How Do Minerals, Vitamins, and Intestinal Microbiota Affect the Development and Progression of Heart Disease in Adult and Pediatric Patients?
Abstract
1. Introduction
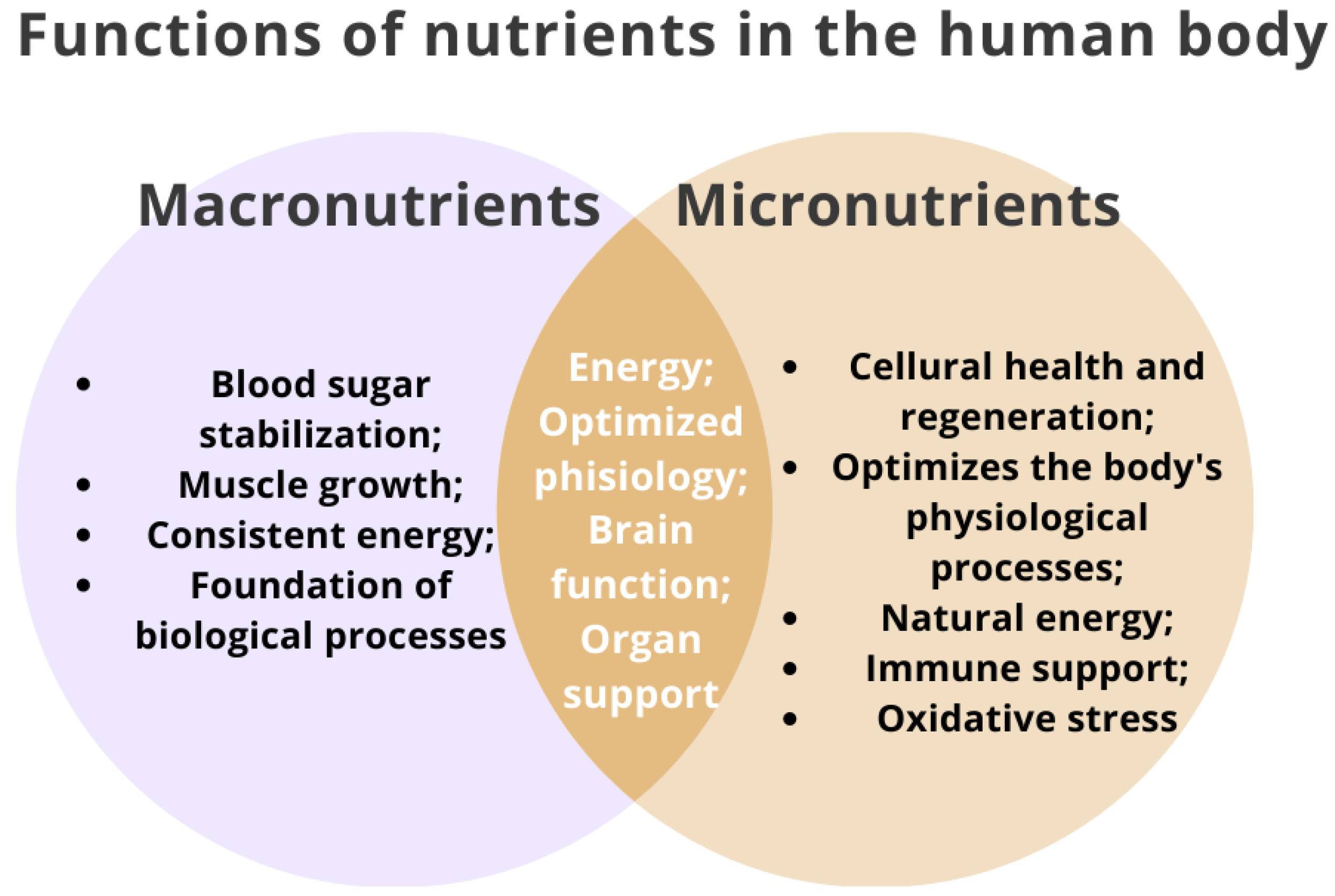
2. Importance of Micronutrients and Vitamins for Human Health
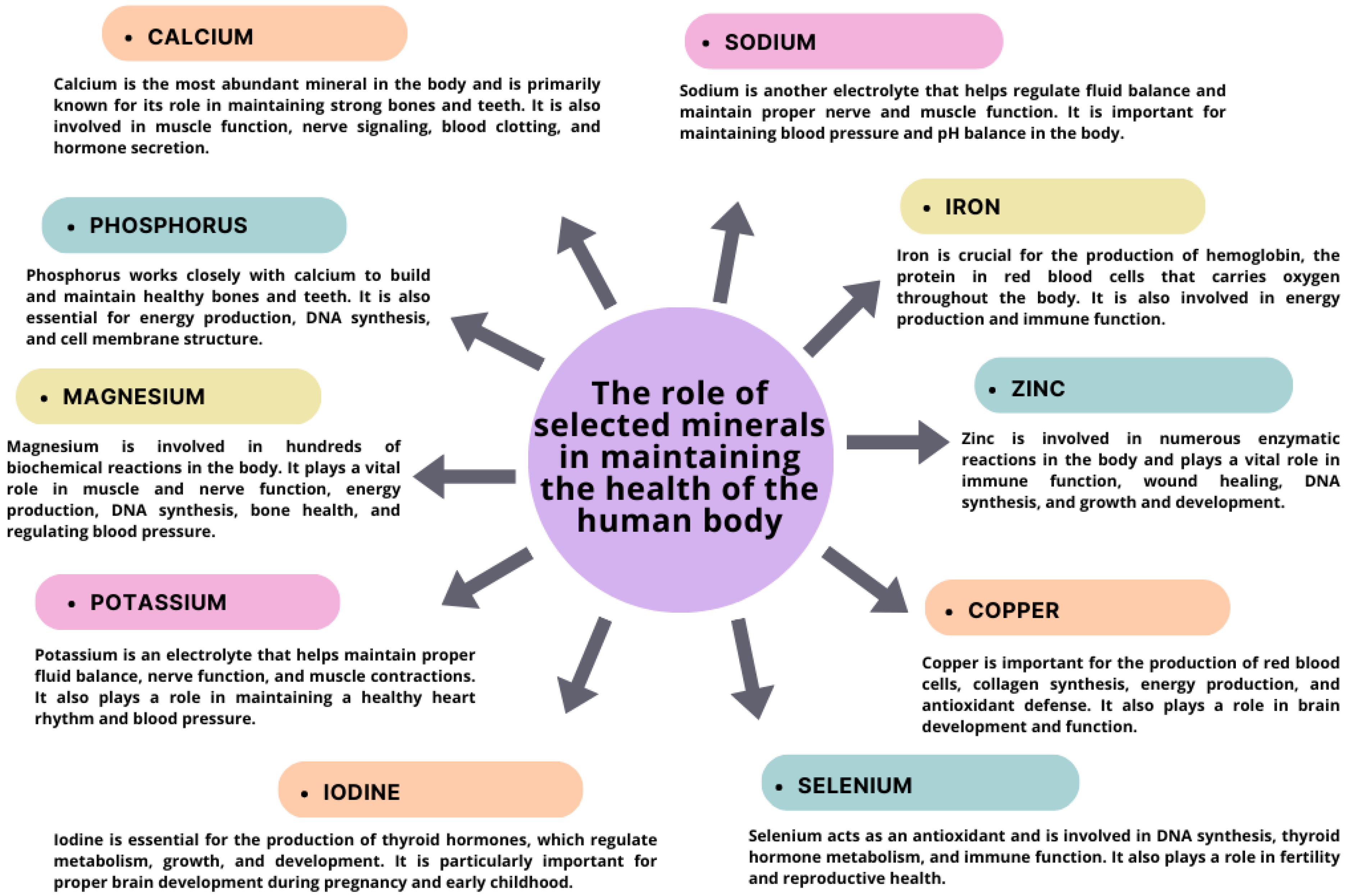
3. The Role of Macro- and Micronutrients in the Course of Heart Disease in Adults
3.1. Importance of Calcium (Ca)
3.2. The Importance of Potassium (K)
3.3. The Importance of Selenium (Se)
3.4. Importance of Copper (Cu)
3.5. Importance of Zinc (Zn)
3.6. The Importance of Iron
3.7. Importance of Magnesium
4. The Role of Vitamins in the Pathogenesis of Heart Disease in Adults
4.1. Fat-Soluble Vitamins
4.1.1. Vitamin A—Retinol
4.1.2. Vitamin D
4.1.3. Vitamin E
4.1.4. Vitamin K
4.2. Water-Soluble Vitamins
4.2.1. Vitamin B3—Niacin
4.2.2. Vitamin B6—Pyridoxine
4.2.3. Vitamin B12 and B9 (Folic Acid)
4.2.4. Vitamin C—Ascorbic Acid
5. The Importance of Macro- and Micronutrients and Vitamins in the Development and Progression of Heart Disease in Pediatric Patients
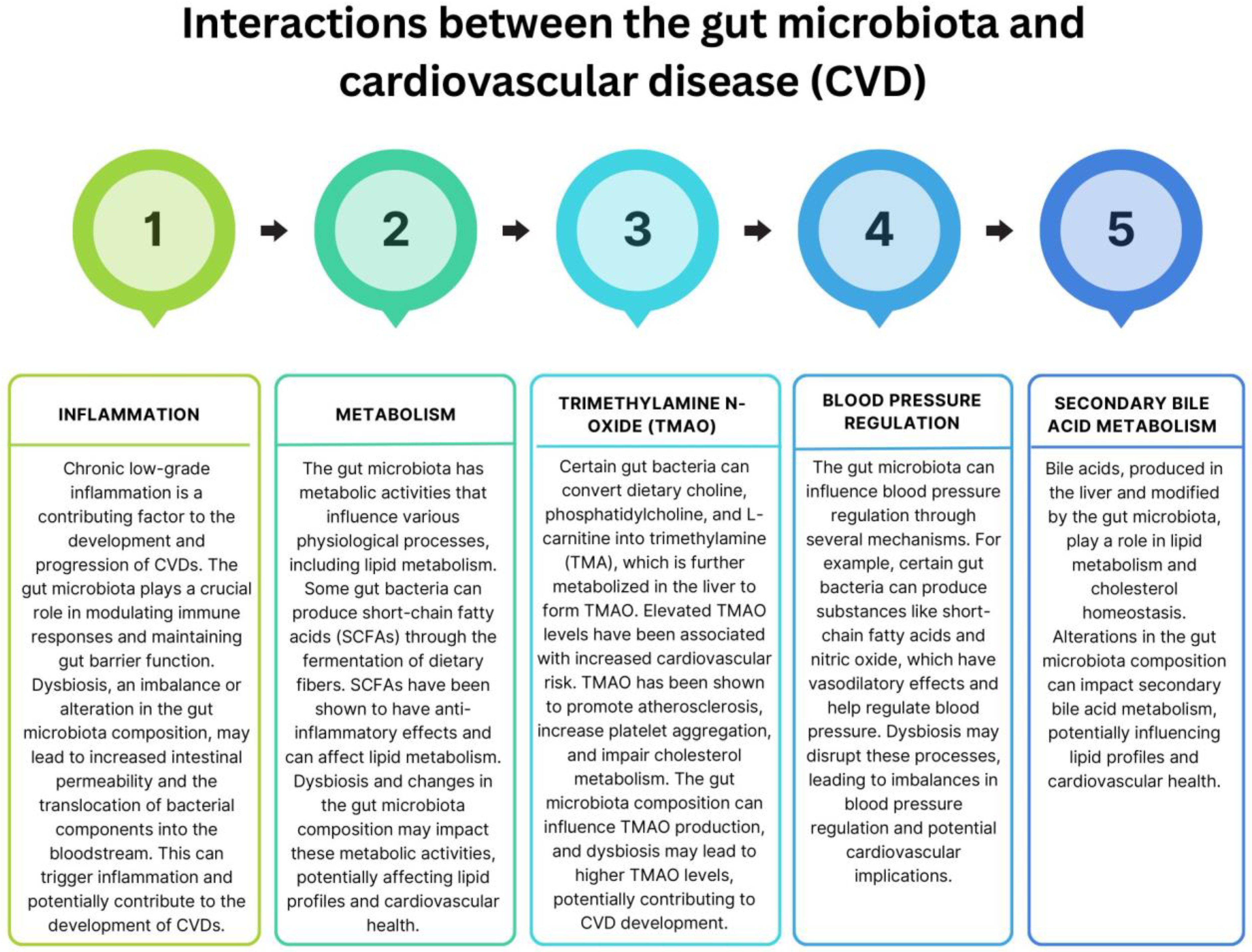
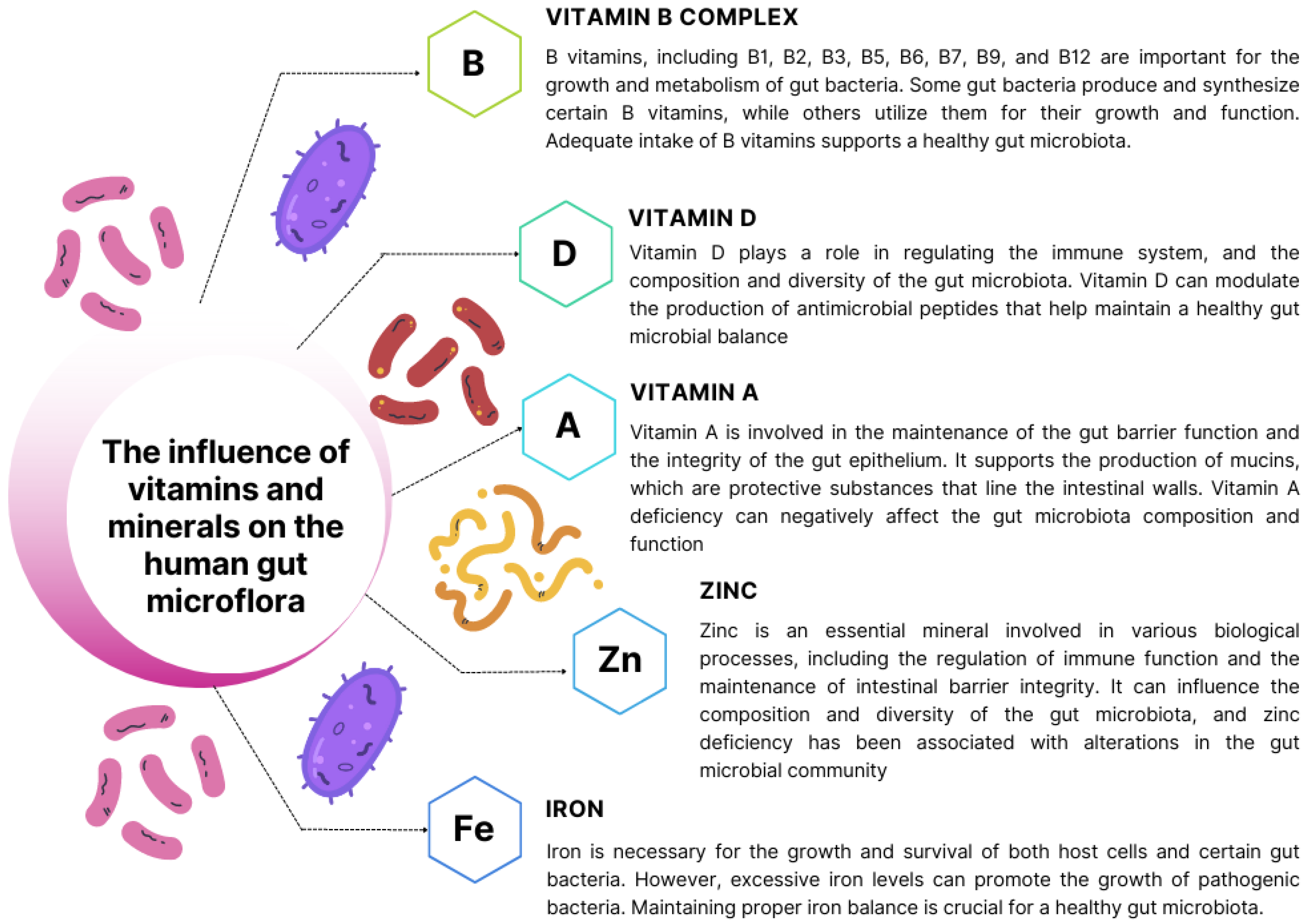
6. Exploring the Significance of Intestinal Microbiota in the Development and Progression of Heart Disease
7. Conclusions and Research Perspectives for the Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olvera Lopez, E.; Ballard, B.D.; Jan, A. Cardiovascular Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Leong, D.P.; Joseph, P.G.; McKee, M.; Anand, S.S.; Teo, K.K.; Schwalm, J.-D.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 2: Prevention and Treatment of Cardiovascular Disease. Circ. Res. 2017, 121, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.D.; Mathers, C.D.; Ezzati, M.; Jamison, D.T.; Murray, C.J.L. Global and Regional Burden of Disease and Risk Factors, 2001: Systematic Analysis of Population Health Data. Lancet 2006, 367, 1747–1757. [Google Scholar] [CrossRef]
- Hajar, R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views 2017, 18, 109–114. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Ayer, J.G.J. Childhood Risk Factors for Adult Cardiovascular Disease and Primary Prevention in Childhood. Heart 2006, 92, 1701–1706. [Google Scholar] [CrossRef]
- Candelino, M.; Tagi, V.M.; Chiarelli, F. Cardiovascular Risk in Children: A Burden for Future Generations. Ital. J. Pediatr. 2022, 48, 57. [Google Scholar] [CrossRef]
- Močnik, M.; Varda, N.M.; Močnik, M.; Varda, N.M. Cardiovascular Risk Factors in Children. In Risk Factors for Cardiovascular Disease; IntechOpen: London, UK, 2021; ISBN 978-1-83968-709-9. [Google Scholar]
- Kolber, M.R.; Scrimshaw, C. Family History of Cardiovascular Disease. Can. Fam. Physician 2014, 60, 1016. [Google Scholar]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Jeong, S.; Choi, S.; Kim, K.; Kim, S.M.; Lee, G.; Park, S.Y.; Kim, Y.; Son, J.S.; Yun, J.; Park, S.M. Effect of Change in Total Cholesterol Levels on Cardiovascular Disease Among Young Adults. J. Am. Heart Assoc. 2018, 7, e008819. [Google Scholar] [CrossRef]
