, J.P.; Dupuy, C.; Carvalho, D.P.; Fortunato, R.S. Redox Signaling in Widespread Health Benefits of Exercise. Antioxid. Redox Signal. 2020, 33, 745–760. [Google Scholar] [CrossRef]
- Petersen, A.M.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Garatachea, N.; Berger, N.A.; Lucia, A. Exercise is the Real Polypill. Physiology 2013, 28, 330–358. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells 2022, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sport. 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Atakan, M.M.; Kosar, S.N.; Guzel, Y.; Tin, H.T.; Yan, X. The Role of Exercise, Diet, and Cytokines in Preventing Obesity and Improving Adipose Tissue. Nutrients 2021, 13, 1459. [Google Scholar] [CrossRef] [PubMed]
- Deslandes, A.; Moraes, H.; Ferreira, C.; Veiga, H.; Silveira, H.; Mouta, R.; Pompeu, F.; Coutinho, E.S.; Laks, J. Exercise and Mental Health: Many Reasons to Move. Neuropsychobiology 2009, 59, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Ruegsegger, G.N.; Booth, F.W. Health Benefits of Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029694. [Google Scholar] [CrossRef]
- An, H.J.; Choi, H.M.; Park, H.S.; Han, J.G.; Lee, E.H.; Park, Y.S.; Um, J.Y.; Hong, S.H.; Kim, H.M. Oral administration of hot water extracts of Chlorella vulgaris increases physical stamina in mice. Ann. Nutr. Metab. 2006, 50, 380–386. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, K.Y.; Jeong, H.J.; Kim, H.M.; Hong, S.H.; Um, J.Y. Effects of hydrolyzed Chlorella vulgaris by malted barley on the immunomodulatory response in ICR mice and in Molt-4 cells. J. Sci. Food Agric. 2010, 90, 1551–1556. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Arakawa, Y.; Kobayashi, M.; Fujishima, M. Influence of Chlorella powder intake during swimming stress in mice. Biochem. Biophys. Res. Commun. 2011, 404, 121–126. [Google Scholar] [CrossRef]
- Umemoto, S.; Otsuki, T. Chlorelladerived multicomponent supplementation increases aerobic endurance capacity in young individuals. J. Clin. Biochem. Nutr. 2014, 55, 143–146. [Google Scholar] [CrossRef]
- Horii, N.; Hasegawa, N.; Fujie, S.; Uchida, M.; Miyamoto-Mikami, E.; Hashimoto, T.; Tabata, I.; Iemitsu, M. High-intensity intermittent exercise training with chlorella intake accelerates exercise performance and muscle glycolytic and oxidative capacity in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R520–R528. [Google Scholar] [CrossRef]
- Horii, N.; Hasegawa, N.; Fujie, S.; Uchida, M.; Iemitsu, K.; Inoue, K.; Iemitsu, M. Effect of combination of chlorella intake and aerobic exercise training on glycemic control in type 2 diabetic rats. Nutrition 2019, 63–64, 45–50. [Google Scholar] [CrossRef]
- Samadi, M.; Shirvani, H.; Shafeie, A.A. Effect of Chlorella vulgaris supplementation with eccentric exercise on serum interleukin 6 and insulin resistance in overweight men. Sport Sci. Health 2020, 16, 543–549. [Google Scholar] [CrossRef]
- Vijayavel, K.; Anbuselvam, C.; Balasubramanian, M.P. Antioxidant effect of the marine algae Chlorella vulgaris against naphthalene-induced oxidative stress in the albino rats. Mol. Cell. Biochem. 2007, 303, 39–44. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, H.J.; Lee, H.J.; Kang, M.H.; Park, Y.K. Six-week supplementation with Chlorella has favorable impact on antioxidant status in Korean male smokers. Nutrition 2010, 26, 175–183. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Y.; Chen, Q.; Yang, H.; Xie, X. Astaxanthin promotes Nrf2/ARE signaling to alleviate renal fibronectin and collagen IV accumulation in diabetic rats. J. Diabetes Res. 2018, 2018, 6730315. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef]
