Shear-Viscosity-Dependent Effect of a Gum-Based Thickening Product on the Safety of Swallowing in Older Patients with Severe Oropharyngeal Dysphagia
Abstract
1. Introduction
2. Materials and Methods
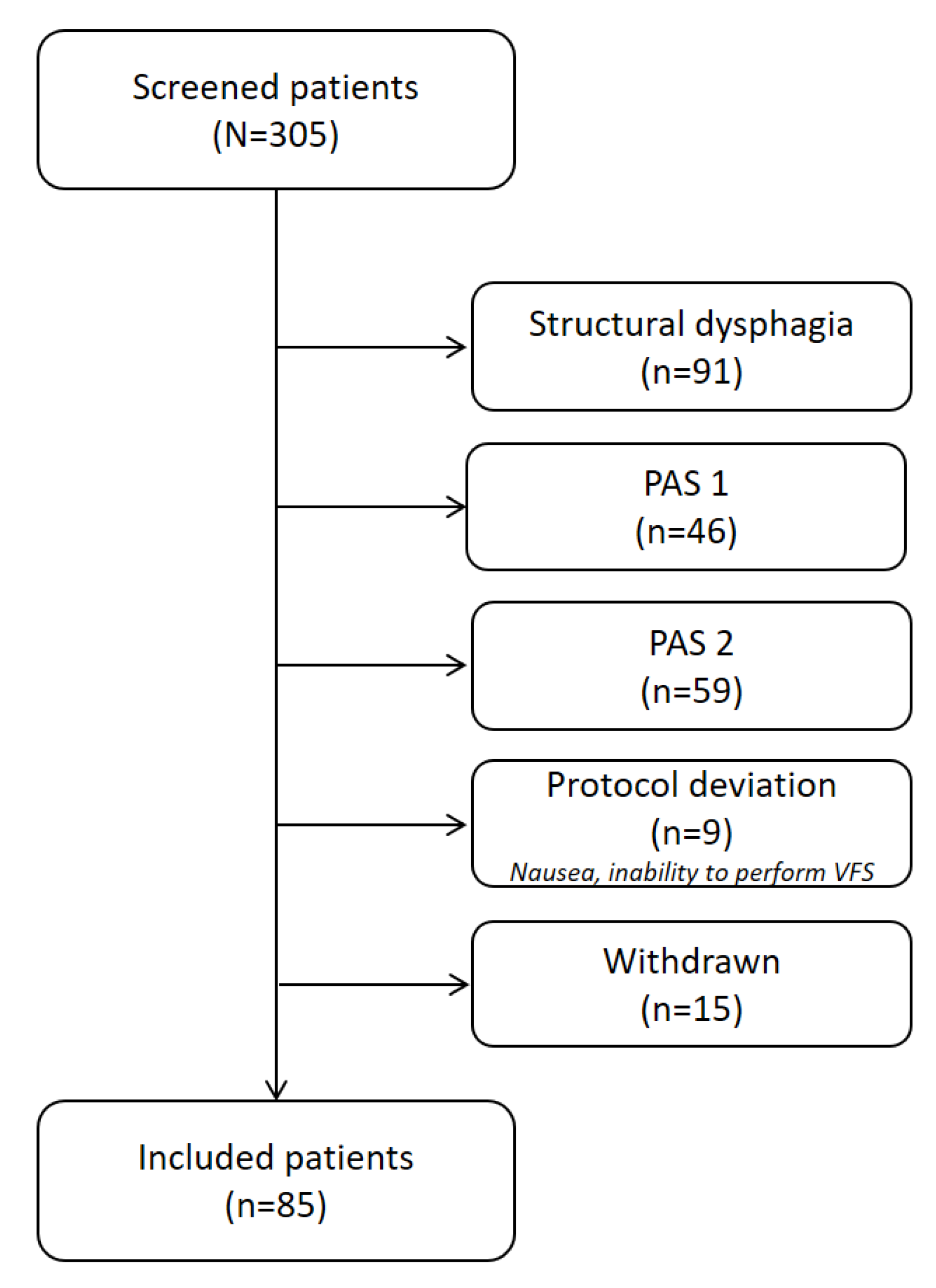
2.1. Study Population
2.2. Material and Methods
2.2.1. Products
2.2.2. Equipment
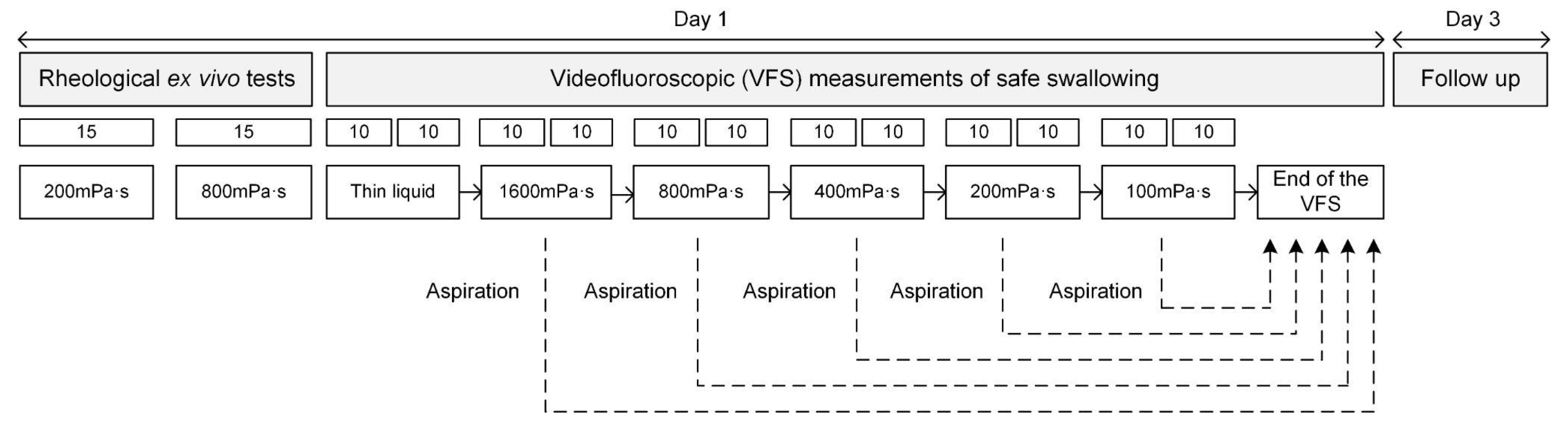
2.3. Experimental Design
2.3.1. Videofluoroscopy
2.3.2. Rheological Ex Vivo Characterisation
2.3.3. Hedonic Scale
