Mendelian Randomization Analysis Reveals Causal Effects of Polyunsaturated Fatty Acids on Subtypes of Diabetic Retinopathy Risk
Abstract
:1. Introduction
2. Materials and Methods
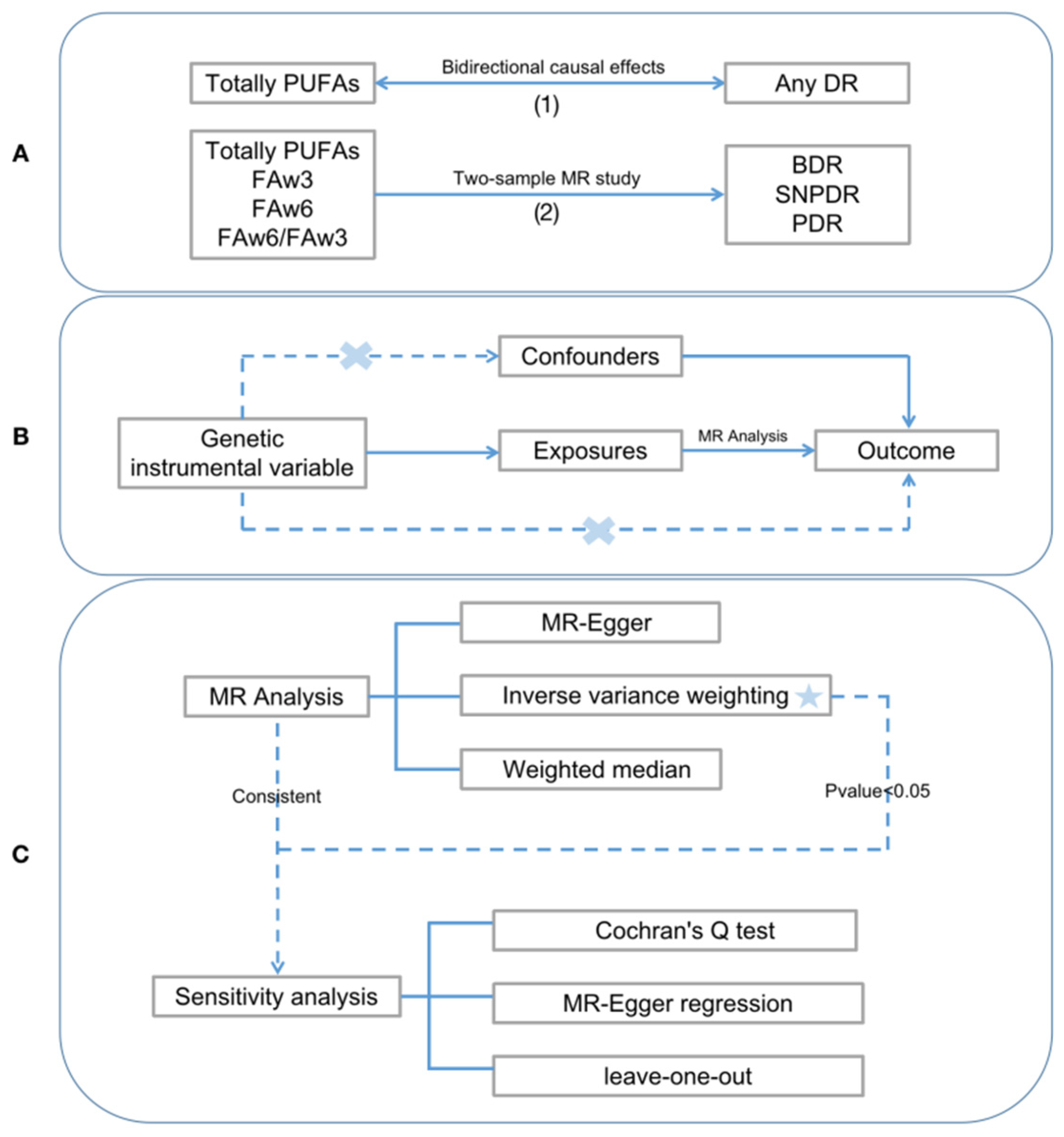
2.1. Study Design
2.2. Data Source
2.2.1. Exposure Data
2.2.2. Outcome Data
2.3. Selection of Genetic Instruments
- (1)
- All SNPs underwent screening at the genome-wide significance threshold (p < 5 × 10−8).
