Probiotics and Polycystic Ovary Syndrome: A Perspective for Management in Adolescents with Obesity
Abstract
1. Introduction
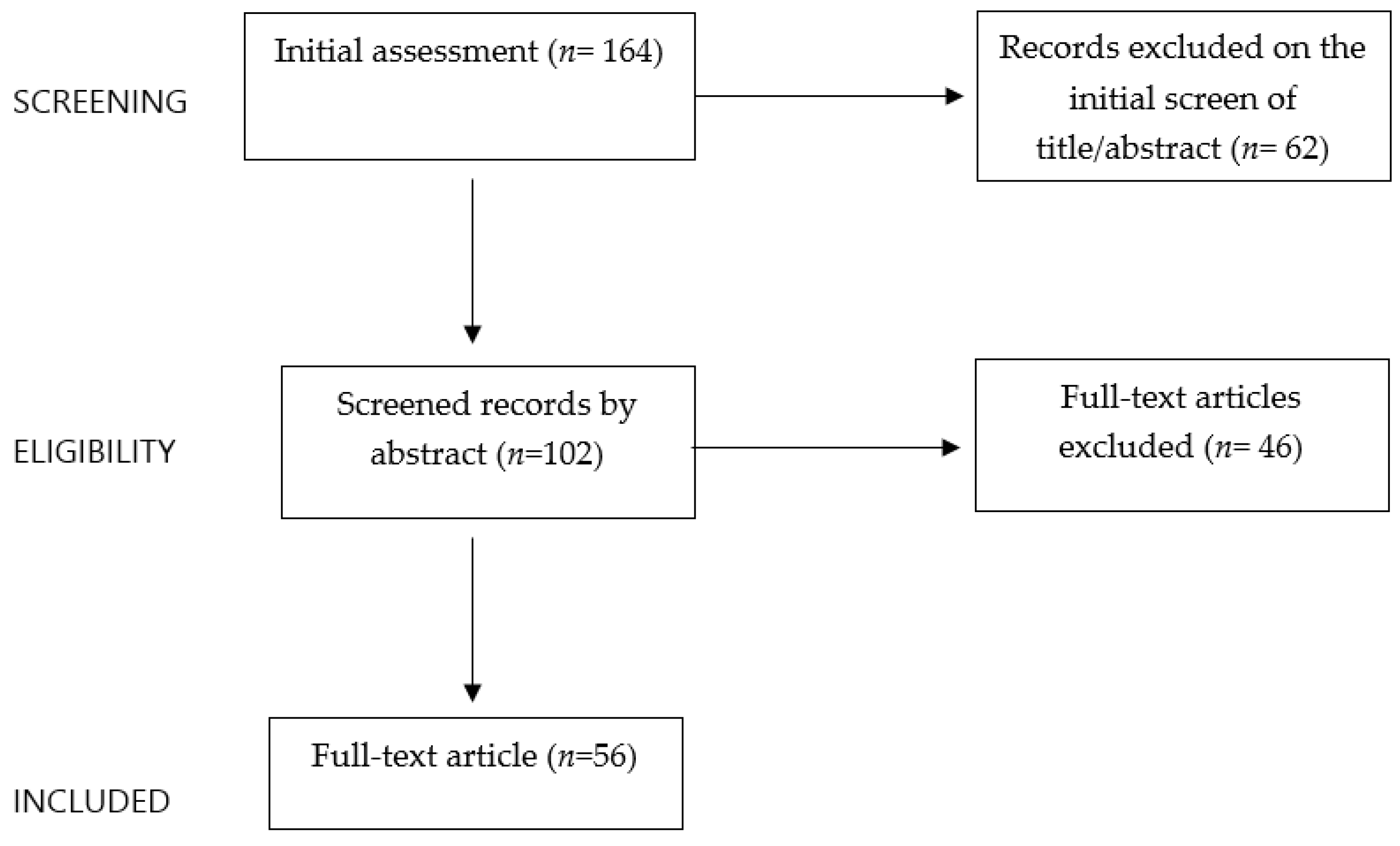
2. Methods
3. Polycystic Ovary Syndrome in Adolescents
4. Polycystic Ovary Syndrome and Obesity
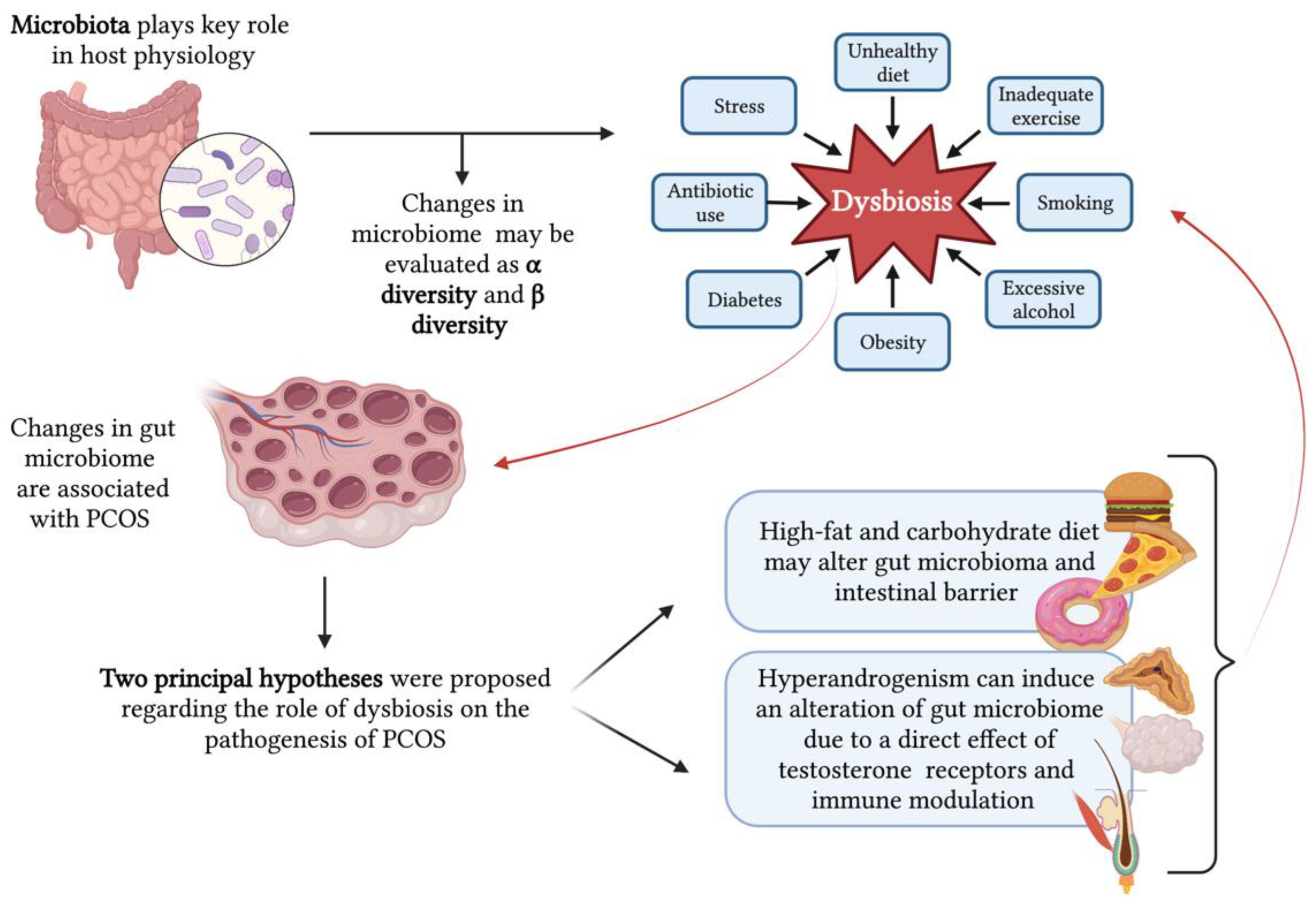
5. Dysbiosis and Polycystic Ovary Syndrome
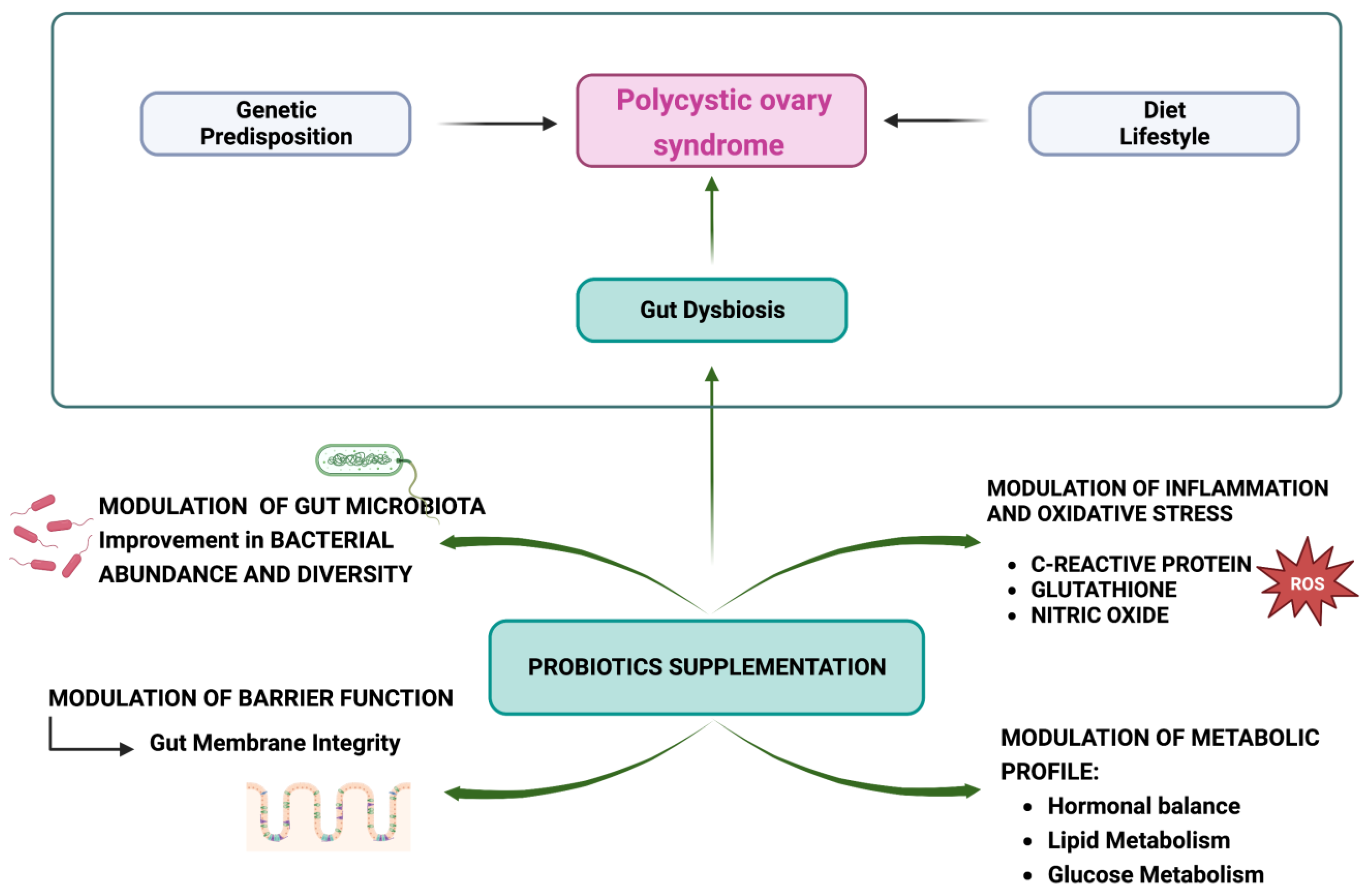
6. Probiotics and Polycystic Ovary Syndrome
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dabadghao, P. Polycystic Ovary Syndrome in Adolescents. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101272. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.E.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic Ovary Syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International PCOS Network; Andersen, M.; et al. Recommendations from the International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef]
- Calcaterra, V.; Verduci, E.; Cena, H.; Magenes, V.C.; Todisco, C.F.; Tenuta, E.; Gregorio, C.; De Giuseppe, R.; Bosetti, A.; Di Profio, E.; et al. Polycystic Ovary Syndrome in Insulin-Resistant Adolescents with Obesity: The Role of Nutrition Therapy and Food Supplements as a Strategy to Protect Fertility. Nutrients 2021, 13, 1848. [Google Scholar] [CrossRef]
- WHO Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 2 June 2023).
- Sommer, A.; Twig, G. The Impact of Childhood and Adolescent Obesity on Cardiovascular Risk in Adulthood: A Systematic Review. Curr. Diabetes Rep. 2018, 18, 91. [Google Scholar] [CrossRef]
- WHO Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 June 2023).
- Barber, T.M.; Hanson, P.; Weickert, M.O.; Franks, S. Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119874042. [Google Scholar] [CrossRef] [PubMed]
- Insenser, M.; Murri, M.; Del Campo, R.; Martínez-García, M.Á.; Fernández-Durán, E.; Escobar-Morreale, H.F. Gut Microbiota and the Polycystic Ovary Syndrome: Influence of Sex, Sex Hormones, and Obesity. J. Clin. Endocrinol. Metab. 2018, 103, 2552–2562. [Google Scholar] [CrossRef]
- Chu, W.; Han, Q.; Xu, J.; Wang, J.; Sun, Y.; Li, W.; Chen, Z.-J.; Du, Y. Metagenomic Analysis Identified Microbiome Alterations and Pathological Association between Intestinal Microbiota and Polycystic Ovary Syndrome. Fertil. Steril. 2020, 113, 1286–1298.e4. [Google Scholar] [CrossRef]
- Zhou, L.; Ni, Z.; Cheng, W.; Yu, J.; Sun, S.; Zhai, D.; Yu, C.; Cai, Z. Characteristic Gut Microbiota and Predicted Metabolic Functions in Women with PCOS. Endocr. Connect. 2020, 9, 63–73. [Google Scholar] [CrossRef]
- Torres, P.J.; Ho, B.S.; Arroyo, P.; Sau, L.; Chen, A.; Kelley, S.T.; Thackray, V.G. Exposure to a Healthy Gut Microbiome Protects against Reproductive and Metabolic Dysregulation in a PCOS Mouse Model. Endocrinology 2019, 160, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut Microbiota-Bile Acid-Interleukin-22 Axis Orchestrates Polycystic Ovary Syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Lynch, S.V. The Gut Microbiome: Relationships with Disease and Opportunities for Therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)—A Novel Theory for the Development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef]
