Cholesterol Paradox in Older People with Type 2 Diabetes Mellitus Regardless of Lipid-Lowering Drug Use: A Cross-Sectional Cohort Study
Abstract
1. Introduction
2. Materials and Methods
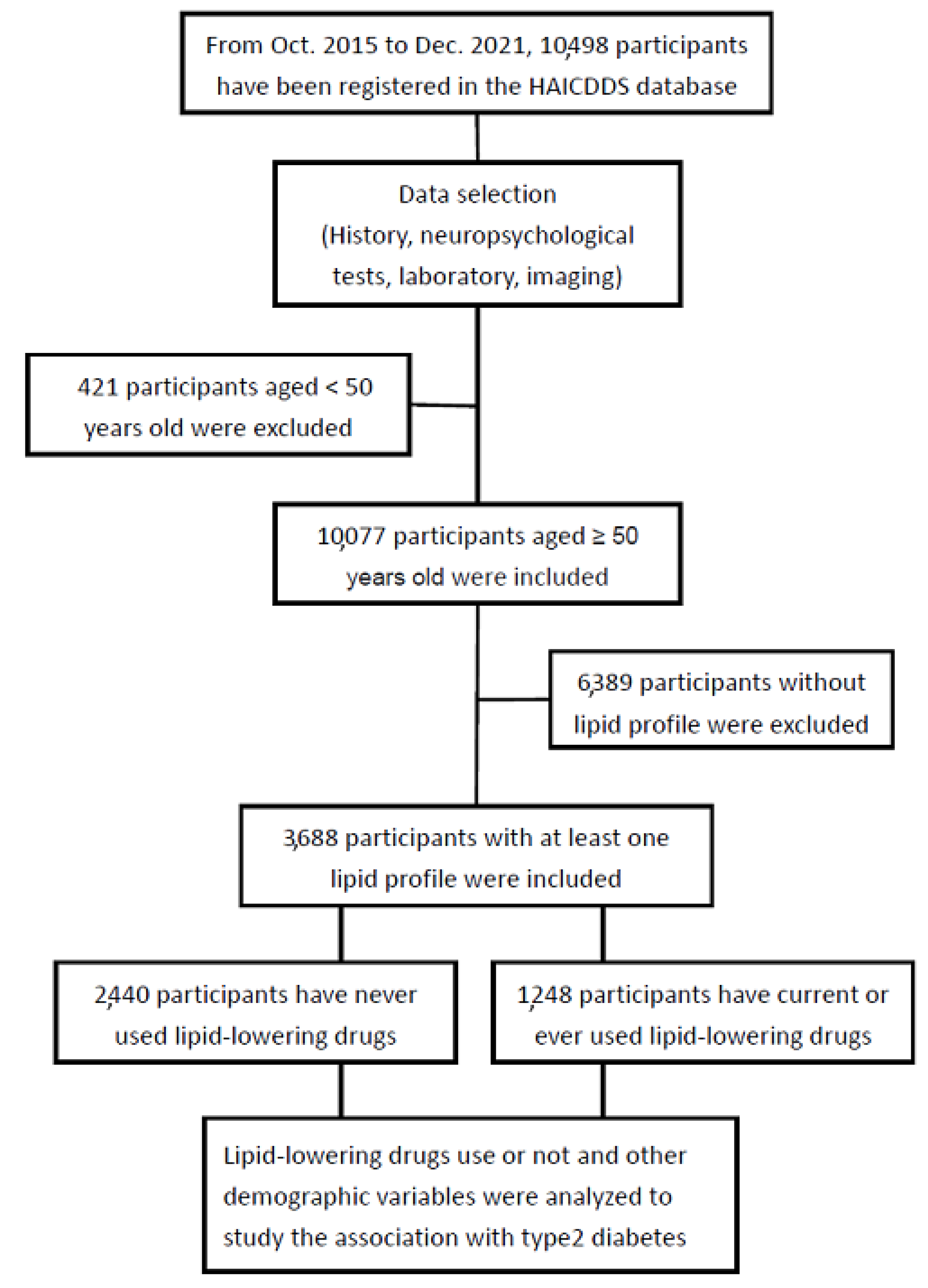
2.1. Participants
2.2. Data Analysis
2.3. Ethical Consideration
3. Results
3.1. Background Information of the Patients
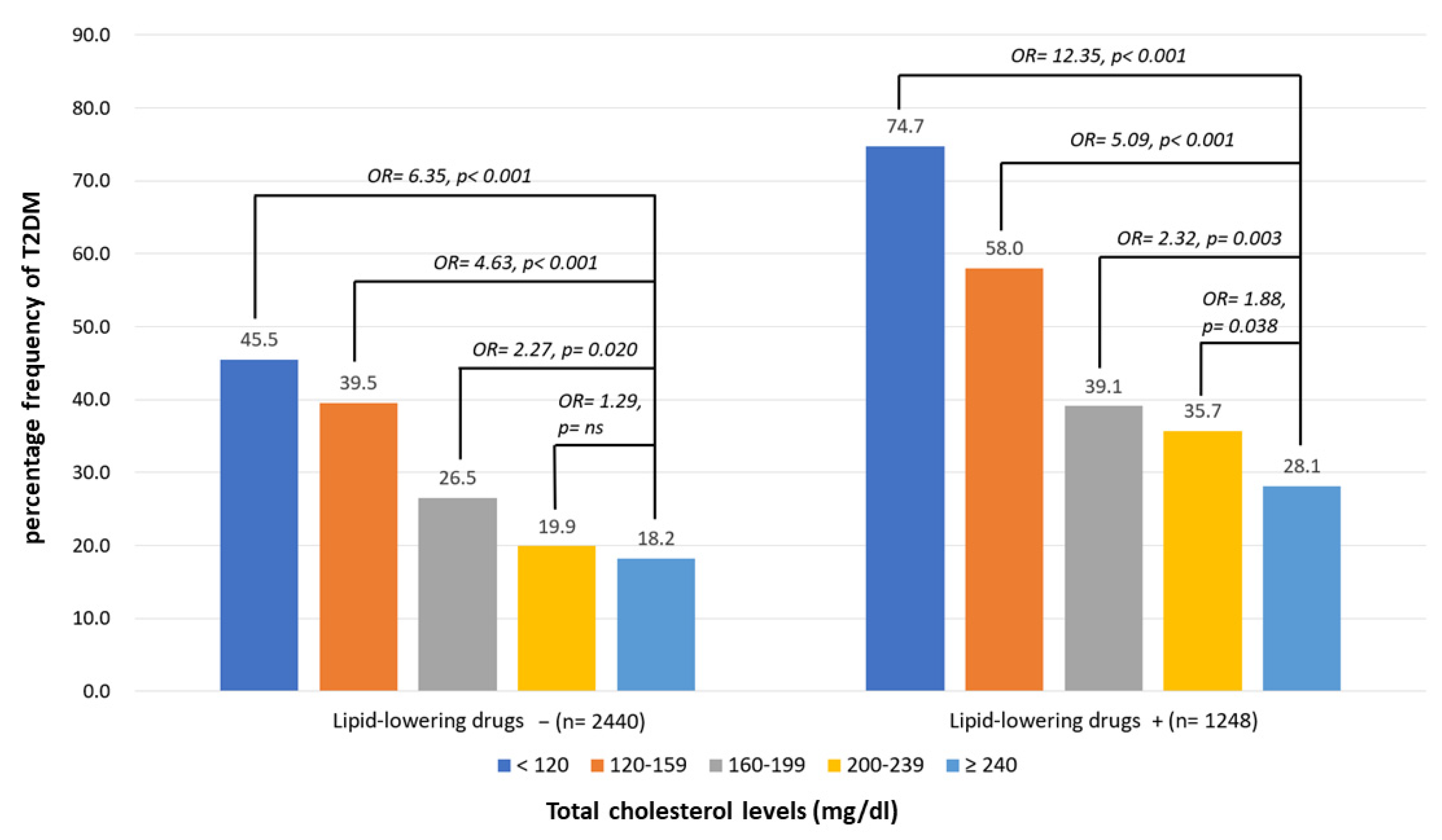
3.2. Percentage Frequencies of T2D According to the Different Levels of TC with or without LAAs
3.3. Percentage Frequencies of T2D According to the Different Levels of LDL-C with or without LAAs
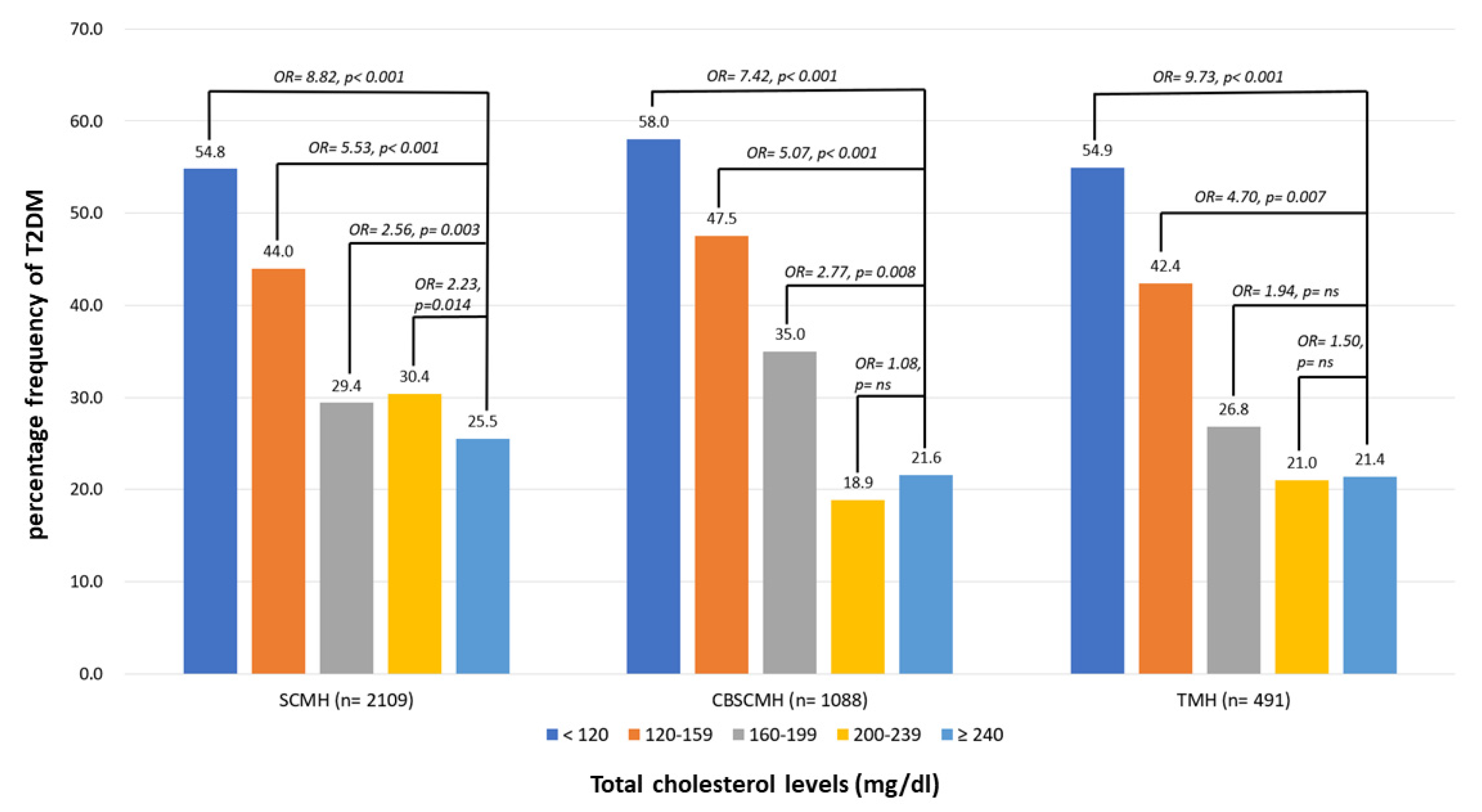
3.4. Percentage Frequencies of T2D According to the Different Levels of TC in Different Centers
3.5. Percentage Frequencies of T2D According to the Different Levels of LDL-C in Different Centers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krause, M.R.; Regen, S.L. The structural role of cholesterol in cell membranes: From condensed bilayers to lipid rafts. Acc. Chem. Res. 2014, 47, 3512–3521. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z.; Shen, W.J.; Azhar, S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 2010, 7, 47. [Google Scholar] [CrossRef]
- Saher, G.; Brugger, B.; Lappe-Siefke, C.; Mobius, W.; Tozawa, R.; Wehr, M.C.; Wieland, F.; Ishibashi, S.; Nave, K.A. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005, 8, 468–475. [Google Scholar] [CrossRef]
- Ravnskov, U. High cholesterol may protect against infections and atherosclerosis. QJM 2003, 96, 927–934. [Google Scholar] [CrossRef]