- (2)
- Using the “ld_clump” R package, linkage disequilibrium between SNPs (R2 < 0.001 and <10,000 from the index variant) was identified to ensure their independence [28].
- (3)
- We aligned effect alleles of outcome-related SNPs with those of exposure-related SNPs based on allelic letters and frequencies and removed palindromic SNP alleles [28].
- (4)
- We used the PhenoScanner database (https://www.phenoscanner.medschl.cam.ac.uk/, accessed on 1 May 2023) to verify whether the SNP loci were associated with other confounding factors [29].
2.4. MR Methods
2.5. Statistical Analysis
3. Results
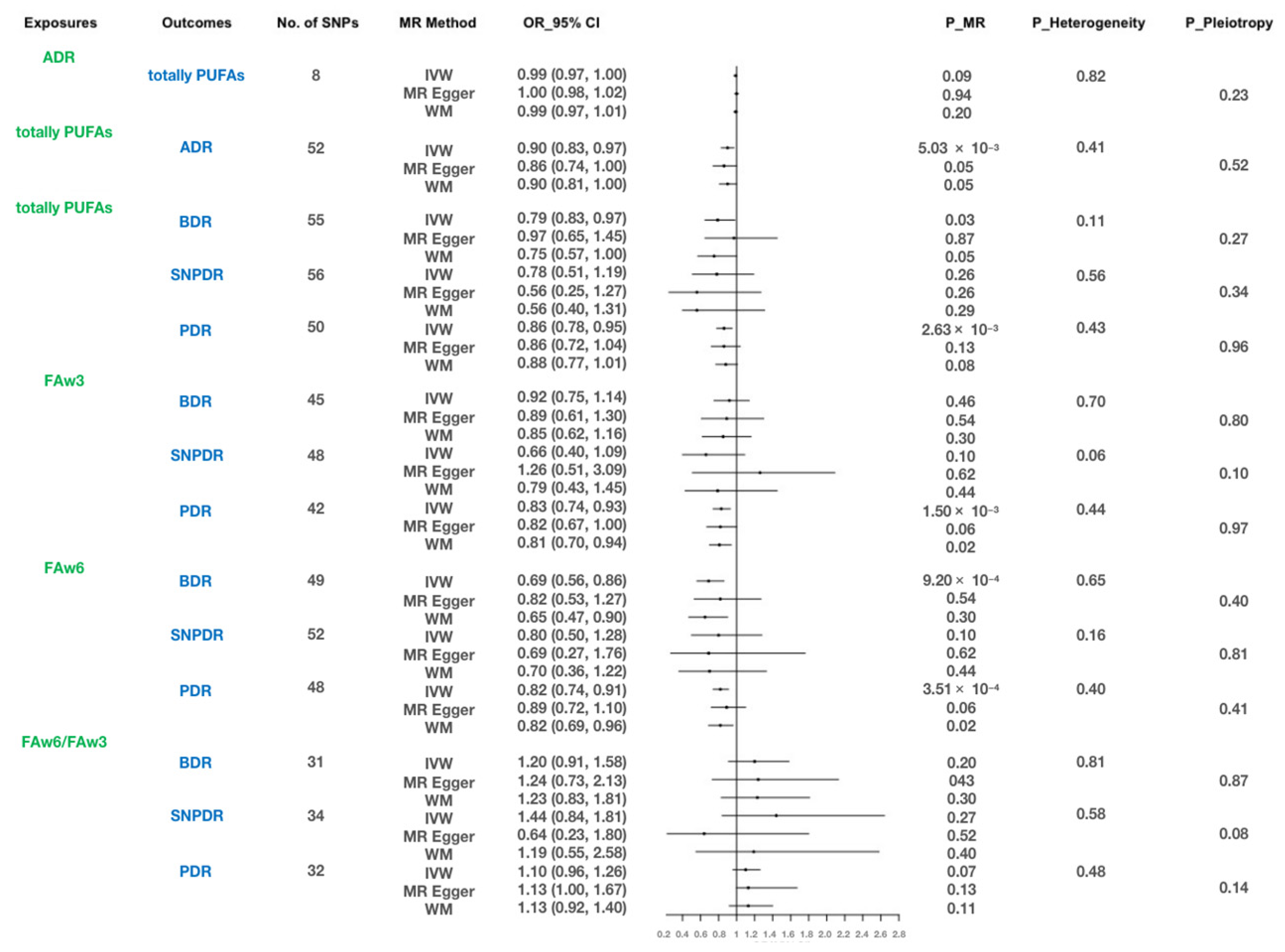
3.1. IVs for PUFAs and DR
3.2. Bidirectional Causal Effects between Total PUFAs and ADR
3.3. Causal Effects of Different Types of PUFAs on Three DR Phenotypes
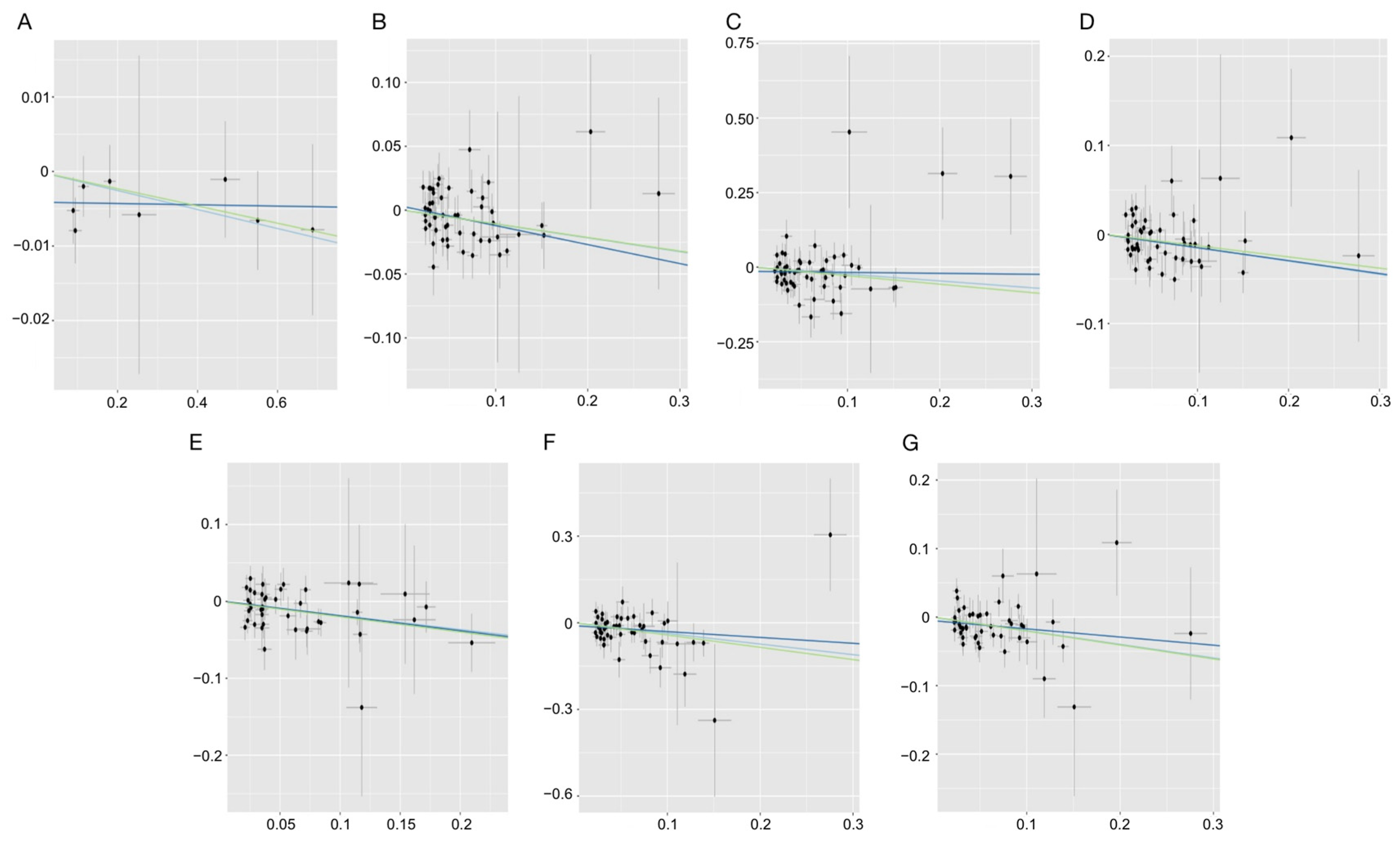
3.4. Sensitivity Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, T.E.; Wong, T.Y. Diabetic retinopathy: Looking forward to 2030. Front. Endocrinol. 2023, 13, 1077669. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simó, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.; Hsih, W.H.; Lin, Y.B.; Wen, C.Y.; Chang, T.J. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J. Diabetes Investig. 2021, 12, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Grauslund, J. Diabetic retinopathy screening in the emerging era of artificial intelligence. Diabetologia 2022, 65, 1415–1423. [Google Scholar] [CrossRef]
- Saccà, S.C.; Cutolo, C.A.; Ferrari, D.; Corazza, P.; Traverso, C.E. The Eye, Oxidative Damage and Polyunsaturated Fatty Acids. Nutrients 2018, 10, 668. [Google Scholar] [CrossRef]
- Layé, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef]
- Duan, H.; Song, W.; Zhao, J.; Yan, W. Polyunsaturated Fatty Acids (PUFAs): Sources, Digestion, Absorption, Application and Their Potential Adjunctive Effects on Visual Fatigue. Nutrients 2023, 15, 2633. [Google Scholar] [CrossRef]
- Swinkels, D.; Baes, M. The essential role of docosahexaenoic acid and its derivatives for retinal integrity. Pharmacol. Ther. 2023, 247, 108440. [Google Scholar] [CrossRef]
- Alsbirk, K.E.; Seland, J.H.; Assmus, J. Diabetic retinopathy and visual impairment in a Norwegian diabetic coast population with a high dietary intake of fish oils. An observational study. Acta Ophthalmol. 2022, 100, e532–e538. [Google Scholar] [CrossRef]