2.3.4. Adverse Events
2.3.5. Outcome Parameters
2.4. Data Analysis
3. Results
3.1. Demographics and Patient’s Clinical Characteristics
3.2. Therapeutic Effect
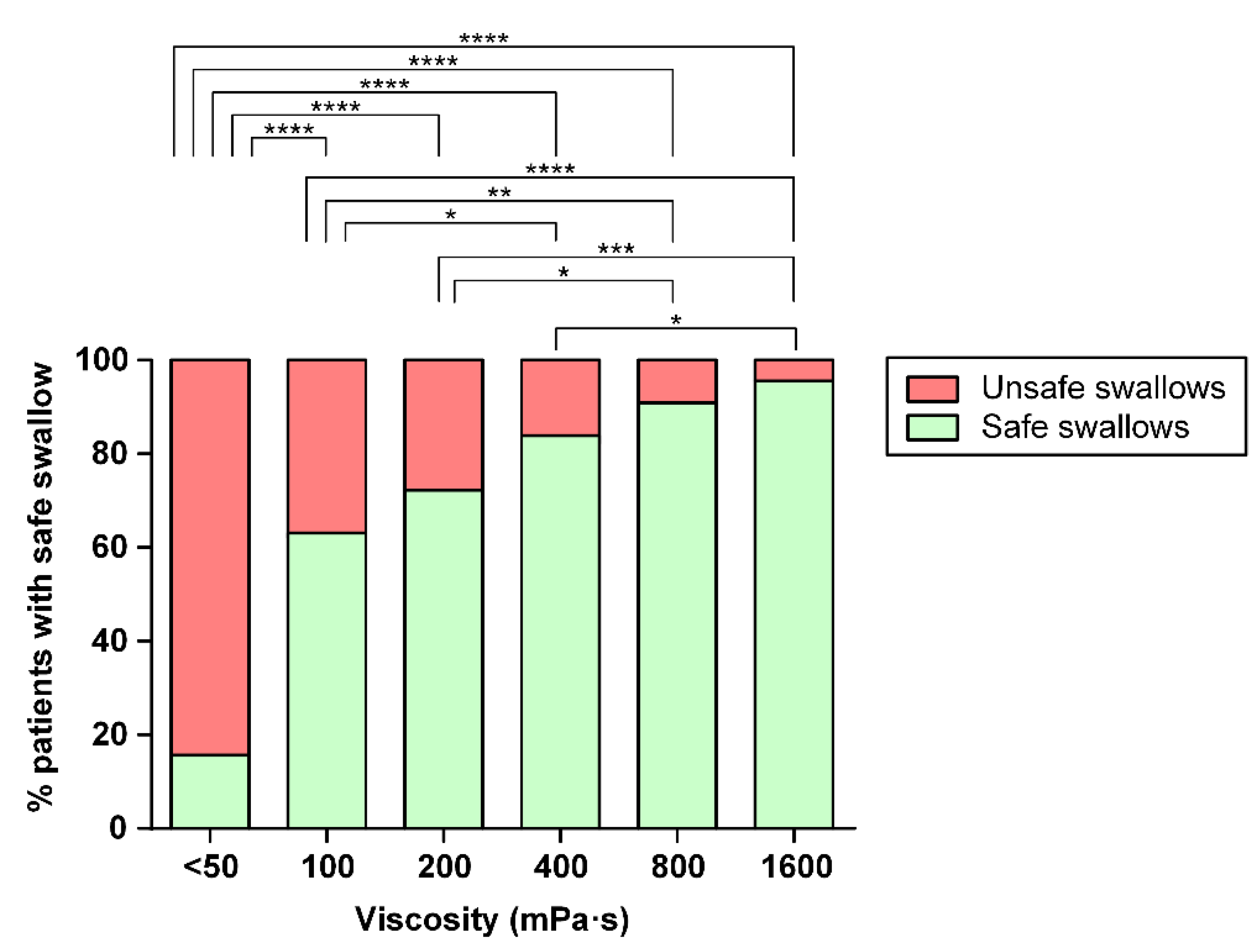
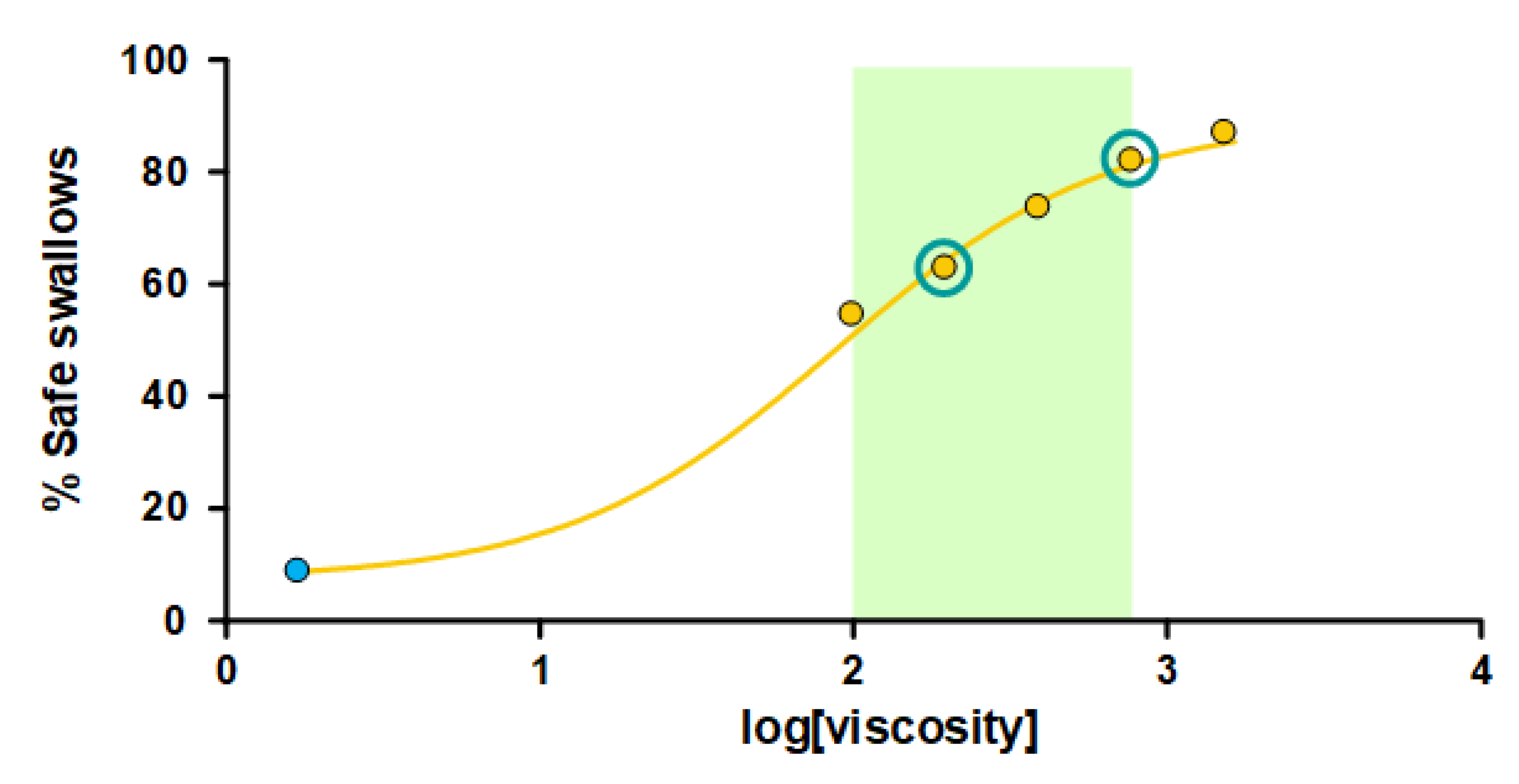
3.2.1. Safety of Swallowing
3.2.2. Efficacy of Swallowing
3.2.3. Oropharyngeal Swallowing Response
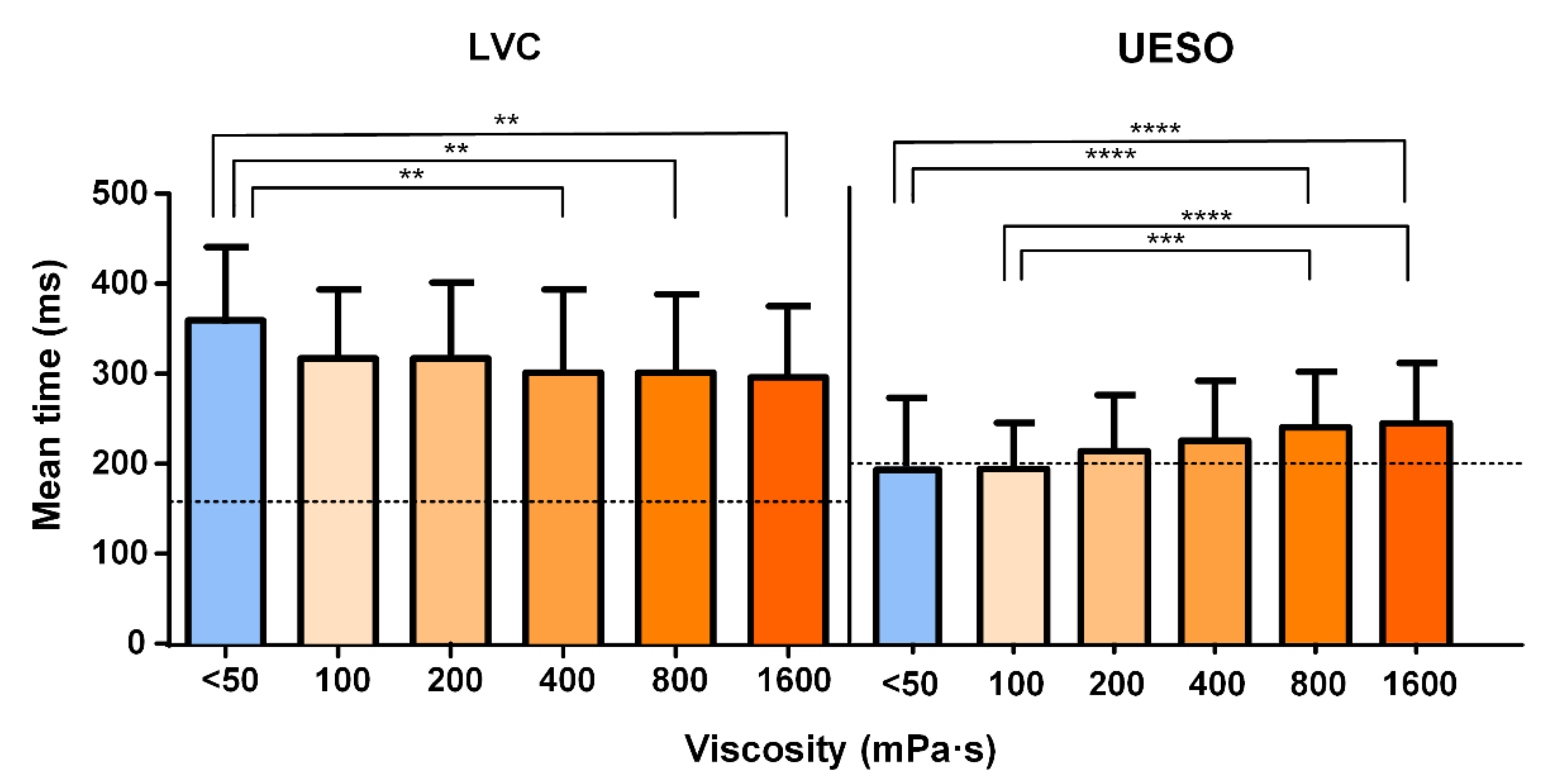
- For the airway protection mechanism, the time to LVC was reduced by increasing the shear viscosity from 360 ± 90.18 at <50 mPa·s to 300 ± 84.16 ms at 1600 mPa·s (Figure 5).
- For the bolus transit, the time to UESO was moderately increased by thickening the fluid, showing significant differences for <50 mPa·s vs. 800 and 1600 mPa·s (p < 0.0001). The mean UESO is represented in Figure 5 with the statistically significant differences obtained for all viscosity levels. The times to LVC and UESO values are presented in Table 3.
- For the kinematics of swallowing, the mean bolus velocity was reduced by increasing the bolus viscosity ≥ 800 mPa·s, ranging from 0.33 ± 0.18 to 0.22 ± 0.08 m/s. Significant differences appeared for <50, 100, and 200 mPa·s vs. 800 and 1600 mPa·s (Supplementary Figure S4). The bolus kinetic energy went from 1.29 ± 1.04 to 3.14 ± 6.00 mJ. Significant differences appeared for <50 and 100 mPa·s when compared to 800 and 1600 mPa·s. Values for the kinematics of swallowing are presented in Table 3.