- Karalis, D.G. Intensive lowering of low-density lipoprotein cholesterol levels for primary prevention of coronary artery disease. Mayo Clin. Proc. 2009, 84, 345–352. [Google Scholar] [CrossRef]
- Grundy, S.M. Promise of low-density lipoprotein-lowering therapy for primary and secondary prevention. Circulation 2008, 117, 569–573; discussion 573. [Google Scholar] [CrossRef]
- Cybulska, B.; Klosiewicz-Latoszek, L.; Penson, P.E.; Nabavi, S.M.; Lavie, C.J.; Banach, M.; International Lipid Expert Panel. How much should LDL cholesterol be lowered in secondary prevention? Clinical efficacy and safety in the era of PCSK9 inhibitors. Prog. Cardiovasc. Dis. 2021, 67, 65–74. [Google Scholar] [CrossRef]
- Burger, A.L.; Pogran, E.; Muthspiel, M.; Kaufmann, C.C.; Jager, B.; Huber, K. New Treatment Targets and Innovative Lipid-Lowering Therapies in Very-High-Risk Patients with Cardiovascular Disease. Biomedicines 2022, 10, 970. [Google Scholar] [CrossRef]
- Jukema, J.W.; Cannon, C.P.; de Craen, A.J.; Westendorp, R.G.; Trompet, S. The controversies of statin therapy: Weighing the evidence. J. Am. Coll. Cardiol. 2012, 60, 875–881. [Google Scholar] [CrossRef]
- Navarese, E.P.; Buffon, A.; Andreotti, F.; Kozinski, M.; Welton, N.; Fabiszak, T.; Caputo, S.; Grzesk, G.; Kubica, A.; Swiatkiewicz, I.; et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am. J. Cardiol. 2013, 111, 1123–1130. [Google Scholar] [CrossRef]
- Corrao, G.; Ibrahim, B.; Nicotra, F.; Soranna, D.; Merlino, L.; Catapano, A.L.; Tragni, E.; Casula, M.; Grassi, G.; Mancia, G. Statins and the risk of diabetes: Evidence from a large population-based cohort study. Diabetes Care 2014, 37, 2225–2232. [Google Scholar] [CrossRef]