- Gong, Y.; Fu, Z.; Liegl, R.; Chen, J.; Hellström, A.; Smith, L.E. ω-3 and ω-6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases. Am. J. Clin. Nutr. 2017, 106, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Kawasaki, R.; Rogers, S.; Man, R.E.K.; Itakura, K.; Xie, J.; Flood, V.; Tsubota, K.; Lamoureux, E.; Wang, J.J. The Associations of Dietary Intake of Polyunsaturated Fatty Acids With Diabetic Retinopathy in Well-Controlled Diabetes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7473–7479. [Google Scholar] [CrossRef]
- Gorusupudi, A.; Chang, F.Y.; Nelson, K.; Hageman, G.S.; Bernstein, P.S. n-3 PUFA Supplementation Alters Retinal Very-Long-Chain-PUFA Levels and Ratios in Diabetic Animal Models. Mol. Nutr. Food Res. 2019, 63, e1801058. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jump, D.B.; Grant, M.B.; Esselman, W.J.; Busik, J.V. Dyslipidemia, but not hyperglycemia, induces inflammatory adhesion molecules in human retinal vascular endothelial cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5016–5022. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jump, D.B.; Esselman, W.J.; Busik, J.V. Inhibition of cytokine signaling in human retinal endothelial cells through modification of caveolae/lipid rafts by docosahexaenoic acid. Investig. Ophthalmol. Vis. Sci. 2007, 48, 18–26. [Google Scholar] [CrossRef]
- Pitale, P.M.; Gorbatyuk, M.S. Diabetic Retinopathy: From Animal Models to Cellular Signaling. Int. J. Mol. Sci. 2022, 23, 1487. [Google Scholar] [CrossRef] [PubMed]
- Arah, O.A. Bias Analysis for Uncontrolled Confounding in the Health Sciences. Annu. Rev. Public Health 2017, 38, 23–38. [Google Scholar] [CrossRef]
- Berlin, I.; Luquiens, A.; Aubin, H.J. Smoking as a confounder of the association of suicidality with serum lipid levels. J. Psychiatry Neurosci. 2016, 41, E24. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Balduzzi, S.; Beyerbach, J.; Bröckelmann, N.; Werner, S.S.; Zähringer, J.; Nagavci, B.; Meerpohl, J.J. Evaluating agreement between bodies of evidence from randomised controlled trials and cohort studies in nutrition research: Meta-epidemiological study. BMJ 2021, 374, n1864. [Google Scholar] [CrossRef]
- Kappelmann, N.; Müller-Myhsok, B.; Kopf-Beck, J. Adapting the randomised controlled trial (RCT) for precision medicine: Introducing the nested-precision RCT (npRCT). Trials 2021, 22, 13. [Google Scholar] [CrossRef]