- Kelley, S.T.; Skarra, D.V.; Rivera, A.J.; Thackray, V.G. The Gut Microbiome Is Altered in a Letrozole-Induced Mouse Model of Polycystic Ovary Syndrome. PLoS ONE 2016, 11, e0146509. [Google Scholar] [CrossRef]
- Akgül, S.; Düzçeker, Y.; Kanbur, N.; Derman, O. Do Different Diagnostic Criteria Impact Polycystic Ovary Syndrome Diagnosis for Adolescents? J. Pediatr. Adolesc. Gynecol. 2018, 31, 258–262. [Google Scholar] [CrossRef]
- Adone, A.; Fulmali, D.G. Polycystic Ovarian Syndrome in Adolescents. Cureus 2023, 15, e34183. [Google Scholar] [CrossRef]
- Naz, M.S.G.; Tehrani, F.R.; Majd, H.A.; Ahmadi, F.; Ozgoli, G.; Fakari, F.R.; Ghasemi, V. The Prevalence of Polycystic Ovary Syndrome in Adolescents: A Systematic Review and Meta-Analysis. Int. J. Reprod. Biomed. 2019, 17, 533–542. [Google Scholar] [CrossRef]
- Aboeldalyl, S.; James, C.; Seyam, E.; Ibrahim, E.M.; Shawki, H.E.-D.; Amer, S. The Role of Chronic Inflammation in Polycystic Ovarian Syndrome-A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 2734. [Google Scholar] [CrossRef]
- Meczekalski, B.; Niwczyk, O.; Kostrzak, A.; Maciejewska-Jeske, M.; Bala, G.; Szeliga, A. PCOS in Adolescents—Ongoing Riddles in Diagnosis and Treatment. J. Clin. Med. 2023, 12, 1221. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; O’Brien, C.; Hawrelak, J.; Gersh, F.L. Polycystic Ovary Syndrome: An Evolutionary Adaptation to Lifestyle and the Environment. Int. J. Environ. Res. Public Health 2022, 19, 1336. [Google Scholar] [CrossRef] [PubMed]
- Steegers-Theunissen, R.P.M.; Wiegel, R.E.; Jansen, P.W.; Laven, J.S.E.; Sinclair, K.D. Polycystic Ovary Syndrome: A Brain Disorder Characterized by Eating Problems Originating during Puberty and Adolescence. Int. J. Mol. Sci. 2020, 21, 8211. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Tal, R. The Role of AMH in the Pathophysiology of Polycystic Ovarian Syndrome. Reprod. Biomed. Online 2016, 33, 15–28. [Google Scholar] [CrossRef]
- Ibáñez, L.; Díaz, R.; López-Bermejo, A.; Marcos, M.V. Clinical Spectrum of Premature Pubarche: Links to Metabolic Syndrome and Ovarian Hyperandrogenism. Rev. Endocr. Metab. Disord. 2009, 10, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Risal, S.; Pei, Y.; Lu, H.; Manti, M.; Fornes, R.; Pui, H.-P.; Zhao, Z.; Massart, J.; Ohlsson, C.; Lindgren, E.; et al. Prenatal Androgen Exposure and Transgenerational Susceptibility to Polycystic Ovary Syndrome. Nat. Med. 2019, 25, 1894–1904. [Google Scholar] [CrossRef]
- Heidarzadehpilehrood, R.; Pirhoushiaran, M.; Abdollahzadeh, R.; Binti Osman, M.; Sakinah, M.; Nordin, N.; Abdul Hamid, H. A Review on CYP11A1, CYP17A1, and CYP19A1 Polymorphism Studies: Candidate Susceptibility Genes for Polycystic Ovary Syndrome (PCOS) and Infertility. Genes 2022, 13, 302. [Google Scholar] [CrossRef]