3.3. Adverse Events
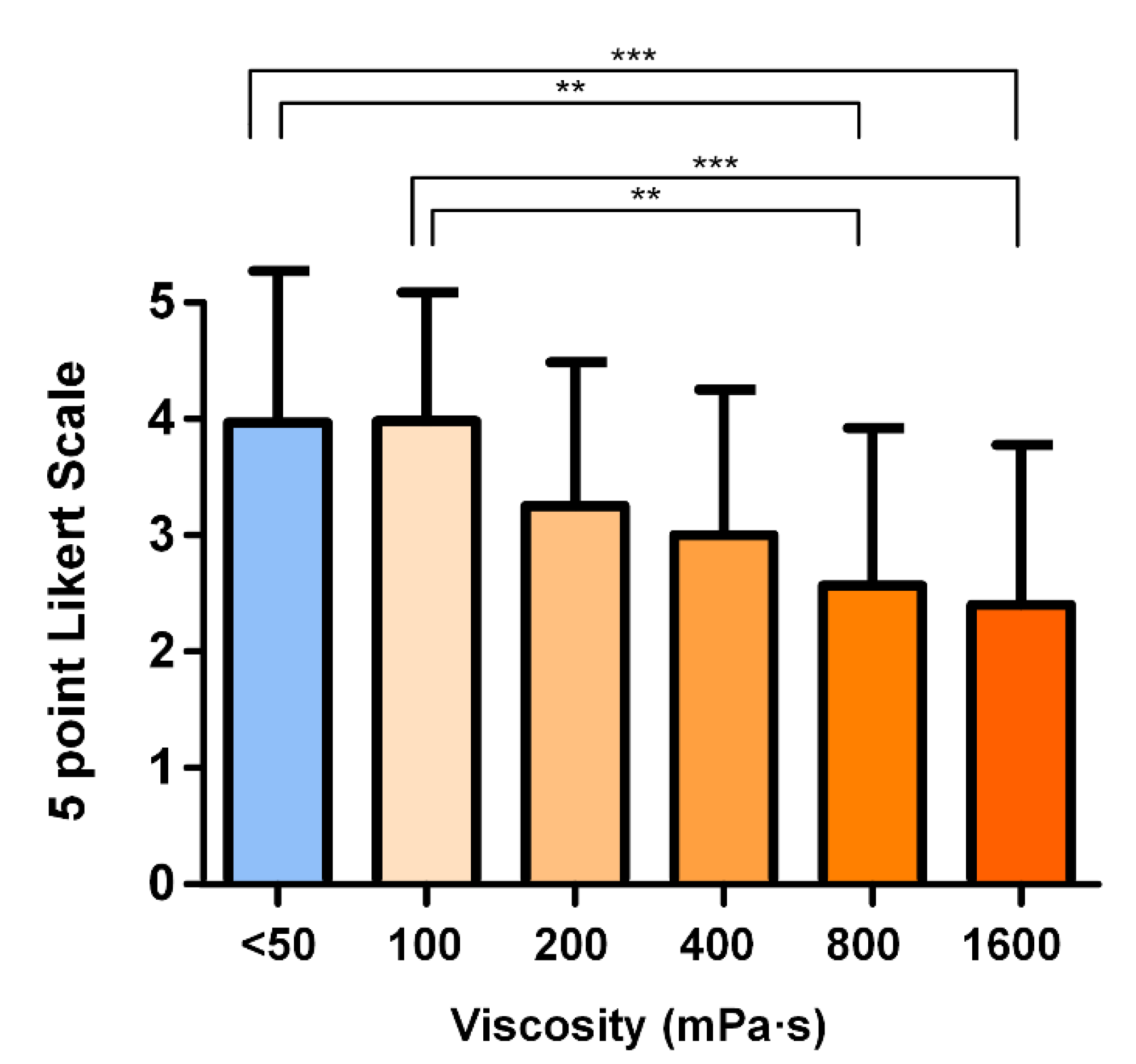
3.4. Hedonic Scale
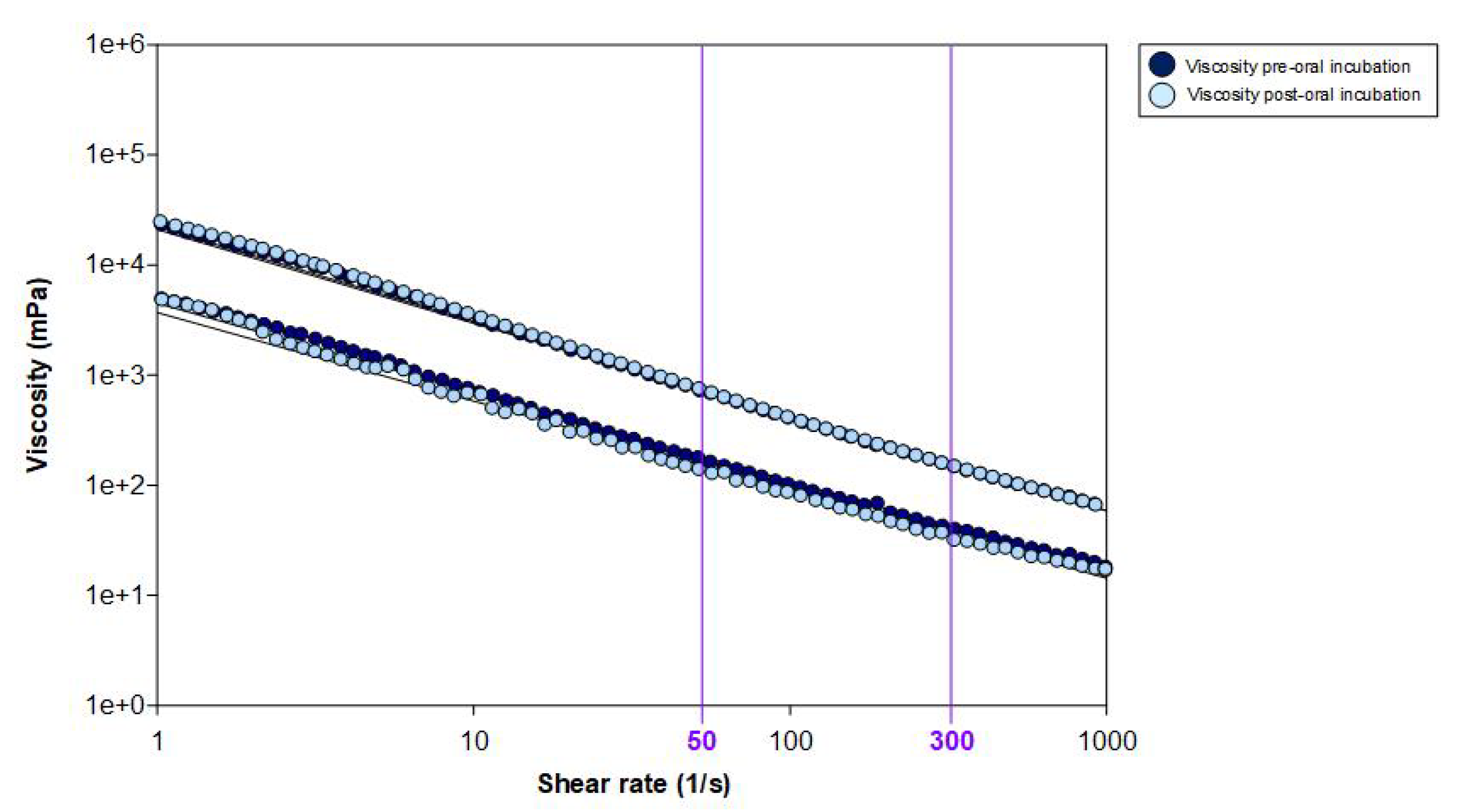
3.5. Rheological Ex Vivo Characterisation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clavé, P.; Shaker, R. Dysphagia: Current reality and scope of the problem. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 5. [Google Scholar] [CrossRef]
- Newman, R.; Vilardell, N.; Clavé, P.; Speyer, R. Effect of Bolus Viscosity on the Safety and Efficacy of Swallowing and the Kinematics of the Swallow Response in Patients with Oropharyngeal Dysphagia: White Paper by the European Society for Swallowing Disorders (ESSD). Dysphagia 2016, 31, 232–249. [Google Scholar] [CrossRef]
- Ortega, O.; Martín, A.; Clavé, P. Diagnosis and Management of Oropharyngeal Dysphagia Among Older Persons, State of the Art. J. Am. Med. Dir. Assoc. 2017, 18, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Barbon, C.E.A.; Steele, C.M. Efficacy of Thickened Liquids for Eliminating Aspiration in Head and Neck Cancer: A Systematic Review. Otolaryngol. Head Neck Surg. 2015, 152, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Bolivar-Prados, M.; Tomsen, N.; Hayakawa, Y.; Kawakami, S.; Miyaji, K.; Kayashita, J.; Clavé, P. Proposal for a Standard Protocol to Assess the Rheological Behavior of Thickening Products for Oropharyngeal Dysphagia. Nutrients 2022, 14, 5023. [Google Scholar] [CrossRef] [PubMed]
- Vilardell, N.; Rofes, L.; Arreola, V.; Speyer, R.; Clavé, P. A Comparative Study Between Modified Starch and Xanthan Gum Thickeners in Post-Stroke Oropharyngeal Dysphagia. Dysphagia 2016, 31, 169–179. [Google Scholar] [CrossRef]
- Bolivar-Prados, M.; Tomsen, N.; Arenas, C.; Ibáñez, L.; Clave, P. A bit thick: Hidden risks in thickening products’ labelling for dysphagia treatment. Food Hydrocoll. 2022, 123, 106960. [Google Scholar] [CrossRef]
- Bolivar-Prados, M.; Rofes, L.; Arreola, V.; Guida, S.; Nascimento, W.V.; Martin, A.; Vilardell, N.; Ortega Fernández, O.; Ripken, D.; Lansink, M.; et al. Effect of a gum-based thickener on the safety of swallowing in patients with poststroke oropharyngeal dysphagia. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2019, 31, e13695. [Google Scholar] [CrossRef]
- Martín, A.; Ortega, O.; Roca, M.; Arús, M.; Clavé Civit, P. Effect of a Minimal-Massive Intervention in Hospitalized Older Patients with Oropharyngeal Dysphagia: A Proof of Concept Study. J. Nutr. Health Aging 2018, 22, 739–747. [Google Scholar] [CrossRef]
- Ortega, O.; Bolívar-Prados, M.; Arreola, V.; Nascimento, W.V.; Tomsen, N.; Gallegos, C.; Brito-de La Fuente, E.; Clavé, P. Therapeutic Effect, Rheological Properties and α-Amylase Resistance of a New Mixed Starch and Xanthan Gum Thickener on Four Different Phenotypes of Patients with Oropharyngeal Dysphagia. Nutrients 2020, 12, 1873. [Google Scholar] [CrossRef]