- Sibi, G.; Rabina, S. Inhibition of Pro-inflammatory Mediators and Cytokines by Chlorella Vulgaris Extracts. Pharmacogn. Res. 2016, 8, 118–122. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef]
- Tran, L.T.; Yuen, V.G.; McNeill, J.H. The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Moll. Cell. Biochem. 2009, 332, 145–159. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2–H12. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, T.; Shimizu, K.; Maeda, S. Changes in arterial stiffness and nitric oxide production with Chlorelladerived multicomponent supplementation in middleaged and older individuals. Clin. Biochem. Nutr. 2015, 57, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, M.; Kato, S.; Tsuneyama, K.; Matsutake, S.; Kamogawa, M.; Hirao, E.; Miyata, A.; Mori, S.; Yamaguchi, N.; Suruga, K.; et al. Phycocyanin prevents hypertension and low serum adiponectin level in a rat model of metabolic syndrome. Nutr. Res. 2013, 33, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, C.M.; Matsuura, C.; Silva, R.S., Jr.; Guimarães, F.F.; Gomes, P.S.C. Acute Effects of L-Arginine Supplementation on Oxygen Consumption Kinetics and Muscle Oxyhemoglobin and Deoxyhemoglobin during Treadmill Running in Male Adults. Int. J. Exerc. Sci. 2019, 12, 444–455. [Google Scholar] [PubMed]
- Otsuki, T.; Shimizu, K.; Zempo-Miyaki, A.; Maeda, S. Changes in salivary flow rate following Chlorella derived multicomponent supplementation. J. Clin. Biochem. Nutr. 2016, 59, 45–48. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae Characterization for Consolidated and New Application in Human Food, Animal Feed and Nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef]
- Waghmare, A.G.; Salve, M.K.; LeBlanc, J.G.; Arya, S.S. Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresour. Bioprocess. 2016, 3, 16. [Google Scholar] [CrossRef]
- Morales, F.E.; Tinsley, G.M.; Gordon, P.M. Acute and Long-Term Impact of High-Protein Diets on Endocrine and Metabolic Function, Body Composition, and Exercise-Induced Adaptations. J. Am. Coll. Nutr. 2017, 36, 295–305. [Google Scholar] [CrossRef]
- Lynch, H.; Johnston, C.; Wharton, C. Plant-Based Diets: Considerations for Environmental Impact, Protein Quality, and Exercise Performance. Nutrients 2018, 10, 1841. [Google Scholar] [CrossRef]
- Li, Y.W.; Li, B. Characterization of structure-antioxidant activity relationship of peptides in free radical systems using QSAR models: Key sequence positions and their amino acid properties. J. Theor. Biol. 2013, 318, 29–43. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Wijesekara, I.; Kim, S.K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef]
- Ko, S.C.; Kim, D.; Jeon, Y.J. Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem. Toxicol. 2012, 50, 2294–2302. [Google Scholar] [CrossRef]
- Sheih, I.C.; Wu, T.-K.; Fang, T.J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Hu, C.J.; Li, F.N.; Duan, Y.H.; Zhang, T.; Li, H.W.; Yin, Y.L.; Wu, G.Y.; Kong, X.F. Dietary supplementation with arginine and glutamic acid alters the expression of amino acid transporters in skeletal muscle of growing pigs. Amino Acids 2019, 51, 1081–1092. [Google Scholar] [CrossRef]