- Ridker, P.M.; Pradhan, A.; MacFadyen, J.G.; Libby, P.; Glynn, R.J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the JUPITER trial. Lancet 2012, 380, 565–571. [Google Scholar] [CrossRef]
- Bang, C.N.; Okin, P.M. Statin treatment, new-onset diabetes, and other adverse effects: A systematic review. Curr. Cardiol. Rep. 2014, 16, 461. [Google Scholar] [CrossRef]
- Everett, B.M.; Mora, S.; Glynn, R.J.; MacFadyen, J.; Ridker, P.M. Safety profile of subjects treated to very low low-density lipoprotein cholesterol levels (<30 mg/dL) with rosuvastatin 20 mg daily (from JUPITER). Am. J. Cardiol. 2014, 114, 1682–1689. [Google Scholar] [CrossRef]
- Liu, L.; Shen, G.; Huang, J.Y.; Yu, Y.L.; Chen, C.L.; Huang, Y.Q.; Feng, Y.Q. U-shaped association between low-density lipid cholesterol and diabetes mellitus in patients with hypertension. Lipids Health Dis. 2019, 18, 163. [Google Scholar] [CrossRef]
- Nunes, J.P. Statins and the cholesterol mortality paradox. Scott. Med. J. 2017, 62, 19–23. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.R.; Choi, E.K.; Han, K.D.; Oh, S. Low Lipid Levels and High Variability are Associated With the Risk of New-Onset Atrial Fibrillation. J. Am. Heart Assoc. 2019, 8, e012771. [Google Scholar] [CrossRef]
- Annoura, M.; Ogawa, M.; Kumagai, K.; Zhang, B.; Saku, K.; Arakawa, K. Cholesterol paradox in patients with paroxysmal atrial fibrillation. Cardiology 1999, 92, 21–27. [Google Scholar] [CrossRef]
- Suzuki, S. “Cholesterol paradox” in atrial fibrillation. Circ. J. 2011, 75, 2749–2750. [Google Scholar] [CrossRef]
