Liposomal Epigallocatechin-3-Gallate for the Treatment of Intestinal Dysbiosis in Children with Autism Spectrum Disorder: A Comprehensive Review
Abstract
1. Introduction
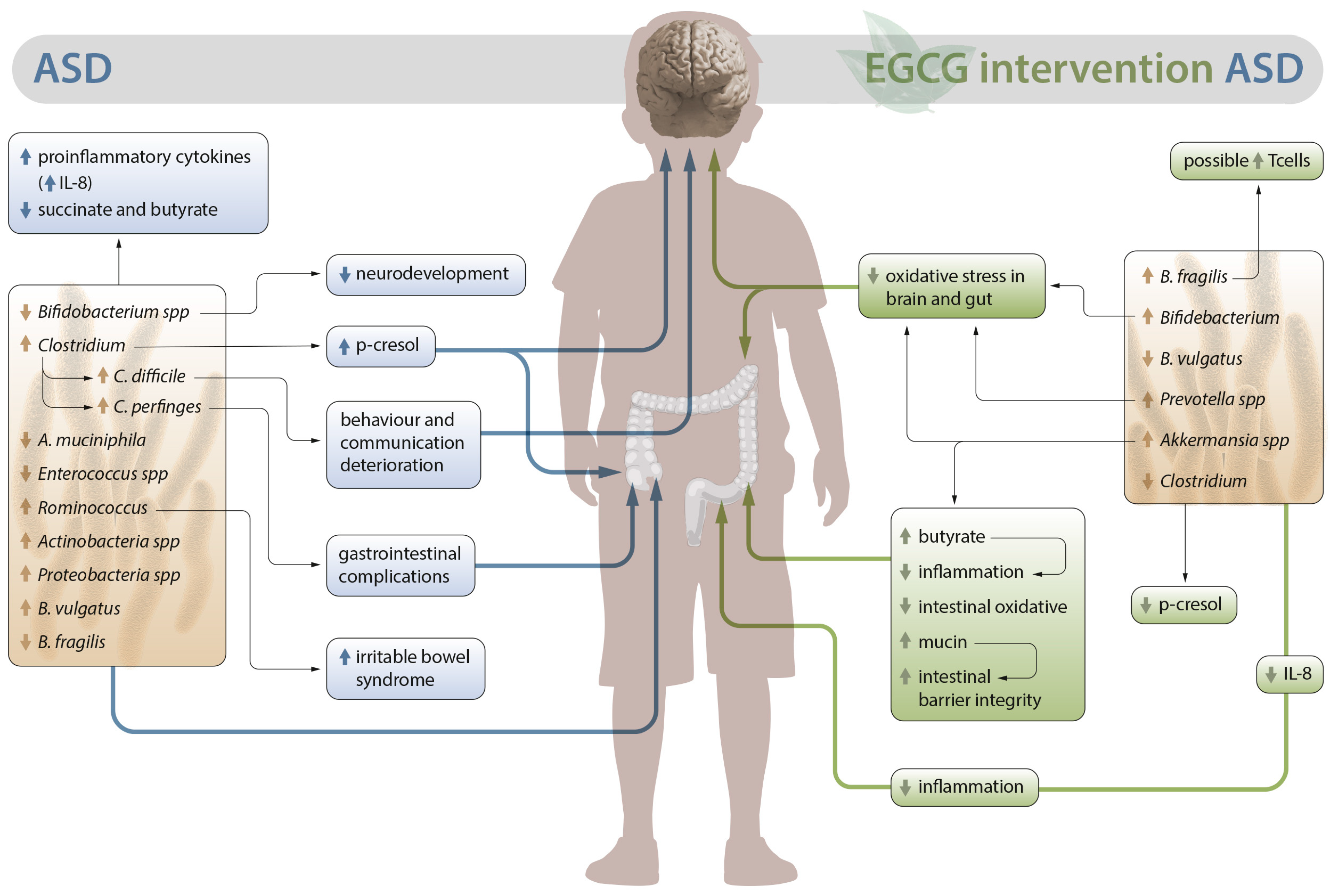
2. Alterations in the Intestinal Microbiota in ASD
3. Polyphenols as a Therapeutic Alternative to ASD: The Epigallocatechin-3-Gallate (EGCG) Option
3.1. Possible Role of EGCG in the Intestinal Microbiota of Patients with ASD
3.2. Outlook on the Anti-Inflammatory and Antioxidant Activity of EGCG in Autism: Neuroprotective Role
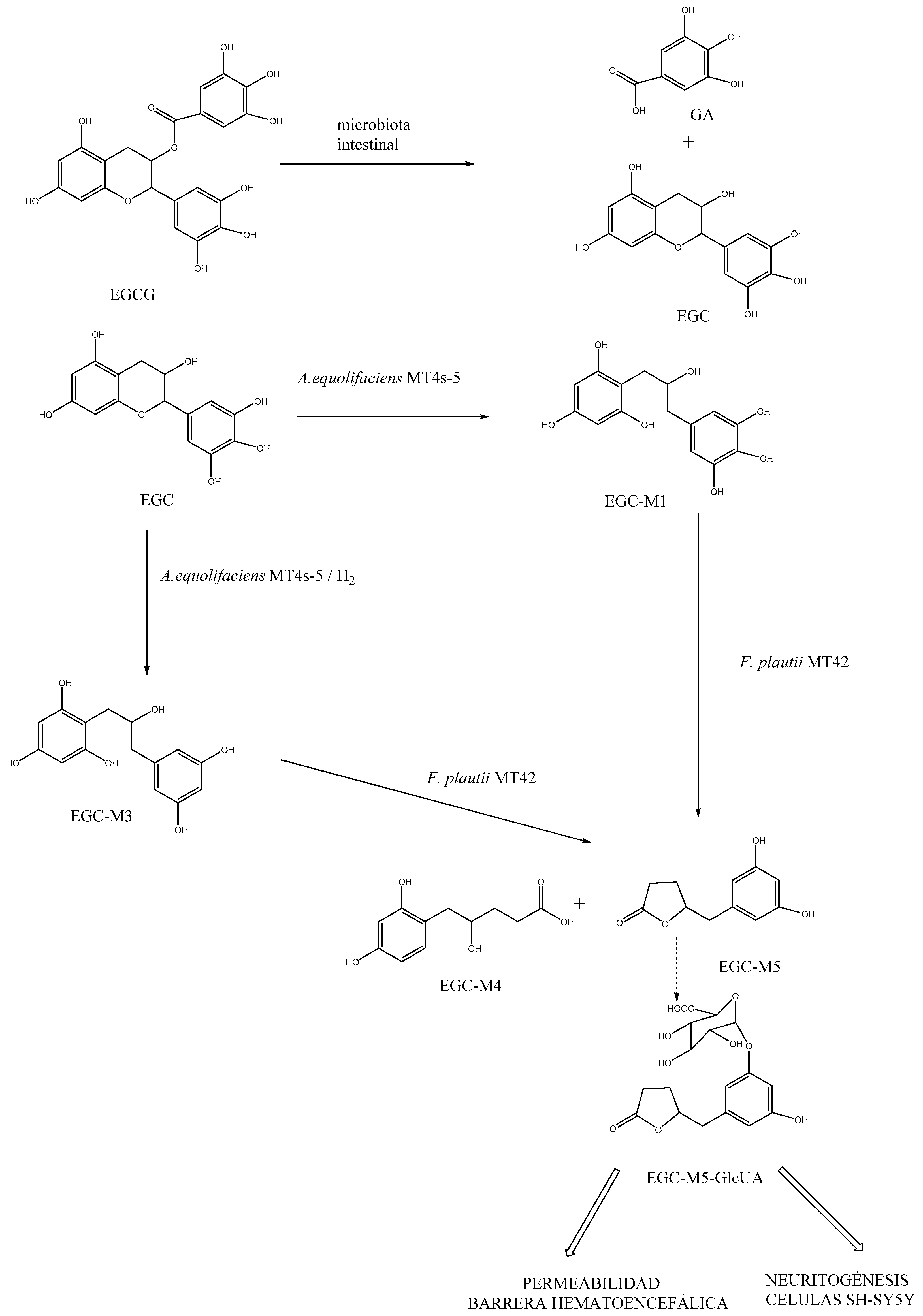
3.3. Role of EGCG in the Metabolic Activity Associated with Dysbiosis in ASD
3.4. Liposomal EGCG
3.5. Potential Adverse Effects of EGCG
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Taylor, M.J.; Rosenqvist, M.A.; Larsson, H.; Gillberg, C.; D’Onofrio, B.M.; Lichtenstein, P.; Lundström, S. Etiology of Autism Spectrum Disorders and Autistic Traits Over Time. JAMA Psychiatry 2020, 77, 936–943. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5 th ed.; 2013; American Psychiatric Association: Washington, DC, USA; Available online: https://dsm.psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596 (accessed on 18 May 2013).