- Gallucci, G.; Tartarone, A.; Lerose, R.; Lalinga, A.V.; Capobianco, A.M. Cardiovascular Risk of Smoking and Benefits of Smoking Cessation. J. Thorac. Dis. 2020, 12, 3866–3876. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.M.; Maddox, T.M. Diabetes and Cardiovascular Disease: Epidemiology, Biological Mechanisms, Treatment Recommendations and Future Research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-X.; Ma, X.-N.; Guan, C.-H.; Li, Y.-D.; Mauricio, D.; Fu, S.-B. Cardiovascular Disease in Type 2 Diabetes Mellitus: Progress toward Personalized Management. Cardiovasc. Diabetol. 2022, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Canada, J.M.; Billingsley, H.E.; Siddiqui, M.S.; Elagizi, A.; Lavie, C.J. Obesity Paradox in Cardiovascular Disease: Where Do We Stand? Vasc. Health Risk Manag. 2019, 15, 89–100. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Cercato, C.; Fonseca, F.A. Cardiovascular Risk and Obesity. Diabetol. Metab. Syndr. 2019, 11, 74. [Google Scholar] [CrossRef]
- Lippi, G.; Henry, B.M.; Sanchis-Gomar, F. Physical Inactivity and Cardiovascular Disease at the Time of Coronavirus Disease 2019 (COVID-19). Eur. J. Prev. Cardiol. 2020, 27, 906–908. [Google Scholar] [CrossRef]
- Anand, S.S.; Hawkes, C.; de Souza, R.J.; Mente, A.; Dehghan, M.; Nugent, R.; Zulyniak, M.A.; Weis, T.; Bernstein, A.M.; Krauss, R.; et al. Food Consumption and Its Impact on Cardiovascular Disease: Importance of Solutions Focused on the Globalized Food System. J. Am. Coll. Cardiol. 2015, 66, 1590–1614. [Google Scholar] [CrossRef]
- Steptoe, A.; Kivimäki, M. Stress and Cardiovascular Disease. Nat. Rev. Cardiol. 2012, 9, 360–370. [Google Scholar] [CrossRef]
- Levine, G.N. Psychological Stress and Heart Disease: Fact or Folklore? Am. J. Med. 2022, 135, 688–696. [Google Scholar] [CrossRef]
- Venkatesh, U.; Sharma, A.; Ananthan, V.A.; Subbiah, P.; Durga, R. CSIR Summer Research training team Micronutrient’s Deficiency in India: A Systematic Review and Meta-Analysis. J. Nutr. Sci. 2021, 10, e110. [Google Scholar] [CrossRef] [PubMed]
- Micronutrients. Available online: https://www.who.int/health-topics/micronutrients (accessed on 9 June 2023).
- Fact Sheets—Malnutrition. Available online: https://www.who.int/news-room/fact-sheets/detail/malnutrition (accessed on 9 June 2023).
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Dring, J.C.; Forma, A.; Chilimoniuk, Z.; Dobosz, M.; Teresiński, G.; Buszewicz, G.; Flieger, J.; Cywka, T.; Januszewski, J.; Baj, J. Essentiality of Trace Elements in Pregnancy, Fertility, and Gynecologic Cancers—A State-of-the-Art Review. Nutrients 2021, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Weyh, C.; Krüger, K.; Peeling, P.; Castell, L. The Role of Minerals in the Optimal Functioning of the Immune System. Nutrients 2022, 14, 644. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Diet and Health. Diet and Health: Implications for Reducing Chronic Disease Risk; National Academies Press: Washington, DC, USA, 1989.
- Tinkov, A.A.; Bogdański, P.; Skrypnik, D.; Skrypnik, K.; Skalny, A.V.; Aaseth, J.; Skalnaya, M.G.; Suliburska, J. Trace Element and Mineral Levels in Serum, Hair, and Urine of Obese Women in Relation to Body Composition, Blood Pressure, Lipid Profile, and Insulin Resistance. Biomolecules 2021, 11, 689. [Google Scholar] [CrossRef]