- Mora, S.; Akinkuolie, A.O.; Sandhu, R.K.; Conen, D.; Albert, C.M. Paradoxical association of lipoprotein measures with incident atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2014, 7, 612–619. [Google Scholar] [CrossRef]
- Harrison, S.; Lip, G.Y.H.; Lane, D.A.; Mastej, M.; Kasperczyk, S.; Banach, M.; Jozwiak, J.J. The cholesterol paradox in atrial fibrillation: Results from the LIPIDOGRAM 2015 study. Eur. Heart J. 2020, 41, ehaa946.0451. [Google Scholar] [CrossRef]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef]
- Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS). Circulation 1998, 97, 1440–1445. [CrossRef]
- Orkaby, A.R.; Driver, J.A.; Ho, Y.L.; Lu, B.; Costa, L.; Honerlaw, J.; Galloway, A.; Vassy, J.L.; Forman, D.E.; Gaziano, J.M.; et al. Association of Statin Use With All-Cause and Cardiovascular Mortality in US Veterans 75 Years and Older. JAMA 2020, 324, 68–78. [Google Scholar] [CrossRef]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.; Seshasai, S.R.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W.; et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Urbano, F.; Bugliani, M.; Filippello, A.; Scamporrino, A.; Di Mauro, S.; Di Pino, A.; Scicali, R.; Noto, D.; Rabuazzo, A.M.; Averna, M.; et al. Atorvastatin but Not Pravastatin Impairs Mitochondrial Function in Human Pancreatic Islets and Rat beta-Cells. Direct Effect of Oxidative Stress. Sci. Rep. 2017, 7, 11863. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Jebari, S.; Larrea-Sebal, A.; Uribe, K.B.; Siddiqi, H.; Ostolaza, H.; Benito-Vicente, A.; Martin, C. Statin Treatment-Induced Development of Type 2 Diabetes: From Clinical Evidence to Mechanistic Insights. Int. J. Mol. Sci. 2020, 21, 4725. [Google Scholar] [CrossRef]