- Hu, C.; Li, F.; Duan, Y.; Kong, X.; Yan, Y.; Deng, J.; Tan, C.; Wu, G.; Yin, Y. Leucine alone or in combination with glutamic acid, but not with arginine, increases biceps femoris muscle and alters muscle AA transport and concentrations in fattening pigs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 791–800. [Google Scholar] [CrossRef]
- Lancha, A.H.J.; Recco, M.B.; Abdallat, D.S.P.; Curi, R. Effect of Aspartate, Asparagine, and Carnitine Supplementation in the Diet on Metabolism of Skeletal Muscle During a Moderate Exercise. Physiol. Behav. 1995, 57, 367–371. [Google Scholar] [CrossRef]
- Sureda, A.; Pons, A. Arginine and Citrulline Supplementation in Sports and Exercise: Ergogenic Nutrients? Med. Sport. Sci. 2013, 59, 18–28. [Google Scholar]
- Silva, E.P., Jr.; Borges, L.S.; Mendes-da-Silva, C.; Hirabara, S.M.; Lambertucci, R.H. L-arginine supplementation improves rats’ antioxidant system and exercise performance. Free Radic. Res. 2017, 51, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Li, H.; Wei, Z.; Lv, K.; Gao, C.; Liu, Y.; Zhao, L. Isolation, structures and biological activities of polysaccharides from Chlorella: A review. Int. J. Biol. Macromol. 2020, 163, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Liu, X.Y.; Xiao, Z.; Huang, Y.F.; Liu, B. Antioxidant activities of polysaccharides obtained from Chlorella pyrenoidosa via different ethanol concentrations. Int. J. Biol. Macromol. 2016, 91, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Yu, F.; Xin, Z.; Zhao, L.; Zhu, X.; Hu, Q. Preparation, identification and their antitumor activities in vitro of polysaccharides from Chlorella pyrenoidosa. Food Chem. 2007, 105, 533–539. [Google Scholar] [CrossRef]
- De Felice, B.; Damiano, S.; Montanino, C.; Del Buono, A.; La Rosa, G.; Guida, B.; Santillo, M. Effect of beta- and alpha-glucans on immune modulating factors expression in enterocyte-like Caco-2 and goblet-like LS 174T cells. Int. J. Biol. Macromol. 2020, 153, 600–607. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Matos, A.P.; Feller, R.; Moecke, E.H.S.; de Oliveira, J.V.; Furigo, A.; Derner, R.B.; Sant’Anna, E.S. Chemical Characterization of Six Microalgae with Potential Utility for Food Application. J. Am. Oil Chem. Soc. 2016, 93, 963–972. [Google Scholar] [CrossRef]
- Guan, Z.W.; Yu, E.Z.; Feng, Q. Soluble Dietary Fiber, One of the Most Important Nutrients for the Gut Microbiota. Molecules 2021, 26, 6802. [Google Scholar] [CrossRef]
- Mao, G.; Li, S.; Orfila, C.; Shen, X.; Zhou, S.; Linhardt, R.J.; Ye, X.; Chen, S. Depolymerized RG-I-enriched pectin from citrus segment membranes modulates gut microbiota, increases SCFA production, and promotes the growth of Bifidobacterium spp., Lactobacillus spp. and Faecalibaculum spp. Food Funct. 2019, 10, 7828–7843. [Google Scholar] [CrossRef]
- Marttinen, M.; Ala-Jaakkola, R.; Laitila, A.; Lehtinen, M.J. Gut Microbiota, Probiotics and Physical Performance in Athletes and Physically Active Individuals. Nutrients 2020, 12, 2936. [Google Scholar] [CrossRef]
- Simpson, H.L.; Campbell, B.J. Review article: Dietary fibre-microbiota interactions. Aliment. Pharm. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- van der Linde, C.; Barone, M.; Turroni, S.; Brigidi, P.; Keleszade, E.; Swann, J.R.; Costabile, A. An In Vitro Pilot Fermentation Study on the Impact of Chlorella pyrenoidosa on Gut Microbiome Composition and Metabolites in Healthy and Coeliac Subjects. Molecules 2021, 26, 2330. [Google Scholar] [CrossRef]