- Hughes, H.K.; Rose, D.; Ashwood, P. The Gut Microbiota and Dysbiosis in Autism Spectrum Disorders. Curr. Neurol. Neurosci. Rep. 2018, 18, 81. [Google Scholar] [CrossRef]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef]
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejía, J.L.; Hansen, L.H.; Leigh Gibson, E.; Nielsen, D.S.; Costabile, A. A Prebiotic Intervention Study in Children with Autism Spectrum Disorders (ASDs). Microbiome 2018, 6, 133. [Google Scholar] [CrossRef]
- World Health Organization. Autism. Available online: https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders (accessed on 26 February 2023).
- Li, Q.; Zhou, J.M. The Microbiota–Gut–Brain Axis and Its Potential Therapeutic Role in Autism Spectrum Disorder. Neuroscience 2016, 324, 131–139. [Google Scholar] [CrossRef]
- Yang, J.; Fu, X.; Liao, X.; Li, Y. Effects of Gut Microbial-Based Treatments on Gut Microbiota, Behavioral Symptoms, and Gastrointestinal Symptoms in Children with Autism Spectrum Disorder: A Systematic Review. Psychiatry Res. 2020, 293, 113471. [Google Scholar] [CrossRef]
- de Theije, C.G.M.; Wopereis, H.; Ramadan, M.; van Eijndthoven, T.; Lambert, J.; Knol, J.; Garssen, J.; Kraneveld, A.D.; Oozeer, R. Altered Gut Microbiota and Activity in a Murine Model of Autism Spectrum Disorders. Brain Behav. Immun. 2014, 37, 197–206. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New Evidences on the Altered Gut Microbiota in Autism Spectrum Disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; Fitzgerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Iovene, M.R.; Bombace, F.; Maresca, R.; Sapone, A.; Iardino, P.; Picardi, A.; Marotta, R.; Schiraldi, C.; Siniscalco, D.; Serra, N.; et al. Intestinal Dysbiosis and Yeast Isolation in Stool of Subjects with Autism Spectrum Disorders. Mycopathologia 2017, 182, 349–363. [Google Scholar] [CrossRef]
- Yadav, M.; Kapoor, A.; Verma, A.; Ambatipudi, K. Functional Significance of Different Milk Constituents in Modulating the Gut Microbiome and Infant Health. J. Agric. Food Chem. 2022, 70, 3929–3947. [Google Scholar] [CrossRef]
- Ding, H.; Yi, X.; Zhang, X.; Wang, H.; Liu, H.; Mou, W.W. Imbalance in the Gut Microbiota of Children with Autism Spectrum Disorders. Front. Cell. Infect. Microbiol. 2021, 11, 496. [Google Scholar] [CrossRef]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal Gut Microbiota Modulates Brain Development and Behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the Microbiota and the Immune System. Science (80-) 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Frye, R.E.; Melnyk, S.; Macfabe, D.F. Unique Acyl-Carnitine Profiles Are Potential Biomarkers for Acquired Mitochondrial Disease in Autism Spectrum Disorder. Transl. Psychiatry 2013, 3, e220. [Google Scholar] [CrossRef]