- Spritzer, P.M.; Marchesan, L.B.; Santos, B.R.; Fighera, T.M. Hirsutism, Normal Androgens and Diagnosis of PCOS. Diagnostics 2022, 12, 1922. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, P.; Li, R.; Wu, Y.; Huang, H. Editorial: Polycystic Ovary Syndrome (PCOS): Mechanism and Management. Front. Endocrinol. 2022, 13, 1030353. [Google Scholar] [CrossRef]
- Chang, S.; Dunaif, A. Diagnosis of Polycystic Ovary Syndrome: Which Criteria to Use and When? Endocrinol. Metab. Clin. N. Am. 2021, 50, 11–23. [Google Scholar] [CrossRef]
- Polson, D.W.; Adams, J.; Wadsworth, J.; Franks, S. Polycystic Ovaries—A Common Finding in Normal Women. Lancet 1988, 1, 870–872. [Google Scholar] [CrossRef]
- Fauser, B.C.J.M.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.E.; et al. Consensus on Women’s Health Aspects of Polycystic Ovary Syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28–38.e25. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L. Perspectives on the International Recommendations for the Diagnosis and Treatment of Polycystic Ovary Syndrome in Adolescence. J. Pediatr. Adolesc. Gynecol. 2020, 33, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Vassalou, H.; Sotiraki, M.; Michala, L. PCOS Diagnosis in Adolescents: The Timeline of a Controversy in a Systematic Review. J. Pediatr. Endocrinol. Metab. 2019, 32, 549–559. [Google Scholar] [CrossRef]
- Peña, A.S.; Metz, M. What Is Adolescent Polycystic Ovary Syndrome? J. Paediatr. Child. Health 2018, 54, 351–355. [Google Scholar] [CrossRef]
- Fulghesu, A.M.; Canu, E.; Casula, L.; Melis, F.; Gambineri, A. Polycystic Ovarian Morphology in Normocyclic Non-Hyperandrogenic Adolescents. J. Pediatr. Adolesc. Gynecol. 2021, 34, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Peña, A.S.; Witchel, S.F.; Hoeger, K.M.; Oberfield, S.E.; Vogiatzi, M.G.; Misso, M.; Garad, R.; Dabadghao, P.; Teede, H. Adolescent Polycystic Ovary Syndrome According to the International Evidence-Based Guideline. BMC Med. 2020, 18, 72. [Google Scholar] [CrossRef]
- Witchel, S.F.; Oberfield, S.; Rosenfield, R.L.; Codner, E.; Bonny, A.; Ibáñez, L.; Pena, A.; Horikawa, R.; Gomez-Lobo, V.; Joel, D.; et al. The Diagnosis of Polycystic Ovary Syndrome during Adolescence. Horm. Res. Paediatr. 2015, 83, 376–389. [Google Scholar] [CrossRef]
- Ibáñez, L.; Oberfield, S.E.; Witchel, S.; Auchus, R.J.; Chang, R.J.; Codner, E.; Dabadghao, P.; Darendeliler, F.; Elbarbary, N.S.; Gambineri, A.; et al. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Horm. Res. Paediatr. 2017, 88, 371–395. [Google Scholar] [CrossRef]
- Witchel, S.F.; Teede, H.J.; Peña, A.S. Curtailing PCOS. Pediatr. Res. 2020, 87, 353–361. [Google Scholar] [CrossRef]
- Cooney, L.G.; Dokras, A. Beyond Fertility: Polycystic Ovary Syndrome and Long-Term Health. Fertil. Steril. 2018, 110, 794–809. [Google Scholar] [CrossRef]
- Trent, M.; Gordon, C.M. Diagnosis and Management of Polycystic Ovary Syndrome in Adolescents. Pediatrics 2020, 145, S210–S218. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S.; DiVasta, A.; Gooding, H. An Update on PCOS in Adolescents. Curr. Opin. Pediatr. 2018, 30, 459–465. [Google Scholar] [CrossRef]
- Pkhaladze, L.; Barbakadze, L.; Kvashilava, N. Myo-Inositol in the Treatment of Teenagers Affected by PCOS. Int. J. Endocrinol. 2016, 2016, 1473612. [Google Scholar] [CrossRef] [PubMed]