- Paseban, M.; Butler, A.E.; Sahebkar, A. Mechanisms of statin-induced new-onset diabetes. J. Cell Physiol. 2019, 234, 12551–12561. [Google Scholar] [CrossRef]
- Da Dalt, L.; Ruscica, M.; Bonacina, F.; Balzarotti, G.; Dhyani, A.; Di Cairano, E.; Baragetti, A.; Arnaboldi, L.; De Metrio, S.; Pellegatta, F.; et al. PCSK9 deficiency reduces insulin secretion and promotes glucose intolerance: The role of the low-density lipoprotein receptor. Eur. Heart J. 2019, 40, 357–368. [Google Scholar] [CrossRef]
- Yi, S.W.; Yi, J.J.; Ohrr, H. Total cholesterol and all-cause mortality by sex and age: A prospective cohort study among 12.8 million adults. Sci. Rep. 2019, 9, 1596. [Google Scholar] [CrossRef]


| Lipid-Lowering Drug − | Lipid-Lowering Drug +, Mean (SD) | |||||
|---|---|---|---|---|---|---|
| Group | Diabetic | Nondiabetic | OR, p | Diabetic | Nondiabetic | OR, p |
| n | 768 | 1672 | 572 | 676 | ||
| Age, year, mean (SD) | 76.2 (8.7) | 76.1 (9.6) | - | 74.2 (8.9) | 74.1 (9.6) | - |
| Male, n (%) | 336 (43.8) | 755 (45.2) | - | 232 (40.6) | 293 (43.3) | - |
| MoCA, mean (SD) | 10.2 (7.6) | 11.5 (8.2) | 0.97, <0.001 | 11.6 (7.5) | 13.3 (8.2) | 0.97, <0.001 |
| IADL, mean (SD) | 3.7 (3.2) | 4.4 (3.3) | 0.95, <0.001 | 4.4 (3.2) | 5.0 (4.4) | 0.93, <0.001 |
| Clinical history, n (%) | ||||||
| Hypertension | 609 (79.3) | 966 (57.8) | 2.81, <0.001 | 493 (86.2) | 527 (78.0) | 1.77, <0.001 |
| Dyslipidemia | 304 (39.6) | 295 (17.6) | 3.10, <0.001 | 438 (76.6) | 466 (68.9) | 1.47, 0.003 |
| CAD | 91 (11.8) | 138 (8.3) | 1.50, <0.001 | 80 (14.0) | 88 (13.0) | 1.10, ns |