- Slattery, J.; Macfabe, D.F.; Frye, R.E. The Significance of the Enteric Microbiome on the Development of Childhood Disease: A Review of Prebiotic and Probiotic Therapies in Disorders of Childhood. Clin. Med. Insights Pediatr. 2016, 10, CMPed.S38338. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Li, H.; Li, B.; Duan, G.; Zhu, C. The Role of Probiotics in Children with Autism Spectrum Disorders: A Study Protocol for a Randomised Controlled Trial. PLoS ONE 2022, 17, e0263109. [Google Scholar] [CrossRef]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-Gut-Microbe Communication in Health and Disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric Acid Production by Culturable Bacteria from the Human Intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. Polyphenols in the Management of Brain Disorders: Modulation of the Microbiota-Gut-Brain Axis. Adv. Food Nutr. Res. 2020, 91, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.; Walker, M.M.; Talley, N.J. The Mucosal Immune System: Master Regulator of Bidirectional Gut–Brain Communications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 143–159. [Google Scholar] [CrossRef]
- Sun, Q.; Cheng, L.; Zhang, X.; Wu, Z.; Weng, P. The Interaction between Tea Polyphenols and Host Intestinal Microorganisms: An Effective Way to Prevent Psychiatric Disorders. Food Funct. 2021, 12, 952–962. [Google Scholar] [CrossRef]
- Argou-Cardozo, I.; Zeidán-Chuliá, F. Clostridium Bacteria and Autism Spectrum Conditions: A Systematic Review and Hypothetical Contribution of Environmental Glyphosate Levels. Med. Sci. 2018, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xu, X.; Li, J.; Li, F. Association between Gut Microbiota and Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Front. Psychiatry 2019, 10, 473. [Google Scholar] [CrossRef]
- Zou, R.; Xu, F.; Wang, Y.; Duan, M.; Guo, M.; Zhang, Q.; Zhao, H.; Zheng, H. Changes in the Gut Microbiota of Children with Autism Spectrum Disorder. Autism Res. 2020, 13, 1614–1625. [Google Scholar] [CrossRef]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; García-Martínez, N.; Sánchez-Samper, E.P.; Martínez-González, A.E. An Approach to Gut Microbiota Profile in Children with Autism Spectrum Disorder. Environ. Microbiol. Rep. 2020, 12, 115–135. [Google Scholar] [CrossRef]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal Microbiota in Children with Autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Xie, X.; Li, L.; Wu, X.; Hou, F.; Chen, Y.; Shi, L.; Liu, Q.; Zhu, K.; Jiang, Q.; Feng, Y.; et al. Alteration of the Fecal Microbiota in Chinese Children with Autism Spectrum Disorder. Autism Res. 2022, 15, 996–1007. [Google Scholar] [CrossRef]
- Williams, B.L.; Hornig, M.; Buie, T.; Bauman, M.L.; Cho Paik, M.; Wick, I.; Bennett, A.; Jabado, O.; Hirschberg, D.L.; Lipkin, W.I. Impaired Carbohydrate Digestion and Transport and Mucosal Dysbiosis in the Intestines of Children with Autism and Gastrointestinal Disturbances. PLoS ONE 2011, 6, e24585. [Google Scholar] [CrossRef]
- Gondalia, S.V.; Palombo, E.A.; Knowles, S.R.; Cox, S.B.; Meyer, D.; Austin, D.W. Molecular Characterisation of Gastrointestinal Microbiota of Children with Autism (with and without Gastrointestinal Dysfunction) and Their Neurotypical Siblings. Autism Res. 2012, 5, 419–427. [Google Scholar] [CrossRef]
- Son, J.S.; Zheng, L.J.; Rowehl, L.M.; Tian, X.; Zhang, Y.; Zhu, W.; Litcher-Kelly, L.; Gadow, K.D.; Gathungu, G.; Robertson, C.E.; et al. Comparison of Fecal Microbiota in Children with Autism Spectrum Disorders and Neurotypical Siblings in the Simons Simplex Collection. PLoS ONE 2015, 10, e0137725. [Google Scholar] [CrossRef]