- D Genazzani, A. Effects of a Combination of Alpha Lipoic Acid and Myo-Inositol on Insulin Dynamics in Overweight/Obese Patients with PCOS. Endocrinol. Metab. Syndr. 2014, 3, 140. [Google Scholar] [CrossRef]
- Stein, I.F.; Leventhal, M.L. Amenorrhea Associated with Bilateral Polycystic Ovaries. Am. J. Obstet. Gynecol. 1935, 29, 181–191. [Google Scholar] [CrossRef]
- Dapas, M.; Dunaif, A. Deconstructing a Syndrome: Genomic Insights Into PCOS Causal Mechanisms and Classification. Endocr. Rev. 2022, 43, 927–965. [Google Scholar] [CrossRef] [PubMed]
- Rebar, R.; Judd, H.L.; Yen, S.S.; Rakoff, J.; Vandenberg, G.; Naftolin, F. Characterization of the Inappropriate Gonadotropin Secretion in Polycystic Ovary Syndrome. J. Clin. Investig. 1976, 57, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.E.; McCourt, B.; Martin, K.A.; Anderson, E.J.; Adams, J.M.; Schoenfeld, D.; Hall, J.E. Determinants of Abnormal Gonadotropin Secretion in Clinically Defined Women with Polycystic Ovary Syndrome1. J. Clin. Endocrinol. Metab. 1997, 82, 2248–2256. [Google Scholar] [CrossRef]
- Dunaif, A. Perspectives in Polycystic Ovary Syndrome: From Hair to Eternity. J. Clin. Endocrinol. Metab. 2016, 101, 759–768. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. The Androgen Excess and PCOS Society Criteria for the Polycystic Ovary Syndrome: The Complete Task Force Report. Fertil. Steril. 2009, 91, 456–488. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Barbotin, A.-L.; Dumont, A.; Catteau-Jonard, S.; Robin, G. Role of Anti-Müllerian Hormone in the Pathogenesis of Polycystic Ovary Syndrome. Front. Endocrinol. 2020, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Dumont, A.; Robin, G.; Catteau-Jonard, S.; Dewailly, D. Role of Anti-Müllerian Hormone in Pathophysiology, Diagnosis and Treatment of Polycystic Ovary Syndrome: A Review. Reprod. Biol. Endocrinol. 2015, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Gilling-Smith, C.; Willis, D.S.; Beard, R.W.; Franks, S. Hypersecretion of Androstenedione by Isolated Thecal Cells from Polycystic Ovaries. J. Clin. Endocrinol. Metab. 1994, 79, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Goldenberg, N. Characteristics of Obesity in Polycystic Ovary Syndrome: Etiology, Treatment, and Genetics. Metabolism 2019, 92, 108–120. [Google Scholar] [CrossRef]
- Alvarez-Blasco, F.; Botella-Carretero, J.I.; Millán, J.L.S.; Escobar-Morreale, H.F. Prevalence and Characteristics of the Polycystic Ovary Syndrome in Overweight and Obese Women. Obstet. Gynecol. 2007, 109, 446. [Google Scholar] [CrossRef]
- Flannery, C.A.; Rackow, B.; Cong, X.; Duran, E.; Selen, D.J.; Burgert, T.S. Polycystic Ovary Syndrome in Adolescence: Impaired Glucose Tolerance Occurs across the Spectrum of BMI: IGT in Adolescent Polycystic Ovary Syndrome. Pediatr. Diabetes 2013, 14, 42–49. [Google Scholar] [CrossRef]