- Birney, E. Mendelian Randomization. Cold Spring Harb. Perspect. Med. 2022, 12, a041302. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Kim, S.J. Inferring causality from observational studies: The role of instrumental variable analysis. Kidney Int. 2021, 99, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.; Yang, H.; Lin, Z.; Xu, L. Causal associations between polyunsaturated fatty acids and kidney function: A bidirectional Mendelian randomization study. Am. J. Clin. Nutr. 2023, 117, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.M.; Holmes, M.V.; Smith, G.D. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef] [PubMed]
- Julkunen, H.; Cichońska, A.; Slagboom, P.E.; Würtz, P.; Nightingale Health UK Biobank Initiative. Metabolic biomarker profiling for identification of susceptibility to severe pneumonia and COVID-19 in the general population. Elife 2021, 10, e63033. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen: Unique genetic insights from combining isolated population and national health register data. medRxiv 2022, 03, 22271360. [Google Scholar]
- Burgess, S.; Davies, N.M.; Thompson, S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 2016, 40, 597–608. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017, 13, e1007081. [Google Scholar]
- Gkatzionis, A.; Burgess, S.; Newcombe, P.J. Statistical methods for cis-Mendelian randomization with two-sample summary-level data. Genet. Epidemiol. 2023, 47, 3–25. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, P.T.; Timpson, N.J.; Dimou, N.K.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Smith, G.D.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Eynard, A.R.; Repossi, G. Role of ω3 polyunsaturated fatty acids in diabetic retinopathy: A morphological and metabolically cross talk among blood retina barriers damage, autoimmunity and chronic inflammation. Lipids Health Dis. 2019, 18, 114. [Google Scholar] [CrossRef]
- Li, J.-S.; Wang, T.; Zuo, J.-J.; Guo, C.-N.; Peng, F.; Zhao, S.-Z.; Li, H.-H.; Hou, X.-Q.; Lan, Y.; Wei, Y.-P.; et al. Association of n-6 PUFAs with the risk of diabetic retinopathy in diabetic patients. Endocr. Connect. 2020, 9, 1191–1201. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Díaz-López, A.; Valls-Pedret, C.; Cofán, M.; García-Layana, A.; Lamuela-Raventós, R.M.; Castaner, O.; Zanon-Moreno, V.; Martinez-Gonzalez, M.A.; Toledo, E.; et al. Dietary Marine ω-3 Fatty Acids and Incident Sight-Threatening Retinopathy in Middle-Aged and Older Individuals with Type 2 Diabetes: Prospective Investigation From the PREDIMED Trial. JAMA Ophthalmol. 2016, 134, 1142–1149. [Google Scholar] [CrossRef]