- Ho, L.K.H.; Tong, V.J.W.; Syn, N.; Nagarajan, N.; Tham, E.H.; Tay, S.K.; Shorey, S.; Tambyah, P.A.; Law, E.C.N. Gut Microbiota Changes in Children with Autism Spectrum Disorder: A Systematic Review. Gut Pathog. 2020, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Nogay, N.H.; Nahikian-Nelms, M. Can We Reduce Autism-Related Gastrointestinal and Behavior Problems by Gut Microbiota Based Dietary Modulation? A Review. Nutr. Neurosci. 2021, 24, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Rho, J.M.; Masino, S.A. Metabolic Dysfunction Underlying Autism Spectrum Disorder and Potential Treatment Approaches. Front. Mol. Neurosci. 2017, 10, 34. [Google Scholar] [CrossRef]
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. Polyphenols as Food Bioactive Compounds in the Context of Autism Spectrum Disorders: A Critical Mini-Review. Neurosci. Biobehav. Rev. 2019, 102, 290–298. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Navajas-Porras, B.; López-Maldonado, A.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Green Tea and Its Relation to Human Gut Microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; Ni, L.; Vincken, J.P. Green and Black Tea Phenolics: Bioavailability, Transformation by Colonic Microbiota, and Modulation of Colonic Microbiota. J. Agric. Food Chem. 2018, 66, 8469–8477. [Google Scholar] [CrossRef]
- Francisco, A.; Tomás-Barberán; González-Sarrías, A.; García-Villalba, R. Dietary Polyphenols: Metabolism and Health Effects; Wiley-Blackwell: Murcia, Spain, 2020. [Google Scholar]
- Peterson, J.; Dwyer, J.; Bhagwat, S.; Haytowitz, D.; Holden, J.; Eldridge, A.L.; Beecher, G.; Aladesanmi, J. Major Flavonoids in Dry Tea. J. Food Compos. Anal. 2005, 18, 487–501. [Google Scholar] [CrossRef]
- Ankolekar, C.; Johnson, D.; Pinto, M.D.S.; Johnson, K.; Labbe, R.; Shetty, K. Inhibitory Potential of Tea Polyphenolics and Influence of Extraction Time against Helicobacter Pylori and Lack of Inhibition of Beneficial Lactic Acid Bacteria. J. Med. Food 2011, 14, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Chung, H.S. Antibacterial Activities of Phenolic Components from Camellia Sinensis L. on Pathogenic Microorganisms. Prev. Nutr. Food Sci. 2007, 12, 135–140. [Google Scholar] [CrossRef]
- Nakayama, M.; Shigemune, N.; Tsugukuni, T.; Jun, H.; Matsushita, T.; Mekada, Y.; Kurahachi, M.; Miyamoto, T. Mechanism of the Combined Anti-Bacterial Effect of Green Tea Extract and NaCl against Staphylococcus Aureus and Escherichia Coli O157:H7. Food Control 2012, 25, 225–232. [Google Scholar] [CrossRef]
- Kohda, C.; Yanagawa, Y.; Shimamura, T. Epigallocatechin Gallate Inhibits Intracellular Survival of Listeria Monocytogenes in Macrophages. Biochem. Biophys. Res. Commun. 2008, 365, 310–315. [Google Scholar] [CrossRef]
- Si, W.; Gong, J.; Tsao, R.; Kalab, M.; Yang, R.; Yin, Y. Bioassay-Guided Purification and Identification of Antimicrobial Components in Chinese Green Tea Extract. J. Chromatogr. A 2006, 1125, 204–210. [Google Scholar] [CrossRef]