- Hickey, M.; Doherty, D.A.; Atkinson, H.; Sloboda, D.M.; Franks, S.; Norman, R.J.; Hart, R. Clinical, Ultrasound and Biochemical Features of Polycystic Ovary Syndrome in Adolescents: Implications for Diagnosis. Hum. Reprod. 2011, 26, 1469–1477. [Google Scholar] [CrossRef]
- Ezeh, U.; Yildiz, B.O.; Azziz, R. Referral Bias in Defining the Phenotype and Prevalence of Obesity in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E1088–E1096. [Google Scholar] [CrossRef]
- Ohashi, K.; Shibata, R.; Murohara, T.; Ouchi, N. Role of Anti-Inflammatory Adipokines in Obesity-Related Diseases. Trends Endocrinol. Metab. 2014, 25, 348–355. [Google Scholar] [CrossRef]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine Dysregulation and Adipose Tissue Inflammation in Human Obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Su, X.; Peng, D. Adipokines as Novel Biomarkers of Cardio-Metabolic Disorders. Clin. Chim. Acta 2020, 507, 31–38. [Google Scholar] [CrossRef]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New Insight into Adiponectin Role in Obesity and Obesity-Related Diseases. BioMed Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the Past Two Decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Lainez, N.M.; Coss, D. Obesity, Neuroinflammation, and Reproductive Function. Endocrinology 2019, 160, 2719–2736. [Google Scholar] [CrossRef]
- Engin, A. The Pathogenesis of Obesity-Associated Adipose Tissue Inflammation. Adv. Exp. Med. Biol. 2017, 960, 221–245. [Google Scholar] [CrossRef]
- Mannerås-Holm, L.; Leonhardt, H.; Kullberg, J.; Jennische, E.; Odén, A.; Holm, G.; Hellström, M.; Lönn, L.; Olivecrona, G.; Stener-Victorin, E.; et al. Adipose Tissue Has Aberrant Morphology and Function in PCOS: Enlarged Adipocytes and Low Serum Adiponectin, but Not Circulating Sex Steroids, Are Strongly Associated with Insulin Resistance. J. Clin. Endocrinol. Metab. 2011, 96, E304–E311. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef] [PubMed]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Regalbuto, C.; Porri, D.; Pelizzo, G.; Mazzon, E.; Vinci, F.; Zuccotti, G.; Fabiano, V.; Cena, H. Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent. Biomolecules 2020, 10, 1324. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef]
- Dewailly, D.; Catteau-Jonard, S.; Reyss, A.-C.; Leroy, M.; Pigny, P. Oligoanovulation with Polycystic Ovaries but Not Overt Hyperandrogenism. J. Clin. Endocrinol. Metab. 2006, 91, 3922–3927. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xie, Y.; Liu, Y.; Long, S.; Mo, Z. Polycystic Ovarian Syndrome: Correlation between Hyperandrogenism, Insulin Resistance and Obesity. Clin. Chim. Acta 2020, 502, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.O.; Carmina, E.; Azziz, R. DHEA, DHEAS and PCOS. J. Steroid Biochem. Mol. Biol. 2015, 145, 213–225. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; O’Brien, C.; Hawrelak, J. A Narrative Review of the Role of Gastrointestinal Dysbiosis in the Pathogenesis of Polycystic Ovary Syndrome. Obstet. Gynecol. Sci. 2022, 65, 14–28. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human Gut Microbiome Viewed across Age and Geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional Interactions between the Gut Microbiota and Host Metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e004947. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Mammadova, G.; Ozkul, C.; Yilmaz Isikhan, S.; Acikgoz, A.; Yildiz, B.O. Characterization of Gut Microbiota in Polycystic Ovary Syndrome: Findings from a Lean Population. Eur. J. Clin. Investig. 2021, 51, e13417. [Google Scholar] [CrossRef]