- Tikhonenko, M.; Lydic, T.A.; Opreanu, M.; Calzi, S.L.; Bozack, S.; McSorley, K.M.; Sochacki, A.L.; Faber, M.S.; Hazra, S.; Duclos, S.; et al. N-3 polyunsaturated Fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PLoS ONE 2013, 8, e55177. [Google Scholar] [CrossRef]
- Yanai, R.; Mulki, L.; Hasegawa, E.; Takeuchi, K.; Sweigard, H.; Suzuki, J.; Gaissert, P.; Vavvas, D.G.; Sonoda, K.-H.; Rothe, M.; et al. Cytochrome P450-generated metabolites derived from ω-3 fatty acids attenuate neovascularization. Proc. Natl. Acad. Sci. USA 2014, 111, 9603–9608. [Google Scholar] [CrossRef] [PubMed]
- Bühler, A.D.; Bucher, F.; Augustynik, M.; Wöhrl, J.; Martin, G.; Schlunck, G.; Agostini, H.; Böhringer, D.; Pütz, G.; Stahl, A. Systemic confounders affecting serum measurements of omega-3 and -6 polyunsaturated fatty acids in patients with retinal disease. BMC Ophthalmol. 2016, 16, 159. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.P.; Weiss, A.; Führer, D.; Krämer, H.J.; Papavassilis, C.; Grimminger, F. Acceleration of experimental diabetic retinopathy in the rat by omega-3 fatty acids. Diabetologia 1996, 39, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, P.; Zhang, R.-D.; Fang, Y.; Jiang, L.-Q.; Fang, X.; Zhao, Y.; Wang, D.-G.; Ni, J.; Pan, H.-F. Mendelian randomization as a tool to gain insights into the mosaic causes of autoimmune diseases. Autoimmun. Rev. 2022, 21, 103210. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar] [CrossRef]
- Busik, J.V. Lipid metabolism dysregulation in diabetic retinopathy. J. Lipid Res. 2021, 62, 100017. [Google Scholar] [CrossRef]
- Aranda, J.V.; Qu, J.; Valencia, G.B.; Beharry, K.D. Pharmacologic interventions for the prevention and treatment of retinopathy of prematurity. Semin. Perinatol. 2019, 43, 360–366. [Google Scholar] [CrossRef]
- Shen, J.; Shen, S.; Das, U.N.; Xu, G. Effect of essential fatty acids on glucose-induced cytotoxicity to retinal vascular endothelial cells. Lipids Health Dis. 2012, 11, 90. [Google Scholar] [CrossRef]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n-3 and n-6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Miles, E.A.; Nebe-Von-Caron, G.; Powell, J.R.; Hurst, T.L.; Newsholme, E.A.; Calder, P.C. Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids 2001, 36, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, S.; Ishikura, Y.; Tateishi, N.; Horikawa, C.; Tokuda, H.; Kontani, M.; Kawashima, H.; Sakakibara, Y.; Kiso, Y.; Shibata, H.; et al. Supplementation of arachidonic acid-enriched oil increases arachidonic acid contents in plasma phospholipids, but does not increase their metabolites and clinical parameters in Japanese healthy elderly individuals: A randomized controlled study. Lipids Health Dis. 2011, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Fu, Z.; Yan, W.; Chen, C.T.; Nilsson, A.K.; Bull, E.; Allen, W.; Yang, J.; Ko, M.; SanGiovanni, J.P.; Akula, J.D.; et al. Omega-3/Omega-6 Long-Chain Fatty Acid Imbalance in Phase I Retinopathy of Prematurity. Nutrients 2022, 14, 1333. [Google Scholar] [CrossRef]
- Wang, K.; Zhong, Y.; Yang, F.; Hu, C.; Liu, X.; Zhu, Y.; Yao, K. Causal Effects of N-6 Polyunsaturated Fatty Acids on Age-related Macular Degeneration: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2021, 106, e3565–e3572. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; Souza, F.d.C.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef]
- Custers Emma, E.M.; Kiliaan Amanda, J. Dietary lipids from body to brain. Prog. Lipid Res. 2022, 85, 101144. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Borges, M.C.; Horta, B.L.; Bowden, J.; Smith, G.D. Inflammatory Biomarkers and Risk of Schizophrenia: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry 2017, 74, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.T.; Dimou, N.; Langenberg, C.; et al. Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomisation (STROBE-MR): Explanation and Elaboration. BMJ 2021, 375, n2233. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Xue, C.; Xu, M.; Li, X. Mendelian Randomization Analysis Reveals Causal Effects of Polyunsaturated Fatty Acids on Subtypes of Diabetic Retinopathy Risk. Nutrients 2023, 15, 4208. https://doi.org/10.3390/nu15194208
Ren S, Xue C, Xu M, Li X. Mendelian Randomization Analysis Reveals Causal Effects of Polyunsaturated Fatty Acids on Subtypes of Diabetic Retinopathy Risk. Nutrients. 2023; 15(19):4208. https://doi.org/10.3390/nu15194208
Chicago/Turabian StyleRen, Shaojie, Chen Xue, Manhong Xu, and Xiaorong Li. 2023. "Mendelian Randomization Analysis Reveals Causal Effects of Polyunsaturated Fatty Acids on Subtypes of Diabetic Retinopathy Risk" Nutrients 15, no. 19: 4208. https://doi.org/10.3390/nu15194208
APA StyleRen, S., Xue, C., Xu, M., & Li, X. (2023). Mendelian Randomization Analysis Reveals Causal Effects of Polyunsaturated Fatty Acids on Subtypes of Diabetic Retinopathy Risk. Nutrients, 15(19), 4208. https://doi.org/10.3390/nu15194208