| Arrythmia | 127 (16.5) | 223 (13.3) | 1.28, 0.039 | 120 (21.0) | 119 (17.6) | 1.25, ns |
| CHF | 66 (8.6) | 97 (5.8) | 1.52, 0.011 | 57 (10.0) | 64 (9.5) | 1.06, ns |
| CVD | 333 (43.4) | 530 (31.7) | 1.67, <0.001 | 249 (43.5) | 261 (38.6) | 1.26, 0.048 |
| Atherosclerosis | 220 (28.6) | 408 (24.4) | 1.25, 0.023 | 238 (41.6) | 337 (49.9) | 0.72, 0.005 |
| Smoking | 165 (21.5) | 374 (22.4) | 0.98, ns | 122 (21.3) | 133 (19.7) | 1.29, ns |
| Exercise | 177 (23.0) | 502 (30.0) | 0.69, <0.001 | 165 (27.1) | 228 (33.7) | 0.73, 0.012 |
| Current medication, n (%) | ||||||
| Antihypertensives | 325 (42.3) | 625 (37.4) | 1.23, 0.021 | 461 (80.6) | 480 (71.0) | 1.70, <0.001 |
| Antidiabetes | 331 (43.1) | 4 (0.2) | 320.24, <0.001 | 492 (86.0) | 18 (2.7) | 235.78, <0.001 |
| Antiplatelets | 326 (42.4) | 573 (34.3) | 1.43, <0.001 | 412 (72.0) | 438 (64.8) | 1.42, 0.005 |
| Anticoagulants | 71 (9.2) | 95 (5.7) | 1.69, 0.001 | 96 (16.8) | 86 (12.7) | 1.40, 0.038 |
| Medical measurement, mean (SD) | ||||||
| TC, mg/dL | 158.3 (33.4) | 171.8 (35.5) | 0.99, <0.001 | 184.6 (40.6) | 164.8 (40.4) | 0.99, <0.001 |
| LDL-C, mg/dL | 94.5 (29.3) | 104.1 (30.5) | 0.99, <0.001 | 112.2 (36.7) | 96.6 (34.4) | 0.99, <0.001 |
| HDL-C, mg/dL | 47.9 (14.9) | 53.0 (15.5) | 0.98, <0.001 | 48.8 (14.9) | 54.1 (15.7) | 0.97, <0.001 |
| TG, mg/dL | 129.5 (74.8) | 113.2 (65.6) | 1.03, <0.001 | 150.4 (96.6) | 132.8 (90.3) | 1.02, <0.001 |
| BMI, kg/m2 | 24.9 (4.0) | 23.5 (3.4) | 1.12, <0.001 | 25.2 (4.1) | 24.4 (3.9) | 1.06, <0.001 |
| SBP, mmHg | 134.8 (20.2) | 133.5 (18.5) | 1.00, ns | 135.9 (17.9) | 135.1 (18.2) | 1.00, ns |