- Lindheim, L.; Bashir, M.; Münzker, J.; Trummer, C.; Zachhuber, V.; Leber, B.; Horvath, A.; Pieber, T.R.; Gorkiewicz, G.; Stadlbauer, V.; et al. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS ONE 2017, 12, e0168390. [Google Scholar] [CrossRef]
- Jobira, B.; Frank, D.N.; Pyle, L.; Silveira, L.J.; Kelsey, M.M.; Garcia-Reyes, Y.; Robertson, C.E.; Ir, D.; Nadeau, K.J.; Cree-Green, M. Obese Adolescents with PCOS Have Altered Biodiversity and Relative Abundance in Gastrointestinal Microbiota. J. Clin. Endocrinol. Metab. 2020, 105, e2134–e2144. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Y.; Qian, C.; Liu, Q.; Cao, W.; Ma, M.; He, R.; Chen, R.; Geng, R.; Liu, Y. Dysbiosis of the Saliva Microbiome in Patients with Polycystic Ovary Syndrome. Front. Cell Infect. Microbiol. 2020, 10, 624504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Z.; Jiang, S.; Bai, X.; Ma, C.; Peng, Q.; Chen, K.; Chang, H.; Fang, T.; Zhang, H. Probiotic Bifidobacterium Lactis V9 Regulates the Secretion of Sex Hormones in Polycystic Ovary Syndrome Patients through the Gut-Brain Axis. mSystems 2019, 4, e00017-19. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, C.; Shi, Y.; Zhang, F.; Li, L.; Wang, X.; Ling, Y.; Fu, H.; Dong, W.; Shen, J.; et al. Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Front. Microbiol. 2017, 8, 324. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Lai, Z.; Sun, L.; Zhang, Z.; Yang, J.; Li, Z.; Lin, J.; Zhang, Z. Structural and Functional Profiles of the Gut Microbial Community in Polycystic Ovary Syndrome with Insulin Resistance (IR-PCOS): A Pilot Study. Res. Microbiol. 2019, 170, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.G.; Thackray, V.G. Intersection of Polycystic Ovary Syndrome and the Gut Microbiome. J. Endocr. Soc. 2021, 5, bvaa177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, Y.; Xi, H.; Chen, L.; Feng, X. Exploration of the Relationship Between Gut Microbiota and Polycystic Ovary Syndrome (PCOS): A Review. Geburtshilfe Frauenheilkd 2020, 80, 161–171. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, B.; Jiang, X.; Li, Z.; Zhao, S.; Cui, L.; Chen, Z.-J. Metabolic Disturbances in Non-Obese Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Fertil. Steril. 2019, 111, 168–177. [Google Scholar] [CrossRef]
- Wolf, W.M.; Wattick, R.A.; Kinkade, O.N.; Olfert, M.D. Geographical Prevalence of Polycystic Ovary Syndrome as Determined by Region and Race/Ethnicity. Int. J. Environ. Res. Public Health 2018, 15, 2589. [Google Scholar] [CrossRef] [PubMed]