- Chen, Y.; Michalak, M.; Agellon, L.B. Importance of Nutrients and Nutrient Metabolism on Human Health. Yale J. Biol. Med. 2018, 91, 95–103. [Google Scholar]
- Tardy, A.-L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential Metals in Health and Disease. Chem.-Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Ryan-Harshman, M.; Aldoori, W. Health Benefits of Selected Minerals. Can. Fam. Physician 2005, 51, 673–675. [Google Scholar]
- Vural, Z.; Avery, A.; Kalogiros, D.I.; Coneyworth, L.J.; Welham, S.J.M. Trace Mineral Intake and Deficiencies in Older Adults Living in the Community and Institutions: A Systematic Review. Nutrients 2020, 12, 1072. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc in Human Health: Effect of Zinc on Immune Cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef]
- Shankar, A.H.; Prasad, A.S. Zinc and Immune Function: The Biological Basis of Altered Resistance to Infection. Am. J. Clin. Nutr. 1998, 68, 447S–463S. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Rink, L. The Immune System and the Impact of Zinc during Aging. Immun. Ageing 2009, 6, 9. [Google Scholar] [CrossRef]
- Ni, S.; Yuan, Y.; Kuang, Y.; Li, X. Iron Metabolism and Immune Regulation. Front. Immunol. 2022, 13, 816282. [Google Scholar] [CrossRef]
- Cherayil, B.J. Iron and Immunity: Immunological Consequences of Iron Deficiency and Overload. Arch. Immunol. Ther. Exp. 2010, 58, 407–415. [Google Scholar] [CrossRef]
- Nairz, M.; Weiss, G. Iron in Infection and Immunity. Mol. Asp. Med. 2020, 75, 100864. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The Influence of Selenium on Immune Responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of Inflammation in the Pathogenesis of Atherosclerosis and Therapeutic Interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef]
- Boban, M.; Bulj, N.; Kolačević Zeljković, M.; Radeljić, V.; Krcmar, T.; Trbusic, M.; Delić-Brkljačić, D.; Alebic, T.; Vcev, A. Nutritional Considerations of Cardiovascular Diseases and Treatments. Nutr. Metab. Insights 2019, 12, 1178638819833705. [Google Scholar] [CrossRef]
- Amare, H.; Hamza, L.; Asefa, H. Malnutrition and Associated Factors among Heart Failure Patients on Follow up at Jimma University Specialized Hospital, Ethiopia. BMC Cardiovasc. Disord. 2015, 15, 128. [Google Scholar] [CrossRef]
- Arikawa, R.; Kanda, D.; Ikeda, Y.; Tokushige, A.; Sonoda, T.; Anzaki, K.; Ohishi, M. Prognostic Impact of Malnutrition on Cardiovascular Events in Coronary Artery Disease Patients with Myocardial Damage. BMC Cardiovasc. Disord. 2021, 21, 479. [Google Scholar] [CrossRef]
- Childs, C.E.; Calder, P.C.; Miles, E.A. Diet and Immune Function. Nutrients 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef]
- Reddy, P.; Jialal, I. Biochemistry, Fat Soluble Vitamins. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lykstad, J.; Sharma, S. Biochemistry, Water Soluble Vitamins. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hunter’s Tropical Medicine and Emerging Infectio—9780323555128. Available online: https://www.us.elsevierhealth.com/hunters-tropical-medicine-and-emerging-infectious-diseases-9780323555128.html (accessed on 14 June 2023).