- Gallegos, C.; Brito-de la Fuente, E.; Clavé, P.; Costa, A.; Assegehegn, G. Chapter Eight—Nutritional Aspects of Dysphagia Management. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 81, pp. 271–318. [Google Scholar] [CrossRef]
- Sworn, G. Rheology Modifiers for the Management of Dysphagia. In Rheology of Biological Soft Matter; Springer: Tokyo, Japan, 2017; pp. 233–263. [Google Scholar] [CrossRef]
- Rofes, L.; Arreola, V.; Romea, M.; Palomera, E.; Almirall, J.; Cabré, M.; Serra-prat, M.; Clavé, P. Pathophysiology of oropharyngeal dysphagia in the frail elderly. Neurogastroenterol. Motil. 2010, 22, 851-e230. [Google Scholar] [CrossRef]
- Rofes, L.; Arreola, V.; Mukherjee, R.; Swanson, J.; Clavé, P. The effects of a xanthan gum-based thickener on the swallowing function of patients with dysphagia. Aliment. Pharmacol. Ther. 2014, 39, 1169–1179. [Google Scholar] [CrossRef]
- Viñas, P.; Bolivar-Prados, M.; Tomsen, N.; Costa, A.; Marin, S.; Riera, S.A.; Barcons, N.; Clavé, P. The Hydration Status of Adult Patients with Oropharyngeal Dysphagia and the Effect of Thickened Fluid Therapy on Fluid Intake and Hydration: Results of Two Parallel Systematic and Scoping Reviews. Nutrients 2022, 14, 2497. [Google Scholar] [CrossRef]
- Cook, I.J.; Kahrilas, P.J. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology 1999, 116, 455–478. [Google Scholar] [CrossRef]
- Robbins, J.; Kays, S.A.; Gangnon, R.E.; Hind, J.A.; Hewitt, A.L.; Gentry, L.R.; Taylor, A.J. The Effects of Lingual Exercise in Stroke Patients with Dysphagia. Arch. Phys. Med. Rehabil. 2007, 88, 150–158. [Google Scholar] [CrossRef]
- The Use of the WHO-UMC System for Standardised Case Causality Assessment. (n.d.). Available online: https://www.who.int/publications/m/item/WHO-causality-assessment (accessed on 28 April 2023).
- Rosenbek, J.C.; Robbins, J.A.; Roecker, E.B.; Coyle, J.L.; Wood, J.L. A Penetration-Aspiration Scale. Dysphagia 1996, 11, 93–98. [Google Scholar] [CrossRef]
- Ortega, O.; Cabré, M.; Clavé, P. Oropharyngeal Dysphagia: Aetiology and Effects of Ageing. J. Gastroenterol. Hepatol. Res. 2014, 3, 1049–1054. [Google Scholar]
- Carrión, S.; Roca, M.; Costa, A.; Arreola, V.; Ortega, O.; Palomera, E.; Serra-Prat, M.; Cabré, M.; Clavé, P. Nutritional status of older patients with oropharyngeal dysphagia in a chronic versus an acute clinical situation. Clin. Nutr. 2017, 36, 1110–1116. [Google Scholar] [CrossRef]
- Marin, S.; Serra-Prat, M.; Ortega, O.; Clavé, P. Healthcare-related cost of oropharyngeal dysphagia and its complications pneumonia and malnutrition after stroke: A systematic review. BMJ Open 2020, 10, e031629. [Google Scholar] [CrossRef]