- Vom Steeg, L.G.; Klein, S.L. Sex Steroids Mediate Bidirectional Interactions Between Hosts and Microbes. Horm. Behav. 2017, 88, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender Bias in Autoimmunity Is Influenced by Microbiota. Immunity 2013, 39, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, J.; Li, W.; Chen, Z.-J.; Du, Y. Androgen-Induced Gut Dysbiosis Disrupts Glucolipid Metabolism and Endocrinal Functions in Polycystic Ovary Syndrome. Microbiome 2021, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Alesi, S.; Ee, C.; Moran, L.J.; Rao, V.; Mousa, A. Nutritional Supplements and Complementary Therapies in Polycystic Ovary Syndrome. Adv. Nutr. 2022, 13, 1243–1266. [Google Scholar] [CrossRef]
- Singh, S.; Pal, N.; Shubham, S.; Sarma, D.K.; Verma, V.; Marotta, F.; Kumar, M. Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J. Clin. Med. 2023, 12, 1454. [Google Scholar] [CrossRef]
- Thackray, V.G. Sex, Microbes, and Polycystic Ovary Syndrome. Trends Endocrinol. Metab. 2019, 30, 54–65. [Google Scholar] [CrossRef]
- Tabrizi, R.; Ostadmohammadi, V.; Akbari, M.; Lankarani, K.B.; Vakili, S.; Peymani, P.; Karamali, M.; Kolahdooz, F.; Asemi, Z. The Effects of Probiotic Supplementation on Clinical Symptom, Weight Loss, Glycemic Control, Lipid and Hormonal Profiles, Biomarkers of Inflammation, and Oxidative Stress in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Probiotics Antimicrob. Proteins 2022, 14, 1–14. [Google Scholar] [CrossRef]
- Bhalla, P.; Rengaswamy, R.; Karunagaran, D.; Suraishkumar, G.K.; Sahoo, S. Metabolic Modeling of Host–Microbe Interactions for Therapeutics in Colorectal Cancer. NPJ Syst. Biol. Appl. 2022, 8, 1. [Google Scholar] [CrossRef]
- Shamasbi, S.G.; Ghanbari-Homayi, S.; Mirghafourvand, M. The Effect of Probiotics, Prebiotics, and Synbiotics on Hormonal and Inflammatory Indices in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2020, 59, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Suri, V.; Sachdeva, N.; Rana, S.V.; Medhi, B.; Sahni, N.; Ahire, J.; Singh, A. Efficacy of Multi-Strain Probiotic along with Dietary and Lifestyle Modifications on Polycystic Ovary Syndrome: A Randomised, Double-Blind Placebo-Controlled Study. Eur. J. Nutr. 2022, 61, 4145–4154. [Google Scholar] [CrossRef] [PubMed]
- Chudzicka-Strugała, I.; Kubiak, A.; Banaszewska, B.; Zwozdziak, B.; Siakowska, M.; Pawelczyk, L.; Duleba, A.J. Effects of Synbiotic Supplementation and Lifestyle Modifications on Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, 2566–2573. [Google Scholar] [CrossRef]
- Heshmati, J.; Farsi, F.; Yosaee, S.; Razavi, M.; Rezaeinejad, M.; Karimie, E.; Sepidarkish, M. The Effects of Probiotics or Synbiotics Supplementation in Women with Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Probiotics Antimicrob. Proteins 2019, 11, 1236–1247. [Google Scholar] [CrossRef]
- Giampaolino, P.; Foreste, V.; Di Filippo, C.; Gallo, A.; Mercorio, A.; Serafino, P.; Improda, F.P.; Verrazzo, P.; Zara, G.; Buonfantino, C.; et al. Microbiome and PCOS: State-of-Art and Future Aspects. Int. J. Mol. Sci. 2021, 22, 2048. [Google Scholar] [CrossRef]