- Savastano, S.; Barrea, L.; Savanelli, M.C.; Nappi, F.; Di Somma, C.; Orio, F.; Colao, A. Low Vitamin D Status and Obesity: Role of Nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 215–225. [Google Scholar] [CrossRef]
- Charen, E.; Harbord, N. Toxicity of Herbs, Vitamins, and Supplements. Adv. Chronic Kidney Dis. 2020, 27, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Gummin, D.D.; Mowry, J.B.; Beuhler, M.C.; Spyker, D.A.; Bronstein, A.C.; Rivers, L.J.; Pham, N.P.T.; Weber, J. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin. Toxicol. 2021, 59, 1282–1501. [Google Scholar] [CrossRef]
- Kamangar, F.; Emadi, A. Vitamin and Mineral Supplements: Do We Really Need Them? Int. J. Prev. Med. 2012, 3, 221–226. [Google Scholar]
- O’Connor, E.A.; Evans, C.V.; Ivlev, I.; Rushkin, M.C.; Thomas, R.G.; Martin, A.; Lin, J.S. Vitamin and Mineral Supplements for the Primary Prevention of Cardiovascular Disease and Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 327, 2334. [Google Scholar] [CrossRef]
- Myung, S.-K.; Kim, H.-B.; Lee, Y.-J.; Choi, Y.-J.; Oh, S.-W. Calcium Supplements and Risk of Cardiovascular Disease: A Meta-Analysis of Clinical Trials. Nutrients 2021, 13, 368. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.R.; Radavelli-Bagatini, S.; Rejnmark, L.; Chen, J.S.; Simpson, J.M.; Lappe, J.M.; Mosekilde, L.; Prentice, R.L.; Prince, R.L. The Effects of Calcium Supplementation on Verified Coronary Heart Disease Hospitalization and Death in Postmenopausal Women: A Collaborative Meta-Analysis of Randomized Controlled Trials: Calcium Supplementation and Chd in postmenopausal women. J. Bone Miner Res. 2015, 30, 165–175. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Sodium Intake for Adults and Children; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2012; ISBN 978-92-4-150483-6. [Google Scholar]
- Chmielewski, J.; Carmody, J.B. Dietary Sodium, Dietary Potassium, and Systolic Blood Pressure in US Adolescents. J. Clin. Hypertens. 2017, 19, 904–909. [Google Scholar] [CrossRef]
- Bastola, M.M.; Locatis, C.; Maisiak, R.; Fontelo, P. Selenium, Copper, Zinc and Hypertension: An Analysis of the National Health and Nutrition Examination Survey (2011–2016). BMC Cardiovasc. Disord. 2020, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Chen, C.; Jin, Y.; Unverzagt, F.W.; Cheng, Y.; Hake, A.M.; Liang, C.; Ma, F.; Su, L.; Liu, J.; Bian, J.; et al. The Association between Selenium and Lipid Levels: A Longitudinal Study in Rural Elderly Chinese. Arch. Gerontol. Geriatr. 2015, 60, 147–152. [Google Scholar] [CrossRef]
- Rayman, M.P.; Stranges, S.; Griffin, B.A.; Pastor-Barriuso, R.; Guallar, E. Effect of Supplementation With High-Selenium Yeast on Plasma Lipids: A Randomized Trial. Ann. Intern. Med. 2011, 154, 656. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, Oxidative Stress, and Human Health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.-F.; Margaritis, I. Dietary Copper and Human Health: Current Evidence and Unresolved Issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Huang, H.; Zhuang, Z.; Chen, R.; Xie, Z.; Xu, C.; Mo, X. The Association between Serum Copper Concentrations and Cardiovascular Disease Risk Factors in Children and Adolescents in NHANES. Environ. Sci. Pollut. Res. 2018, 25, 16951–16958. [Google Scholar] [CrossRef]
- Eshak, E.S.; Iso, H.; Yamagishi, K.; Maruyama, K.; Umesawa, M.; Tamakoshi, A. Associations between Copper and Zinc Intakes from Diet and Mortality from Cardiovascular Disease in a Large Population-Based Prospective Cohort Study. J. Nutr. Biochem. 2018, 56, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Hu, P.; Zhang, D. Associations Between Copper and Zinc and Risk of Hypertension in US Adults. Biol. Trace Elem. Res. 2018, 186, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Topuzoglu, G.; Erbay, A.R.; Karul, A.B.; Yensel, N. Concentations of Copper, Zinc, and Magnesium in Sera from Patients with Idiopathic Dilated Cardiomyopathy. Biol. Trace Elem. Res. 2003, 95, 11–18. [Google Scholar] [CrossRef]
- Soinio, M.; Marniemi, J.; Laakso, M.; Pyörälä, K.; Lehto, S.; Rönnemaa, T. Serum Zinc Level and Coronary Heart Disease Events in Patients With Type 2 Diabetes. Diabetes Care 2007, 30, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Suliburska, J.; Bogdański, P.; Pupek-Musialik, D.; Krejpcio, Z. Dietary intake and serum and hair concentrations of minerals and their relationship with serum lipids and glucose levels in hypertensive and obese patients with insulin resistance. Biol. Trace Elem. Res. 2010, 139, 137–150. [Google Scholar] [CrossRef]
- Maret, W. The Redox Biology Of Redox-inert Zinc Ions. Free. Radic. Biol. Med. 2019, 134, 311–326. [Google Scholar] [CrossRef]
- Joo, Y.S.; Kim, H.W.; Lee, S.; Nam, K.H.; Yun, H.-R.; Jhee, J.H.; Han, S.H.; Yoo, T.-H.; Kang, S.-W.; Park, J.T. Dietary Zinc Intake and Incident Chronic Kidney Disease. Clin. Nutr. 2021, 3, 1039–1045. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S.; Hosseini-Esfahani, F.; Azizi, F. Gender Differences In the Relationship Between Serum Zinc Concentration And Metabolic Syndrome. Ann. Hum. Biol. 2014, 5, 436–442. [Google Scholar] [CrossRef]
- Rostan, E.F.; DeBuys, H.V.; Madey, D.L.; Pinnell, S.R. Evidence Supporting Zinc as an Important Antioxidant for Skin. Int. J. Dermatol. 2002, 41, 606–611. [Google Scholar] [CrossRef]
- Richter, P.; Faroon, O.; Pappas, R.S. Cadmium and Cadmium/Zinc Ratios and Tobacco-Related Morbidities. Int. J. Environ. Res. Public Health 2017, 14, 1154. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc Deficiency and Cellular Oxidative Stress: Prognostic Implications In Cardiovascular Diseases. Acta Pharmacol. Sin. 2018, 7, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.F.; Wei, Y.S. Associations between Serum Bilirubin Levels and Essential Trace Elements Status in an Adult Population. Oncotarget 2017, 8, 81315–81320. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.A.; Evans, C.V.; Ivlev, I.; Rushkin, M.C.; Thomas, R.G.; Martin, A.; Lin, J.S. Vitamin, Mineral, and Multivitamin Supplementation for the Primary Prevention of Cardiovascular Disease and Cancer: A Systematic Evidence Review for the U.S. Preventive Services Task Force; U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2021.
- Avni, T.; Leibovici, L.; Gafter-Gvili, A. Iron Supplementation for the Treatment of Chronic Heart Failure and Iron Deficiency: Systematic Review and Meta-Analysis. Eur. J. Heart Fail. 2012, 14, 423–429. [Google Scholar] [CrossRef]
- Tomasoni, D.; Adamo, M.; Lombardi, C.M.; Metra, M. Highlights in Heart Failure. ESC Heart Fail. 2019, 6, 1105–1127. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, R.D.; Barry, A.R.; Hawkins, N.M.; Ellis, U.M. Pharmacotherapy for Heart Failure with Reduced Ejection Fraction and health-related Quality of Life: A Systematic Review and meta-analysis. Eur. J Heart Fail. 2021, 23, 578–589. [Google Scholar] [CrossRef]
- Khan, M.S.; Khan, F.; Fonarow, G.C.; Sreenivasan, J.; Greene, S.J.; Khan, S.U.; Usman, M.S.; Vaduganathan, M.; Fudim, M.; Anker, S.D.; et al. Dietary Interventions and Nutritional Supplements for Heart Failure: A Systematic Appraisal and Evidence Map. Eur. J Heart Fail. 2021, 23, 1468–1476. [Google Scholar] [CrossRef]
- McDonagh, T.; Damy, T.; Doehner, W.; Lam, C.S.P.; Sindone, A.; Van Der Meer, P.; Cohen-Solal, A.; Kindermann, I.; Manito, N.; Pfister, O.; et al. Screening, Diagnosis and Treatment of Iron Deficiency in Chronic Heart Failure: Putting the 2016 European Society of Cardiology Heart Failure Guidelines into Clinical Practice. Eur. J. Heart Fail. 2018, 20, 1664–1672. [Google Scholar] [CrossRef]
- King, D.E.; Mainous, A.G.; Geesey, M.E.; Woolson, R.F. Dietary Magnesium and C-Reactive Protein Levels. J. Am. Coll. Nutr. 2005, 24, 166–171. [Google Scholar] [CrossRef]