- Attrill, S.; White, S.; Murray, J.; Hammond, S.; Doeltgen, S. Impact of oropharyngeal dysphagia on healthcare cost and length of stay in hospital: A systematic review. BMC Health Serv. Res. 2018, 18, 594. [Google Scholar] [CrossRef] [PubMed]
- Kayashita, J.; Fujishima, I.; Fujitani, J.; Hironaka, S.; Kojo, A.; Mizukami, M.; Senda, N.; Moriwaki, M.; Watanabe, E. The Japanese Dysphagia Diet of 2021 by the Japanese Society of Dysphagia Rehabilitation. Jpn. J. Compr. Rehabil. Sci. 2022, 13, 64–77. [Google Scholar] [CrossRef]
- Cichero, J.A.Y.; Steele, C.; Duivestein, J.; Clavé, P.; Chen, J.; Kayashita, J.; Dantas, R.; Lecko, C.; Speyer, R.; Lam, P.; et al. The Need for International Terminology and Definitions for Texture-Modified Foods and Thickened Liquids Used in Dysphagia Management: Foundations of a Global Initiative. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, E.; Yamagata, Y.; Fujitani, J.; Fujishima, I.; Takahashi, K.; Uyama, R.; Ogoshi, H.; Kojo, A.; Maeda, H.; Ueda, K.; et al. The Criteria of Thickened Liquid for Dysphagia Management in Japan. Dysphagia 2018, 33, 26–32. [Google Scholar] [CrossRef]
- Funami, T. Next target for food hydrocolloid studies: Texture design of foods using hydrocolloid technology. Food Hydrocoll. 2011, 25, 1904–1914. [Google Scholar] [CrossRef]
- Rofes, L.; Ortega, O.; Vilardell, N.; Mundet, L.; Clavé, P. Spatiotemporal characteristics of the pharyngeal event-related potential in healthy subjects and older patients with oropharyngeal dysfunction. Neurogastroenterol. Motil. 2017, 29, e12916. [Google Scholar] [CrossRef]
- Nakauma, M.; Ishihara, S.; Funami, T.; Nishinari, K. Swallowing profiles of food polysaccharide solutions with different flow behaviors. Food Hydrocoll. 2011, 25, 1165–1173. [Google Scholar] [CrossRef]
- Funami, T.; Ishihara, S.; Nakauma, M.; Kohyama, K.; Nishinari, K. Texture design for products using food hydrocolloids. Food Hydrocoll. 2012, 26, 412–420. [Google Scholar] [CrossRef]
| Target Viscosity (mPa·s) at 50 s−1 | Tsururinko Quickly (g) | Final Volume (mL) | Dissolvent (mL) |
|---|---|---|---|
| VFS doses | |||
| <50 | - | 50 | 1:1 (water:Omnipaque) |
| 100 | 0.58 | 50 | 1:1 (water:Omnipaque) |
| 200 | 1 | 50 | 1:1 (water:Omnipaque) |
| 400 | 1.45 | 50 | 1:1 (water:Omnipaque) |
| 800 | 2.45 | 50 | 1:1 (water:Omnipaque) |
| 1600 | 4.3 | 50 | 1:1 (water:Omnipaque) |
| Rheological test doses | |||
| 200 | 2 | 100 | Water |
| 800 | 5.8 | 100 | Water |
| Prevalence (%) | Viscosity Level (mPa·s) | |||||
|---|---|---|---|---|---|---|
| <50 | 100 | 200 | 400 | 800 | 1600 | |