- Kwok, K.O.; Fries, L.R.; Silva-Zolezzi, I.; Thakkar, S.K.; Iroz, A.; Blanchard, C. Effects of Probiotic Intervention on Markers of Inflammation and Health Outcomes in Women of Reproductive Age and Their Children. Front. Nutr. 2022, 9, 889040. [Google Scholar] [CrossRef]
- Ghanei, N.; Rezaei, N.; Amiri, G.A.; Zayeri, F.; Makki, G.; Nasseri, E. The Probiotic Supplementation Reduced Inflammation in Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Funct. Foods 2018, 42, 306–311. [Google Scholar] [CrossRef]
- Miao, C.; Guo, Q.; Fang, X.; Chen, Y.; Zhao, Y.; Zhang, Q. Effects of Probiotic and Synbiotic Supplementation on Insulin Resistance in Women with Polycystic Ovary Syndrome: A Meta-Analysis. J. Int. Med. Res. 2021, 49, 030006052110317. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, E.; De Pergola, G.; Rosania, R.; Loverro, G. Obesity as Disruptor of the Female Fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef]

| Criteria | Pena AS et al., 2020 | Ibanez L. et al., 2017 | Witchel S. et al., 2015 |
|---|---|---|---|
| Menstrual Irregularity | Strong correlation with the timing of menarche:
| Irregular cycles two years post-menarche. | Cycles <20 days and >45 days two years post-menarche. |
| Amenorrhea | Primary amenorrhea is defined as amenorrhea at 15 years of age or 3 years post-thelarche. | Primary amenorrhea is defined as amenorrhea in girls who completed puberty. | Primary amenorrhea is defined as amenorrhea in girls who completed puberty. |
| Clinical hyperandrogenism | Hirsutism is defined as a modified Ferriman Gallway score of 4–6 and/or severe acne. | Hirsutism and/or moderate to severe acne unresponsive to topical therapy. | Moderate to severe hirsutism and acne unresponsive to topical therapy. |
| Biochemical hyperandrogenism | In females with irregular cycles without hyperandrogenism testosterone, free testosterone of free androgen index can help with diagnosis. | Confirmation test in girls with hyperandrogenism using high-quality assays. | Elevation of total testosterone and/or free testosterone in girls with hyperandrogenism. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Rossi, V.; Massini, G.; Casini, F.; Zuccotti, G.; Fabiano, V. Probiotics and Polycystic Ovary Syndrome: A Perspective for Management in Adolescents with Obesity. Nutrients 2023, 15, 3144. https://doi.org/10.3390/nu15143144
Calcaterra V, Rossi V, Massini G, Casini F, Zuccotti G, Fabiano V. Probiotics and Polycystic Ovary Syndrome: A Perspective for Management in Adolescents with Obesity. Nutrients. 2023; 15(14):3144. https://doi.org/10.3390/nu15143144
Chicago/Turabian StyleCalcaterra, Valeria, Virginia Rossi, Giulia Massini, Francesca Casini, Gianvincenzo Zuccotti, and Valentina Fabiano. 2023. "Probiotics and Polycystic Ovary Syndrome: A Perspective for Management in Adolescents with Obesity" Nutrients 15, no. 14: 3144. https://doi.org/10.3390/nu15143144
APA StyleCalcaterra, V., Rossi, V., Massini, G., Casini, F., Zuccotti, G., & Fabiano, V. (2023). Probiotics and Polycystic Ovary Syndrome: A Perspective for Management in Adolescents with Obesity. Nutrients, 15(14), 3144. https://doi.org/10.3390/nu15143144