- Saris, N.-E.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, M.; Yang, L.; Xu, H.; Song, W.; Qian, Y.; Zhao, M. Quantitative Association Between Serum/Dietary Magnesium and Cardiovascular Disease/Coronary Heart Disease Risk: A Dose–Response Meta-Analysis of Prospective Cohort Studies. J. Cardiovasc. Pharmacol. 2019, 74, 516–527. [Google Scholar] [CrossRef]
- Shechter, M. Magnesium and Cardiovascular System. Magnes. Res. 2010, 23, 60–72. [Google Scholar] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Dietary Magnesium Intake and Risk of Stroke: A Meta-Analysis of Prospective Studies. Am. J. Clin. Nutr. 2012, 95, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, L.C.; Imamura, F.; Wu, J.H.; De Oliveira Otto, M.C.; Chiuve, S.E.; Mozaffarian, D. Circulating and Dietary Magnesium and Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Prospective Studies. Am. J. Clin. Nutr. 2013, 98, 160–173. [Google Scholar] [CrossRef]
- Porter, K.; Hoey, L.; Hughes, C.; Ward, M.; McNulty, H. Causes, Consequences and Public Health Implications of Low B-Vitamin Status in Ageing. Nutrients 2016, 8, 725. [Google Scholar] [CrossRef] [PubMed]
- Hemminger, A.; Wills, B.K. Vitamin B6 Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Achan, V.; Tran, C.T.L.; Arrigoni, F.; Whitley, G.S.J.; Leiper, J.M.; Vallance, P. All- Trans -Retinoic Acid Increases Nitric Oxide Synthesis by Endothelial Cells: A Role for the Induction of Dimethylarginine Dimethylaminohydrolase. Circ. Res. 2002, 90, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Reifen, R. Vitamin A as an Anti-Inflammatory Agent. Proc. Nutr. Soc. 2002, 61, 397–400. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Zhou, C.; Zhang, Z.; He, P.; Li, Q.; Liu, C.; Qin, X. Inverse Association between Dietary Vitamin A Intake and New-Onset Hypertension. Clin. Nutr. 2021, 40, 2868–2875. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, H.; Song, Y.; Lin, T.; Zhou, Z.; Guo, H.; Liu, L.; Wang, B.; Liu, C.; Li, J.; et al. Plasma Retinol and the Risk of First Stroke in Hypertensive Adults: A Nested Case-Control Study. Am. J. Clin. Nutr. 2019, 109, 449–456. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D Deficiency: A Worldwide Problem with Health Consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is Vitamin D Deficiency a Major Global Public Health Problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef]
- Chen, S.; Swier, V.J.; Boosani, C.S.; Radwan, M.M.; Agrawal, D.K. Vitamin D Deficiency Accelerates Coronary Artery Disease Progression in Swine. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1651–1659. [Google Scholar] [CrossRef]
- Holick, M.F. High Prevalence of Vitamin D Inadequacy and Implications for Health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Al Mheid, I.; Quyyumi, A.A. Vitamin D and Cardiovascular Disease. J. Am. Coll. Cardiol. 2017, 70, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Adamopoulos, C.; Basdra, E.K.; Papavassiliou, A.G. Role of Vitamin D in Atherosclerosis. Circulation 2013, 128, 2517–2531. [Google Scholar] [CrossRef] [PubMed]
- Trehan, N.; Afonso, L.; Levine, D.L.; Levy, P.D. Vitamin D Deficiency, Supplementation, and Cardiovascular Health. Crit. Pathw. Cardiol. A J. Evid.-Based Med. 2017, 16, 109–118. [Google Scholar] [CrossRef]
- Mancuso, P.; Rahman, A.; Hershey, S.D.; Dandu, L.; Nibbelink, K.A.; Simpson, R.U. 1,25-Dihydroxyvitamin-D3 Treatment Reduces Cardiac Hypertrophy and Left Ventricular Diameter in Spontaneously Hypertensive Heart Failure-Prone (Cp/+) Rats Independent of Changes in Serum Leptin. J. Cardiovasc. Pharmacol. 2008, 51, 559–564. [Google Scholar] [CrossRef]
- Seibert, E.; Lehmann, U.; Riedel, A.; Ulrich, C.; Hirche, F.; Brandsch, C.; Dierkes, J.; Girndt, M.; Stangl, G.I. Vitamin D3 Supplementation Does Not Modify Cardiovascular Risk Profile of Adults with Inadequate Vitamin D Status. Eur. J. Nutr. 2017, 56, 621–634. [Google Scholar] [CrossRef]
- Gholami, F.; Moradi, G.; Zareei, B.; Rasouli, M.A.; Nikkhoo, B.; Roshani, D.; Ghaderi, E. The Association between Circulating 25-Hydroxyvitamin D and Cardiovascular Diseases: A Meta-Analysis of Prospective Cohort Studies. BMC Cardiovasc. Disord. 2019, 19, 248. [Google Scholar] [CrossRef]
- Scragg, R.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Sluyter, J.; Murphy, J.; Khaw, K.-T.; Camargo, C.A. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 608. [Google Scholar] [CrossRef]
- Özer, N.K.; Azzi, A. Effect of Vitamin E on the Development of Atherosclerosis. Toxicology 2000, 148, 179–185. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Zingg, J.-M.; Azzi, A. Vitamin E Reduces the Uptake of Oxidized LDL by Inhibiting CD36 Scavenger Receptor Expression in Cultured Aortic Smooth Muscle Cells. Circulation 2000, 102, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, A.; Taddei, M.; Tamburini, I.; Bergamini, E.; Azzi, A.; Zingg, J.-M. Antagonistic Effects of Oxidized Low Density Lipoprotein and α-Tocopherol on CD36 Scavenger Receptor Expression in Monocytes. J. Biol. Chem. 2006, 281, 6489–6497. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Sami, S.A.; Salser, W.A.; Khan, F.A. Dose-Dependent Suppression of Serum Cholesterol by Tocotrienol-Rich Fraction (TRF25) of Rice Bran in Hypercholesterolemic Humans. Atherosclerosis 2002, 161, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Weber, S.U.; Rimbach, G. Molecular Aspects of α-Tocotrienol Antioxidant Action and Cell Signalling. J. Nutr. 2001, 131, 369S–373S. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond Tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef]
- Azzi, A.; Ricciarelli, R.; Zingg, J.-M. Non-Antioxidant Molecular Functions of α-Tocopherol (Vitamin E). FEBS Lett. 2002, 519, 8–10. [Google Scholar] [CrossRef]
- Ramanathan, N.; Tan, E.; Loh, L.J.; Soh, B.S.; Yap, W.N. Tocotrienol Is a Cardioprotective Agent against Ageing-Associated Cardiovascular Disease and Its Associated Morbidities. Nutr. Metab. 2018, 15, 6. [Google Scholar] [CrossRef]
- Eshak, E.S.; Iso, H.; Yamagishi, K.; Cui, R.; Tamakoshi, A. Dietary Intakes of Fat Soluble Vitamins as Predictors of Mortality from Heart Failure in a Large Prospective Cohort Study. Nutrition 2018, 47, 50–55. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.J.; Robitaille, L.; Eintracht, S.; MacNamara, E.; Hoffer, L.J. Effects of Vitamin C and Vitamin D Administration on Mood and Distress in Acutely Hospitalized Patients. Am. J. Clin. Nutr. 2013, 98, 705–711. [Google Scholar] [CrossRef]
- Sozen, E.; Demirel, T.; Ozer, N.K. Vitamin E: Regulatory Role in the Cardiovascular System: Vitamin E: Regulatory Role in the Cardiovascular System. IUBMB Life 2019, 71, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Perri, L.; Di Castelnuovo, A.; Iacoviello, L.; De Gaetano, G.; Violi, F. Supplementation with Vitamin E Alone Is Associated with Reduced Myocardial Infarction: A Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 354–363. [Google Scholar] [CrossRef]
- Henríquez-Sánchez, P.; Sánchez-Villegas, A.; Ruano-Rodríguez, C.; Gea, A.; Lamuela-Raventós, R.M.; Estruch, R.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Schröder, H.; et al. Dietary Total Antioxidant Capacity and Mortality in the PREDIMED Study. Eur. J. Nutr. 2016, 55, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Guo, F.; Zhang, Y.; Yuan, Y.; Chen, D.; Bai, G. Vitamin A, D, and E Levels and Reference Ranges for Pregnant Women: A Cross-Sectional Study 2017–2019. Front. Nutr. 2021, 8, 628902. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Booth, S.L.; Massaro, J.M.; Jacques, P.F.; D’Agostino, R.B.; Dawson-Hughes, B.; Ordovas, J.M.; O’Donnell, C.J.; Kathiresan, S.; Keaney, J.F.; et al. Vitamin K and Vitamin D Status: Associations with Inflammatory Markers in the Framingham Offspring Study. Am. J. Epidemiol. 2007, 167, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.; Cushman, M.; Booth, S.; Burke, G.; Chen, H.; Kritchevsky, S. Associations between Vitamin K Status and Haemostatic and Inflammatory Biomarkers in Community-Dwelling Adults: The Multi-Ethnic Study of Atherosclerosis. Thromb. Haemost. 2014, 112, 438–444. [Google Scholar] [CrossRef]