| Safe swallows | 16.25 | 62.90 | 71.43 | 82.28 | 90.36 | 95.24 |
| Penetrations | 45.00 | 30.65 | 17.14 | 7.60 | 7.23 | 3.57 |
| Aspirations | 38.75 | 6.45 | 11.43 | 10.13 | 2.41 | 1.19 |
| Mean PAS ± SD | 4.91 ± 2.16 | 2.55 ± 1.87 | 2.47 ± 1.92 | 2.11 ± 1.97 | 1.53 ± 1.14 | 1.31 ± 0.92 |
| Oral residue Coating Pooling | 46.25 | 58.06 | 71.43 | 68.35 | 65.06 | 69.05 |
| 41.25 | 50.00 | 64.29 | 59.49 | 49.40 | 52.38 | |
| 5.00 | 8.07 | 7.14 | 8.86 | 15.66 | 16.67 | |
| Pharyngeal residue Coating Pooling | 11.25 | 11.29 | 14.29 | 12.66 | 16.87 | 20.24 |
| 8.75 | 8.07 | 11.43 | 8.86 | 13.25 | 14.29 | |
| 2.50 | 3.23 | 2.86 | 3.80 | 3.61 | 5.95 | |
| OSR and Kinematics | Viscosity Level (mPa·s) | |||||
|---|---|---|---|---|---|---|
| <50 | 100 | 200 | 400 | 800 | 1600 | |
| LVC (ms) | 360 ± 90.18 | 323 ± 76.67 | 324 ± 86.40 | 305 ± 93.85 | 307 ± 89.16 | 300 ± 84.16 |
| UESO (ms) | 186 ± 78.20 | 188 ± 50.35 | 208 ± 61.54 | 214 ± 66.85 | 231 ± 59.60 | 236 ± 65.60 |
| Mean velocity (m/s) | 0.34 ± 0.23 | 0.28 ± 0.11 | 0.27 ± 0.09 | 0.27 ± 0.15 | 0.23 ± 0.08 | 0.23 ± 0.10 |
| KE (mJ) | 3.14 ± 6.00 | 2.01 ± 2.43 | 1.76 ± 1.30 | 1.75 ± 1.60 | 1.29 ± 1.04 | 1.39 ± 1.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolivar-Prados, M.; Hayakawa, Y.; Tomsen, N.; Arreola, V.; Nascimento, W.; Riera, S.; Kawakami, S.; Miyaji, K.; Takeda, Y.; Kayashita, J.; et al. Shear-Viscosity-Dependent Effect of a Gum-Based Thickening Product on the Safety of Swallowing in Older Patients with Severe Oropharyngeal Dysphagia. Nutrients 2023, 15, 3279. https://doi.org/10.3390/nu15143279
Bolivar-Prados M, Hayakawa Y, Tomsen N, Arreola V, Nascimento W, Riera S, Kawakami S, Miyaji K, Takeda Y, Kayashita J, et al. Shear-Viscosity-Dependent Effect of a Gum-Based Thickening Product on the Safety of Swallowing in Older Patients with Severe Oropharyngeal Dysphagia. Nutrients. 2023; 15(14):3279. https://doi.org/10.3390/nu15143279
Chicago/Turabian StyleBolivar-Prados, Mireia, Yuki Hayakawa, Noemi Tomsen, Viridiana Arreola, Weslania Nascimento, Stephanie Riera, Satomi Kawakami, Kazuhiro Miyaji, Yasuhiro Takeda, Jun Kayashita, and et al. 2023. "Shear-Viscosity-Dependent Effect of a Gum-Based Thickening Product on the Safety of Swallowing in Older Patients with Severe Oropharyngeal Dysphagia" Nutrients 15, no. 14: 3279. https://doi.org/10.3390/nu15143279
APA StyleBolivar-Prados, M., Hayakawa, Y., Tomsen, N., Arreola, V., Nascimento, W., Riera, S., Kawakami, S., Miyaji, K., Takeda, Y., Kayashita, J., & Clavé, P. (2023). Shear-Viscosity-Dependent Effect of a Gum-Based Thickening Product on the Safety of Swallowing in Older Patients with Severe Oropharyngeal Dysphagia. Nutrients, 15(14), 3279. https://doi.org/10.3390/nu15143279