- Lv, K.L.; Yuan, Q.X.; Li, H.; Li, T.T.; Ma, H.Q.; Gao, C.H.; Zhang, S.Y.; Liu, Y.H.; Zhao, L.Y. Chlorella pyrenoidosa Polysaccharides as a Prebiotic to Modulate Gut Microbiota: Physicochemical Properties and Fermentation Characteristics In Vitro. Foods 2022, 11, 725. [Google Scholar] [CrossRef]
- Jin, J.B.; Cha, J.W.; Shin, I.S.; Jeon, J.Y.; Cha, K.H.; Pan, C.H. Supplementation with Chlorella vulgaris, Chlorella protothecoides, and Schizochytrium sp. increases propionate-producing bacteria in in vitro human gut fermentation. J. Sci. Food Agric. 2020, 100, 2938–2945. [Google Scholar] [CrossRef]
- Ścieszka, S.; Klewicka, E. Influence of the Microalga Chlorella vulgaris on the Growth and Metabolic Activity of Lactobacillus spp. Bacteria. Foods 2020, 9, 959. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Nomaguchi, T.; Mori, Y.; Ito, M.; Nakamura, Y.; Fujishima, M.; Murakami, S.; Yamada, T.; Fukuda, S. The Nutritional Efficacy of Chlorella Supplementation Depends on the Individual Gut Environment: A Randomised Control Study. Front. Nutr. 2021, 8, 648073. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Yao, L.; Gerde, J.A.; Lee, S.L.; Wang, T.; Harrata, K.A. Microalgae lipid characterization. J. Agric. Food Chem. 2015, 63, 1773–1787. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sanchez, N.F.; Valadez-Blanco, R.; Hernandez-Carlos, B.; Torres-Arino, A.; Guadarrama-Mendoza, P.C.; Salas-Coronado, R. Lipids rich in ω-3 polyunsaturated fatty acids from microalgae. Appl. Microbiol. Biotechnol. 2016, 100, 8667–8684. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sommerfeld, M.; Hu, Q.A. Microwave-assisted Nile red method for in vivo quantification of neutral lipids in microalgae. Bioresour. Technol. 2011, 102, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef]
- Pingitore, A.; Lima, G.P.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal. Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef]
- Zhang, W.; Fu, F.; Tie, R.; Liang, X.Y.; Tian, F.; Xing, W.J.; Li, J.; le Ji, L.; Xing, J.L.; Sun, X.; et al. Alpha-linolenic acid intake prevents endothelial dysfunction in high-fat diet-fed streptozotocin rats and underlying mechanisms. Vasa Eur. J. Vasc. Med. 2013, 42, 421–428. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Yang, H.; Zhang, P.; Wu, F.; Li, Y.; Zhou, Y.; Zhang, X.; Ma, H.; Zhang, W.; et al. α-Linolenic acid but not linolenic acid protects against hypertension: Critical role of SIRT3 and autophagic flux. Cell Death Dis. 2020, 11, 83. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids and antioxidants in edible wild plants. Biol. Res. 2004, 37, 263–277. [Google Scholar] [CrossRef]
- Pal, M.; Ghosh, M. Prophylactic effect of alpha-linolenic acid and alpha-eleostearic acid against MeHg induced oxidative stress, DNA damage and structural changes in RBC membrane. Food Chem. Toxicol. 2012, 50, 2811–2818. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.; Bloomer, R.J. Acute exercise and oxidative stress: A 30 year history. Dyn. Med. 2009, 8, 1. [Google Scholar] [CrossRef]
- Cai, D.; Frantz, J.D.; Tawa, N.E., Jr.; Melendez, P.A.; Oh, B.C.; Lidov, H.G.; Hasselgren, P.O.; Frontera, W.R.; Lee, J.; Glass, D.J.; et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 2004, 119, 285–298. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Q.W.; Zheng, P.P.; Zhang, J.S.; Huang, F.R. DHA inhibits protein degradation more efficiently than EPA by regulating the PPARγ/NFκB pathway in C2C12 myotubes. BioMed Res. Int. 2013, 2013, 318981. [Google Scholar] [CrossRef]