| DBP, mmHg | 71.8 (12.8) | 73.0 (12.3) | 0.99, ns | 73.3 (12.7) | 74.9 (12.4) | 0.99, 0.042 |
| HR, bpm | 80.8 (13.8) | 78.2 (13.0) | 1.02, 0.002 | 80.4 (13.5) | 78.1 (13.2) | 1.02, 0.002 |
| Fasting glucose, mg/dL | 152.2 (88.9) | 103.0 (24.1) | 1.03, <0.001 | 144.1 (61.4) | 103.1 (22.2) | 1.04, <0.001 |
| HbA1C, mg/dL | 6.9 (1.4) | 5.6 (0.5) | 7.78, <0.001 | 7.0 (1.5) | 5.8 (0.8) | 5.97, <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-Y.; Chang, W.-L.; Wei, C.-Y.; Liu, C.-H.; Tzeng, R.-C.; Chiu, P.-Y. Cholesterol Paradox in Older People with Type 2 Diabetes Mellitus Regardless of Lipid-Lowering Drug Use: A Cross-Sectional Cohort Study. Nutrients 2023, 15, 3270. https://doi.org/10.3390/nu15143270
Wang T-Y, Chang W-L, Wei C-Y, Liu C-H, Tzeng R-C, Chiu P-Y. Cholesterol Paradox in Older People with Type 2 Diabetes Mellitus Regardless of Lipid-Lowering Drug Use: A Cross-Sectional Cohort Study. Nutrients. 2023; 15(14):3270. https://doi.org/10.3390/nu15143270
Chicago/Turabian StyleWang, Tzu-Yuan, Wei-Lun Chang, Cheng-Yu Wei, Chung-Hsiang Liu, Ray-Chang Tzeng, and Pai-Yi Chiu. 2023. "Cholesterol Paradox in Older People with Type 2 Diabetes Mellitus Regardless of Lipid-Lowering Drug Use: A Cross-Sectional Cohort Study" Nutrients 15, no. 14: 3270. https://doi.org/10.3390/nu15143270
APA StyleWang, T.-Y., Chang, W.-L., Wei, C.-Y., Liu, C.-H., Tzeng, R.-C., & Chiu, P.-Y. (2023). Cholesterol Paradox in Older People with Type 2 Diabetes Mellitus Regardless of Lipid-Lowering Drug Use: A Cross-Sectional Cohort Study. Nutrients, 15(14), 3270. https://doi.org/10.3390/nu15143270