- Berkner, K.L.; Runge, K.W. The Physiology of Vitamin K Nutriture and Vitamin K-dependent Protein Function in Atherosclerosis. J. Thromb. Haemost. 2004, 2, 2118–2132. [Google Scholar] [CrossRef]
- Shea, M.K.; Booth, S.L.; Weiner, D.E.; Brinkley, T.E.; Kanaya, A.M.; Murphy, R.A.; Simonsick, E.M.; Wassel, C.L.; Vermeer, C.; Kritchevsky, S.B. Circulating Vitamin K Is Inversely Associated with Incident Cardiovascular Disease Risk among Those Treated for Hypertension in the Health, Aging, and Body Composition Study (Health ABC). J. Nutr. 2017, 147, 888–895. [Google Scholar] [CrossRef]
- Wei, F.-F.; Trenson, S.; Verhamme, P.; Vermeer, C.; Staessen, J.A. Vitamin K–Dependent Matrix Gla Protein as Multifaceted Protector of Vascular and Tissue Integrity. Hypertension 2019, 73, 1160–1169. [Google Scholar] [CrossRef]
- Al-Aly, Z. Medial Vascular Calcification in Diabetes Mellitus and Chronic Kidney Disease: The Role of Inflammation. Cardiovasc. Haematol. Disord.-Drug Targets 2007, 7, 1–6. [Google Scholar] [CrossRef]
- Ramirez-Cabral, N.Y.Z.; Kumar, L.; Shabani, F. Global Alterations in Areas of Suitability for Maize Production from Climate Change and Using a Mechanistic Species Distribution Model (CLIMEX). Sci. Rep. 2017, 7, 5910. [Google Scholar] [CrossRef]
- Carlson, L.A. Nicotinic Acid: The Broad-Spectrum Lipid Drug. A 50th Anniversary Review. J Intern. Med. 2005, 258, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.; Foord, S.M.; Fraser, N.J.; Barnes, A.A.; Elshourbagy, N.; Eilert, M.; Ignar, D.M.; Murdock, P.R.; Steplewski, K.; Green, A.; et al. Molecular Identification of High and Low Affinity Receptors for Nicotinic Acid. J. Biol. Chem. 2003, 278, 9869–9874. [Google Scholar] [CrossRef]
- Soga, T.; Kamohara, M.; Takasaki, J.; Matsumoto, S.; Saito, T.; Ohishi, T.; Hiyama, H.; Matsuo, A.; Matsushime, H.; Furuichi, K. Molecular Identification of Nicotinic Acid Receptor. Biochem. Biophys. Res. Commun. 2003, 303, 364–369. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Zhao, S.-P. Niacin Promotes Cholesterol Efflux through Stimulation of the PPARγ-LXRα-ABCA1 Pathway in 3T3-L1 Adipocytes. Pharmacology 2009, 84, 282–287. [Google Scholar] [CrossRef]
- Knowles, H.J.; Poole, R.T.; Workman, P.; Harris, A.L. Niacin Induces PPARγ Expression and Transcriptional Activation in Macrophages via HM74 and HM74a-Mediated Induction of Prostaglandin Synthesis Pathways. Biochem. Pharmacol. 2006, 71, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Oram, J.F.; Lawn, R.M.; Garvin, M.R.; Wade, D.P. ABCA1 Is the CAMP-Inducible Apolipoprotein Receptor That Mediates Cholesterol Secretion from Macrophages. J. Biol. Chem. 2000, 275, 34508–34511. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Wang, N.; Tall, A.R. Role of HDL, ABCA1, and ABCG1 Transporters in Cholesterol Efflux and Immune Responses. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 139–143. [Google Scholar] [CrossRef]
- Takahashi, Y.; Miyata, M.; Zheng, P.; Imazato, T.; Horwitz, A.; Smith, J.D. Identification of CAMP Analogue Inducible Genes in RAW264 Macrophages. Biochim. Biophys. Acta (BBA)—Gene Struct. Expr. 2000, 1492, 385–394. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, J.; Li, J.; Dong, S.; Wu, Z. Effect of Niacin on LXRα and PPARγ Expression and HDL-Induced Cholesterol Efflux in Adipocytes of Hypercholesterolemic Rabbits. Int. J. Cardiol. 2008, 124, 172–178. [Google Scholar] [CrossRef]
- Huang, S.-C.; Wei, J.C.-C.; Wu, D.J.; Huang, Y.-C. Vitamin B6 Supplementation Improves Pro-Inflammatory Responses in Patients with Rheumatoid Arthritis. Eur. J. Clin. Nutr. 2010, 64, 1007–1013. [Google Scholar] [CrossRef]
- Zhang, P.; Tsuchiya, K.; Kinoshita, T.; Kushiyama, H.; Suidasari, S.; Hatakeyama, M.; Imura, H.; Kato, N.; Suda, T. Vitamin B6 Prevents IL-1β Protein Production by Inhibiting NLRP3 Inflammasome Activation. J. Biol. Chem. 2016, 291, 24517–24527. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Briarava, M.; Pilati, P. Vitamin B6 and Cancer Risk: A Field Synopsis and Meta-Analysis. JNCI J. Natl. Cancer Inst. 2017, 109, djw230. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Park, K. Dietary Vitamin B6 Intake Associated with a Decreased Risk of Cardiovascular Disease: A Prospective Cohort Study. Nutrients 2019, 11, 1484. [Google Scholar] [CrossRef] [PubMed]
- Page, J.H.; Ma, J.; Chiuve, S.E.; Stampfer, M.J.; Selhub, J.; Manson, J.E.; Rimm, E.B. Plasma Vitamin B 6 and Risk of Myocardial Infarction in Women. Circulation 2009, 120, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Kumrungsee, T.; Zhang, P.; Yanaka, N.; Suda, T.; Kato, N. Emerging Cardioprotective Mechanisms of Vitamin B6: A Narrative Review. Eur. J. Nutr. 2022, 61, 605–613. [Google Scholar] [CrossRef]
- Holmes, M.V.; Newcombe, P.; Hubacek, J.A.; Sofat, R.; Ricketts, S.L.; Cooper, J.; Breteler, M.M.; Bautista, L.E.; Sharma, P.; Whittaker, J.C.; et al. Effect Modification by Population Dietary Folate on the Association between MTHFR Genotype, Homocysteine, and Stroke Risk: A Meta-Analysis of Genetic Studies and Randomised Trials. Lancet 2011, 378, 584–594. [Google Scholar] [CrossRef]
- Klerk, M.; Verhoef, P.; Clarke, R.; Blom, H.J.; Kok, F.J.; Schouten, E.G.; The Mthfr Studies Collaboration Group. MTHFR 677C→T Polymorphism and Risk of Coronary Heart Disease: A Meta-Analysis. JAMA 2002, 288, 2023. [Google Scholar] [CrossRef]
- Wald, D.S. Homocysteine and Cardiovascular Disease: Evidence on Causality from a Meta-Analysis. BMJ 2002, 325, 1202–1206. [Google Scholar] [CrossRef]
- Homocysteine Studies Collaboration. Homocysteine and Risk of Ischemic Heart Disease and Stroke: A Meta-Analysis. JAMA 2002, 288, 2015. [Google Scholar] [CrossRef]
- Collaboration, H.L.T. Lowering Blood Homocysteine with Folic Acid Based Supplements: Meta-Analysis of Randomised Trials. Homocysteine Lowering Trialists’ Collaboration. BMJ 1998, 316, 894–898. [Google Scholar] [CrossRef]
- Lehotský, J.; Tothová, B.; Kovalská, M.; Dobrota, D.; Beňová, A.; Kalenská, D.; Kaplán, P. Role of Homocysteine in the Ischemic Stroke and Development of Ischemic Tolerance. Front. Neurosci. 2016, 10, 538. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Zadeh, A.A.; Shah, P.K. Homocysteine Hypothesis for Atherothrombotic Cardiovascular Disease. J. Am. Coll. Cardiol. 2006, 48, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Pushpakumar, S.; Kundu, S.; Sen, U. Endothelial Dysfunction: The Link Between Homocysteine and Hydrogen Sulfide. Curr. Med. Chem. 2014, 21, 3662–3672. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, L.L.; Fu, R.; Rogers, K.; Freeman, M.; Helfand, M. Homocysteine Level and Coronary Heart Disease Incidence: A Systematic Review and Meta-Analysis. Mayo Clin. Proc. 2008, 83, 1203–1212. [Google Scholar] [CrossRef]
- Miao, L.; Deng, G.-X.; Yin, R.-X.; Nie, R.-J.; Yang, S.; Wang, Y.; Li, H. No Causal Effects of Plasma Homocysteine Levels on the Risk of Coronary Heart Disease or Acute Myocardial Infarction: A Mendelian Randomization Study. Eur. J. Prev. Cardiol. 2021, 28, 227–234. [Google Scholar] [CrossRef]
- Zhao, J.V.; Schooling, C.M. Homocysteine-Reducing B Vitamins and Ischemic Heart Disease: A Separate-Sample Mendelian Randomization Analysis. Eur. J. Clin. Nutr. 2017, 71, 267–273. [Google Scholar] [CrossRef]
- Verhaar, M.C.; Stroes, E.; Rabelink, T.J. Folates and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 6–13. [Google Scholar] [CrossRef]
- Ashor, A.W.; Siervo, M.; Lara, J.; Oggioni, C.; Mathers, J.C. Antioxidant Vitamin Supplementation Reduces Arterial Stiffness in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Nutr. 2014, 144, 1594–1602. [Google Scholar] [CrossRef]