- Stark, A.H.; Crawford, M.A.; Reifen, R. Update on alpha-linolenic acid. Nutr. Rev. 2008, 66, 326–332. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Cornish, S.M.; Chilibeck, P.D. Alpha-linolenic acid supplementation and resistance training in older adults. Appl. Physiol. Nutr. Metab. 2009, 34, 49–59. [Google Scholar] [CrossRef]
- Robinson, S.M.; Reginster, J.Y.; Rizzoli, R.; Shaw, S.C.; Kanis, J.A.; Bautmans, I.; Bischoff-Ferrari, H.; Bruyere, O.; Cesari, M.; Dawson-Hughes, B.; et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin. Nutr. 2018, 37, 1121–1132. [Google Scholar] [CrossRef]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef]
- Storlien, L.H.; Pan, D.A.; Kriketos, A.D.; O’Connor, J.; Caterson, I.D.; Cooney, G.J.; Jenkins, A.B.; Baur, L.A. Skeletal Muscle Membrane Lipids and Insulin Resistance. Lipids 1996, 31, S261–S265. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Micha, R.; Wu, J.H.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef] [PubMed]
- Cabout, M.; Alssema, M.; Nijpels, G.; Stehouwer, C.D.A.; Zock, P.L.; Brouwer, I.A.; Elshorbagy, A.K.; Refsum, H.; Dekker, J.M. Circulating linoleic acid and alpha-linolenic acid and glucose metabolism: The Hoorn Study. Eur. J. Nutr. 2017, 56, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Lyudinina, A.Y.; Bushmanova, E.A.; Varlamova, N.G.; Bojko, E.R. Dietary and plasma blood alpha-linolenic acid as modulators of fat oxidation and predictors of aerobic performance. J. Int. Soc. Sport. Nutr. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, J.E. Alpha-linolenic acid: Functions and effects on linoleic acid metabolism and eicosanoid-mediated reactions. Adv. Food Nutr. Res. 1991, 35, 1–184. [Google Scholar] [CrossRef]
- Pan, D.A.; Storlien, L.H. Dietary lipid profile is a determinant of tissue phospholipid fatty acid composition and rate of weight gain in rats. J. Nutr. 1993, 123, 512–519. [Google Scholar] [CrossRef]
- Borkman, M.; Storlien, L.H.; Pan, D.A.; Jenkins, A.B.; Chisholm, D.J.; Campbell, L.V. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N. Engl. J. Med. 1993, 328, 238–244. [Google Scholar] [CrossRef]
- Mickleborough, T.D. Omega-3 Polyunsaturated Fatty Acids in Physical Performance Optimization. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 83–96. [Google Scholar] [CrossRef]
- Gurney, T.; Spendiff, O. Algae Supplementation for Exercise Performance: Current Perspectives and Future Directions for Spirulina and Chlorella. Front. Nutr. 2022, 9, 865741. [Google Scholar] [CrossRef]
- Hosseini, A.M.; Keshavarz, S.A.; Nasli-Esfahani, E.; Amiri, F.; Janani, L. The effects of Chlorella supplementation on glycemic control, lipid profile and anthropometric measures on patients with type 2 diabetes mellitus. Eur. J. Nutr. 2021, 60, 3131–3141. [Google Scholar] [CrossRef]
- Sales, K.M.; Reimer, R.A. Unlocking a novel determinant of athletic performance: The role of the gut microbiota, short-chain fatty acids, and “biotics” in exercise. J. Sport Health Sci. 2023, 12, 36–44. [Google Scholar] [CrossRef]
| Main Components | |
|---|---|
| Macronutrients | |
| Proteins | All the essential amino acids |
| Carbohydrates | α- and β-glucans, fiber |
| Lipids | PUFAs (linoleic acid, alpha-linolenic acid) |
| Micronutrients | |
| Minerals | Na+, K+, Fe2+, Ca2+, Mg2+, Mn2+, Zn2+, Co2+ |
| Vitamins | A, B1, B2, B6, B12, C, E, K1, folic and pantothenic acids, niacin |
| Pigments | Chlorophylls, carotenoids, lutein |
| Subjects | Supplementation Protocol | Exercise Protocols and Tests | Results |
|---|---|---|---|
| ICR mice, male Healthy | C. vulgaris (Hot water extract) Dosage: 0.05–0.15 g/kg/day Intervention: 1 week | Training: -- Forced swimming test (6′, measured the total duration of immobility. | ↓ Immobility time, BUN, CK, and LDH No effect: Glc, TP [30] |
| ICR mice, male 4 weeks old Healthy | C. vulgaris (Hydrolyzed) Dosage: 10 mL/kg/day Intervention: 2 weeks | Training: -- Forced swimming test (6′, measured the total duration of immobility. | ↑ IFN-γ, IL-2 (in Molt-4 cells) ↓ Immobility time, BUN [31] |
| BALB/c mice, male 6 weeks old Healthy | Chlorella powder in chow Dosage: 0.5%, 1 mg/kg/day Intervention: 2 weeks | Training: -- Forced swimming test (Measured the maximum swimming time). | ↑ Swimming time, FFA, Glc, TG, and lactic acid. ↓ Oxidoreductase activity and the leukotriene synthesis pathway [32] |
| Men and women ≈21 years old Healthy | Chlorella tablets Dosage: 15 tablets, 2 times/day Intervention: 4 weeks | Training: -- Maximal exercise test (Incremental cycling to exhaustion). | ↑ VO2 peak [33] |
| Sprague-Dawley rats, male 12 weeks old Healthy | Chlorella powder in chow Dosage: 0.5% Intervention: 6 weeks | Training: HIIE (14 × 20″ swim, with a 10″ pause between series, bearing a weight, 4 days/week, for 6 week). Exercise performance test (Maximal number of HIIE). | ↑ number HIIE sessions, the expression of MCT1, MCT4, and PPARγ coactivator-1α, and the activities of LDH, CS, and COX in the red region of gastrocnemius. No effect: MCT1 expression and LDH, CS, and COX activities in the white region of gastrocnemius [34] |
| OLETF rats, male 20 weeks old Type 2 diabetics | Chlorella powder in chow Dosage: 0.5% Intervention: 8 week | Training: Aerobic exercise (running on the treadmill for 1 h, 25 m/min, 5 days/week, during 8 weeks). | ↑ insulin sensitivity index concomitant with muscle PI3K activity, Akt phosphorylation, and GLUT4 translocation levels ↓ fasting blood glucose, insulin levels, and total glucose AUC during the OGTT [35] |
| Men ≈23 years old Overweight | C. vulgaris tablets Dosage: 300 mg, 4 times/day Intervention: 1 week | Training: -- Acute eccentric exercise protocol (20´ treadmill run at a speed of 9 km/h with a negative 10% slope, 1 week after supplementation) | ↓ IL-6 levels and insulin resistance, 24 h after acute eccentric exercise test [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo, K.; Santocildes, G.; Torrella, J.R.; Magalhães, J.; Pagès, T.; Viscor, G.; Torres, J.L.; Ramos-Romero, S. Bioactivity of Macronutrients from Chlorella in Physical Exercise. Nutrients 2023, 15, 2168. https://doi.org/10.3390/nu15092168
Lorenzo K, Santocildes G, Torrella JR, Magalhães J, Pagès T, Viscor G, Torres JL, Ramos-Romero S. Bioactivity of Macronutrients from Chlorella in Physical Exercise. Nutrients. 2023; 15(9):2168. https://doi.org/10.3390/nu15092168
Chicago/Turabian StyleLorenzo, Karenia, Garoa Santocildes, Joan Ramon Torrella, José Magalhães, Teresa Pagès, Ginés Viscor, Josep Lluís Torres, and Sara Ramos-Romero. 2023. "Bioactivity of Macronutrients from Chlorella in Physical Exercise" Nutrients 15, no. 9: 2168. https://doi.org/10.3390/nu15092168
APA StyleLorenzo, K., Santocildes, G., Torrella, J. R., Magalhães, J., Pagès, T., Viscor, G., Torres, J. L., & Ramos-Romero, S. (2023). Bioactivity of Macronutrients from Chlorella in Physical Exercise. Nutrients, 15(9), 2168. https://doi.org/10.3390/nu15092168