- Myint, P.K.; Luben, R.N.; Wareham, N.J.; Khaw, K.-T. Association Between Plasma Vitamin C Concentrations and Blood Pressure in the European Prospective Investigation Into Cancer-Norfolk Population-Based Study. Hypertension 2011, 58, 372–379. [Google Scholar] [CrossRef]
- Pfister, R.; Sharp, S.J.; Luben, R.; Wareham, N.J.; Khaw, K.-T. Plasma Vitamin C Predicts Incident Heart Failure in Men and Women in European Prospective Investigation into Cancer and Nutrition–Norfolk Prospective Study. Am. Heart J. 2011, 162, 246–253. [Google Scholar] [CrossRef]
- Baker, T.A.; Milstien, S.; Katusic, Z.S. Effect of Vitamin C on the Availability of Tetrahydrobiopterin in Human Endothelial Cells. J. Cardiovasc. Pharmacol. 2001, 37, 333–338. [Google Scholar] [CrossRef]
- Ye, Z.; Song, H. Antioxidant Vitamins Intake and the Risk of Coronary Heart Disease: Meta-Analysis of Cohort Studies. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Iso, H.; Date, C.; Kikuchi, S.; Watanabe, Y.; Wada, Y.; Inaba, Y.; Tamakoshi, A.; The JACC Study Group. Dietary Intakes of Antioxidant Vitamins and Mortality From Cardiovascular Disease: The Japan Collaborative Cohort Study (JACC) Study. Stroke 2011, 42, 1665–1672. [Google Scholar] [CrossRef]
- Ye, Y.; Li, J.; Yuan, Z. Effect of Antioxidant Vitamin Supplementation on Cardiovascular Outcomes: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2013, 8, e56803. [Google Scholar] [CrossRef]
- Knekt, P.; Ritz, J.; Pereira, M.A.; O’Reilly, E.J.; Augustsson, K.; Fraser, G.E.; Goldbourt, U.; Heitmann, B.L.; Hallmans, G.; Liu, S.; et al. Antioxidant Vitamins and Coronary Heart Disease Risk: A Pooled Analysis of 9 Cohorts. Am. J. Clin. Nutr. 2004, 80, 1508–1520. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Guallar, E.; Appel, L.J.; Miller, E.R. Effects of Vitamin C Supplementation on Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2012, 95, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Dai, P.; Wang, H. Effects of Vitamin C Supplementation on Essential Hypertension: A Systematic Review and Meta-Analysis. Medicine 2020, 99, e19274. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Rashidy-Pour, A.; Parohan, M.; Zargar, M.S.; Shab-Bidar, S. Dietary and Circulating Vitamin C, Vitamin E, β-Carotene and Risk of Total Cardiovascular Mortality: A Systematic Review and Dose–Response Meta-Analysis of Prospective Observational Studies. Public Health Nutr. 2019, 22, 1872–1887. [Google Scholar] [CrossRef]
- Valls, N.; Gormaz, J.G.; Aguayo, R.; González, J.; Brito, R.; Hasson, D.; Libuy, M.; Ramos, C.; Carrasco, R.; Prieto, J.C.; et al. Amelioration of Persistent Left Ventricular Function Impairment through Increased Plasma Ascorbate Levels Following Myocardial Infarction. Redox Rep. 2016, 21, 75–83. [Google Scholar] [CrossRef]
- Barak, O.F.; Caljkusic, K.; Hoiland, R.L.; Ainslie, P.N.; Thom, S.R.; Yang, M.; Jovanov, P.; Dujic, Z. Differential Influence of Vitamin C on the Peripheral and Cerebral Circulation after Diving and Exposure to Hyperoxia. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 315, R759–R767. [Google Scholar] [CrossRef]
- Johnson, B.D.; Mather, K.J.; Newcomer, S.C.; Mickleborough, T.D.; Wallace, J.P. Vitamin C Prevents the Acute Decline of Flow-Mediated Dilation after Altered Shear Rate Patterns. Appl. Physiol. Nutr. Metab. 2013, 38, 268–274. [Google Scholar] [CrossRef]
- Wallace, J.P. Antioxidant Vitamin C Prevents Decline in Endothelial Function during Sitting. Med. Sci. Monit. 2015, 21, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, S.; Prior, M.; Rigoni, A.; Zecchetto, S.; Rulfo, F.; Arosio, E. Ascorbic Acid Prevents Vascular Dysfunction Induced by Oral Glucose Load in Healthy Subjects. Eur. J. Intern. Med. 2012, 23, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Batista, G.M.S.; Rocha, H.N.M.; Storch, A.S.; Garcia, V.P.; Teixeira, G.F.; Mentzinger, J.; Gomes, E.A.C.; Velasco, L.L.; Nóbrega, A.C.L.; Rocha, N.G. Ascorbic Acid Inhibits Vascular Remodeling Induced by Mental Stress in Overweight/Obese Men. Life Sci. 2020, 250, 117554. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.H.; Rhea, E.M.; Qu, Z.-C.; Hecker, M.R.; May, J.M. Intracellular Ascorbate Tightens the Endothelial Permeability Barrier through Epac1 and the Tubulin Cytoskeleton. Am. J. Physiol.-Cell Physiol. 2016, 311, C652–C662. [Google Scholar] [CrossRef]
- Ulker, E.; Parker, W.H.; Raj, A.; Qu, Z.; May, J.M. Ascorbic Acid Prevents VEGF-Induced Increases in Endothelial Barrier Permeability. Mol. Cell. Biochem. 2016, 412, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sarmiento, J.; Salazar-Peláez, L.M.; Carcillo, J.A. The Endothelial Glycocalyx: A Fundamental Determinant of Vascular Permeability in Sepsis. Pediatr. Crit. Care Med. 2020, 21, e291–e300. [Google Scholar] [CrossRef]
- Li, Z.; Yin, M.; Zhang, H.; Ni, W.; Pierce, R.W.; Zhou, H.J.; Min, W. BMX Represses Thrombin-PAR1–Mediated Endothelial Permeability and Vascular Leakage During Early Sepsis. Circ. Res. 2020, 126, 471–485. [Google Scholar] [CrossRef]
- Suhail, S.; Zajac, J.; Fossum, C.; Lowater, H.; McCracken, C.; Severson, N.; Laatsch, B.; Narkiewicz-Jodko, A.; Johnson, B.; Liebau, J.; et al. Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review. Protein J. 2020, 39, 644–656. [Google Scholar] [CrossRef]
- Schönrich, G.; Raftery, M.J.; Samstag, Y. Devilishly Radical NETwork in COVID-19: Oxidative Stress, Neutrophil Extracellular Traps (NETs), and T Cell Suppression. Adv. Biol. Regul. 2020, 77, 100741. [Google Scholar] [CrossRef]
- Demir, F.; Kayali, S.; Gokcebay, D.G. Does Iron Deficiency Affect the Heart in Children? J. Pediatr. Hematol. Oncol. 2022, 44, 84–88. [Google Scholar] [CrossRef]
- Savarese, G.; von Haehling, S.; Butler, J.; Cleland, J.G.F.; Ponikowski, P.; Anker, S.D. Iron Deficiency and Cardiovascular Disease. Eur. Heart J. 2023, 44, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Cropp, G.J.A. Cardiovascular Function in Children with Severe Anemia. Circulation 1969, 39, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.C.; Schlueter, E.L.; Arndt, F.; Dodge-Khatami, A.; Weil, J.; Mir, T.S. Prevalence of Anemia in Children with Congestive Heart Failure Due to Dilated Cardiomyopathy. Int. J. Pediatr. 2012, 2012, 452909. [Google Scholar] [CrossRef]
- Hadi, H.A.; Carr, C.S.; Al Suwaidi, J. Endothelial Dysfunction: Cardiovascular Risk Factors, Therapy, and Outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar] [PubMed]
- Jui, E.; Singampalli, K.L.; Shani, K.; Ning, Y.; Connell, J.P.; Birla, R.K.; Bollyky, P.L.; Caldarone, C.A.; Keswani, S.G.; Grande-Allen, K.J. The Immune and Inflammatory Basis of Acquired Pediatric Cardiac Disease. Front. Cardiovasc. Med. 2021, 8, 701224. [Google Scholar] [CrossRef]
- Knez, M.; Glibetic, M. Zinc as a Biomarker of Cardiovascular Health. Front. Nutr. 2021, 8, 686078. [Google Scholar] [CrossRef]
- Kelishadi, R.; Alikhassy, H.; Amiri, M. Zinc and Copper Status in Children with High Family Risk of Premature Cardiovascular Disease. Ann. Saudi Med. 2002, 22, 291–294. [Google Scholar] [CrossRef]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and Vitamin D: Skeletal and Extraskeletal Health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef]
- Sunyecz, J.A. The Use of Calcium and Vitamin D in the Management of Osteoporosis. Ther. Clin. Risk Manag. 2008, 4, 827–836. [Google Scholar] [CrossRef]
- Mandarino, N.R.; Júnior, F.d.C.M.; Salgado, J.V.L.; Lages, J.S.; Filho, N.S. Is Vitamin D Deficiency a New Risk Factor for Cardiovascular Disease? Open Cardiovasc. Med. J. 2015, 9, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.; Appel, L.J.; Michos, E.D. Vitamin D, Calcium, and Cardiovascular Disease: A“D”Vantageous or “D”Etrimental? An Era of Uncertainty. Curr. Atheroscler. Rep. 2017, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, B.S. Role of the Gut Microbiota in Human Nutrition and Metabolism. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S4), 9–17. [Google Scholar] [CrossRef]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the Gut Microbiome and Gastrointestinal Health in Humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Hossain, K.S.; Amarasena, S.; Mayengbam, S. B Vitamins and Their Roles in Gut Health. Microorganisms 2022, 10, 1168. [Google Scholar] [CrossRef] [PubMed]
- Uebanso, T.; Shimohata, T.; Mawatari, K.; Takahashi, A. Functional Roles of B-Vitamins in the Gut and Gut Microbiome. Mol. Nutr. Food Res. 2020, 64, e2000426. [Google Scholar] [CrossRef]
- Wan, Z.; Zheng, J.; Zhu, Z.; Sang, L.; Zhu, J.; Luo, S.; Zhao, Y.; Wang, R.; Zhang, Y.; Hao, K.; et al. Intermediate Role of Gut Microbiota in Vitamin B Nutrition and Its Influences on Human Health. Front. Nutr. 2022, 9, 1031502. [Google Scholar] [CrossRef]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The Potential Role of Vitamin D Supplementation as a Gut Microbiota Modifier in Healthy Individuals. Sci. Rep. 2020, 10, 21641. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Lutfor, A.B.; Razzaque, M.S. Vitamin D and the Host-Gut Microbiome: A Brief Overview. Acta Histochem. Cytochem. 2020, 53, 33–42. [Google Scholar] [CrossRef]
- Bellerba, F.; Muzio, V.; Gnagnarella, P.; Facciotti, F.; Chiocca, S.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Serrano, D.; Raimondi, S.; et al. The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients 2021, 13, 3378. [Google Scholar] [CrossRef]
- Iyer, N.; Vaishnava, S. Vitamin A at the Interface of Host–Commensal–Pathogen Interactions. PLoS Pathog. 2019, 15, e1007750. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Buys, E.M. Insights into the Role of Bacteria in Vitamin A Biosynthesis: Future Research Opportunities. Crit. Rev. Food Sci. Nutr. 2019, 59, 3211–3226. [Google Scholar] [CrossRef]
- Scarpellini, E.; Balsiger, L.M.; Maurizi, V.; Rinninella, E.; Gasbarrini, A.; Giostra, N.; Santori, P.; Abenavoli, L.; Rasetti, C. Zinc and Gut Microbiota in Health and Gastrointestinal Disease under the COVID-19 Suggestion. Biofactors 2022, 48, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Tako, E. Chronic Dietary Zinc Deficiency Alters Gut Microbiota Composition and Function. Proceedings 2020, 61, 16. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, F.; Or-Rashid, H.; Mamun, A.A.; Rahaman, S.; Islam, M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, M.; Rout, A.; Kingsley, T.; Kirchoff, R.; Singh, A.; Verma, V.; Kant, R.; Chaudhary, R. Role of Gut Microbiota in Cardiovascular Diseases. World J. Cardiol. 2020, 12, 110–122. [Google Scholar] [CrossRef]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef]
- Kazemian, N.; Mahmoudi, M.; Halperin, F.; Wu, J.C.; Pakpour, S. Gut Microbiota and Cardiovascular Disease: Opportunities and Challenges. Microbiome 2020, 8, 36. [Google Scholar] [CrossRef]
- Trøseid, M.; Andersen, G.Ø.; Broch, K.; Hov, J.R. The Gut Microbiome in Coronary Artery Disease and Heart Failure: Current Knowledge and Future Directions. eBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef]
- Canyelles, M.; Borràs, C.; Rotllan, N.; Tondo, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Gut Microbiota-Derived TMAO: A Causal Factor Promoting Atherosclerotic Cardiovascular Disease? Int. J. Mol. Sci. 2023, 24, 1940. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, M. Trimethylamine N-Oxide Generated by the Gut Microbiota Is Associated with Vascular Inflammation: New Insights into Atherosclerosis. Mediat. Inflamm. 2020, 2020, 4634172. [Google Scholar] [CrossRef]
- Masenga, S.K.; Hamooya, B.; Hangoma, J.; Hayumbu, V.; Ertuglu, L.A.; Ishimwe, J.; Rahman, S.; Saleem, M.; Laffer, C.L.; Elijovich, F.; et al. Recent Advances in Modulation of Cardiovascular Diseases by the Gut Microbiota. J. Hum. Hypertens. 2022, 36, 952–959. Available online: https://www.nature.com/articles/s41371-022-00698-6 (accessed on 14 June 2023). [CrossRef]
- Callender, C.; Attaye, I.; Nieuwdorp, M. The Interaction between the Gut Microbiome and Bile Acids in Cardiometabolic Diseases. Metabolites 2022, 12, 65. [Google Scholar] [CrossRef]
- Pushpass, R.-A.G.; Alzoufairi, S.; Jackson, K.G.; Lovegrove, J.A. Circulating Bile Acids as a Link between the Gut Microbiota and Cardiovascular Health: Impact of Prebiotics, Probiotics and Polyphenol-Rich Foods. Nutr. Res. Rev. 2022, 35, 161–180. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, R.; Zhang, Y.; Pan, D.; Zhu, Y.; Zhang, X.; Yang, R.; Jiang, R.; Xu, Y.; Qin, H. Dysbiosis Signatures of Gut Microbiota in Coronary Artery Disease. Physiol. Genom. 2018, 50, 893–903. [Google Scholar] [CrossRef]
- Kau, A.L.; Planer, J.D.; Liu, J.; Rao, S.; Yatsunenko, T.; Trehan, I.; Manary, M.J.; Liu, T.-C.; Stappenbeck, T.S.; Maleta, K.M.; et al. Functional Characterization of IgA-Targeted Bacterial Taxa from Undernourished Malawian Children That Produce Diet-Dependent Enteropathy. Sci. Transl. Med. 2015, 7, 276ra24. [Google Scholar] [CrossRef] [PubMed]
- Ronan, V.; Yeasin, R.; Claud, E.C. Childhood Development and the Microbiome—The Intestinal Microbiota in Maintenance of Health and Development of Disease During Childhood Development. Gastroenterology 2021, 160, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Tun, H.M.; Bridgman, S.L.; Chari, R.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Sears, M.R.; et al. Roles of Birth Mode and Infant Gut Microbiota in Intergenerational Transmission of Overweight and Obesity From Mother to Offspring. JAMA Pediatr. 2018, 172, 368. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Carmen Collado, M.; Salminen, S.; Isolauri, E. Early Differences in Fecal Microbiota Composition in Children May Predict Overweight. Am. J. Clin. Nutr. 2008, 87, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzales, M.; Mistretta, T.-A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M.; et al. Structure and Function of the Healthy Pre-Adolescent Pediatric Gut Microbiome. Microbiome 2015, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.; Su, J.; Koprowski, S.; Hsu, A.; Tweddell, J.S.; Rafiee, P.; Gross, G.J.; Salzman, N.H.; Baker, J.E. Intestinal Microbiota Determine Severity of Myocardial Infarction in Rats. FASEB J. 2012, 26, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.; Su, J.; Hsu, A.; Gross, G.J.; Salzman, N.H.; Baker, J.E. Intestinal Microbial Metabolites Are Linked to Severity of Myocardial Infarction in Rats. PLoS ONE 2016, 11, e0160840. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brecht, P.; Dring, J.C.; Yanez, F.; Styczeń, A.; Mertowska, P.; Mertowski, S.; Grywalska, E. How Do Minerals, Vitamins, and Intestinal Microbiota Affect the Development and Progression of Heart Disease in Adult and Pediatric Patients? Nutrients 2023, 15, 3264. https://doi.org/10.3390/nu15143264
Brecht P, Dring JC, Yanez F, Styczeń A, Mertowska P, Mertowski S, Grywalska E. How Do Minerals, Vitamins, and Intestinal Microbiota Affect the Development and Progression of Heart Disease in Adult and Pediatric Patients? Nutrients. 2023; 15(14):3264. https://doi.org/10.3390/nu15143264
Chicago/Turabian StyleBrecht, Peet, James Curtis Dring, Felipe Yanez, Agnieszka Styczeń, Paulina Mertowska, Sebastian Mertowski, and Ewelina Grywalska. 2023. "How Do Minerals, Vitamins, and Intestinal Microbiota Affect the Development and Progression of Heart Disease in Adult and Pediatric Patients?" Nutrients 15, no. 14: 3264. https://doi.org/10.3390/nu15143264
APA StyleBrecht, P., Dring, J. C., Yanez, F., Styczeń, A., Mertowska, P., Mertowski, S., & Grywalska, E. (2023). How Do Minerals, Vitamins, and Intestinal Microbiota Affect the Development and Progression of Heart Disease in Adult and Pediatric Patients? Nutrients, 15(14), 3264. https://doi.org/10.3390/nu15143264