- Bancirova, M. Comparison of the Antioxidant Capacity and the Antimicrobial Activity of Black and Green Tea. Food Res. Int. 2010, 43, 1379–1382. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Zhao, Q.; He, Y.; Niu, J.; Debnath, A.K.; Wu, S.; Jiang, S. Theaflavin Derivatives in Black Tea and Catechin Derivatives in Green Tea Inhibit HIV-1 Entry by Targeting Gp41. Biochim. Biophys. Acta 2005, 1723, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Tsai, H.L.; Peng, C.W. EGCG Debilitates the Persistence of EBV Latency by Reducing the DNA Binding Potency of Nuclear Antigen 1. Biochem. Biophys. Res. Commun. 2012, 417, 1093–1099. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of Dietary Compounds, Especially Polyphenols, with the Intestinal Microbiota: A Review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-Gallate (EGCG): Chemical and Biomedical Perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Trovò, L.; Fuchs, C.; De Rosa, R.; Barbiero, I.; Tramarin, M.; Ciani, E.; Rusconi, L.; Kilstrup-Nielsen, C. The Green Tea Polyphenol Epigallocatechin-3-Gallate (EGCG) Restores CDKL5-Dependent Synaptic Defects in Vitro and in Vivo. Neurobiol. Dis. 2020, 138, 104791. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, P.; Melchias, G.; Vasanth, N.; Manivasagam, T. Dose-Dependent Amelioration of Epigallocatechin-3-Gallate against Sodium Valproate Induced Autistic Rats. Int. J. Pharm. Pharm. Sci. 2017, 9, 203–206. [Google Scholar] [CrossRef]
- Taliou, A.; Zintzaras, E.; Lykouras, L.; Francis, K. An Open-Label Pilot Study of a Formulation Containing the Anti-Inflammatory Flavonoid Luteolin and Its Effects on Behavior in Children with Autism Spectrum Disorders. Clin. Ther. 2013, 35, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Tsilioni, I.; Taliou, A.; Francis, K.; Theoharides, T.C. Children with Autism Spectrum Disorders, Who Improved with a Luteolin-Containing Dietary Formulation, Show Reduced Serum Levels of TNF and IL-6. Transl. Psychiatry 2015, 5, e647. [Google Scholar] [CrossRef]
- Bertolino, B.; Crupi, R.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Beneficial Effects of Co-Ultramicronized Palmitoylethanolamide/Luteolin in a Mouse Model of Autism and in a Case Report of Autism. CNS Neurosci. Ther. 2017, 23, 87–98. [Google Scholar] [CrossRef]
- Nasiry, D.; Khalatbary, A.R. Natural Polyphenols for the Management of Autism Spectrum Disorder: A Review of Efficacy and Molecular Mechanisms. Nutr. Neurosci. 2023, 2023, 1–11. [Google Scholar] [CrossRef]
- Scholey, A.; Downey, L.A.; Ciorciari, J.; Pipingas, A.; Nolidin, K.; Finn, M.; Wines, M.; Catchlove, S.; Terrens, A.; Barlow, E.; et al. Acute Neurocognitive Effects of Epigallocatechin Gallate (EGCG). Appetite 2012, 58, 767–770. [Google Scholar] [CrossRef]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of Tea Phenolics and Their Aromatic Fecal Bacterial Metabolites on Intestinal Microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef]
- Liu, Z.; De Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.P. Reciprocal Interactions between Epigallocatechin-3-Gallate (EGCG) and Human Gut Microbiota in Vitro. J. Agric. Food Chem. 2020, 68, 9804–9815. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, M.; Xue, J.; Xiang, L.; Li, Y.; Xiao, J.; Xiao, G.; Wang, H.L. EGCG Ameliorates Neuronal and Behavioral Defects by Remodeling Gut Microbiota and TotM Expression in Drosophila Models of Parkinson’s Disease. FASEB J. 2020, 34, 5931–5950. [Google Scholar] [CrossRef]
- Ushiroda, C.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Higashimura, Y.; Yasukawa, Z.; Okubo, T.; Inoue, R.; Honda, A.; et al. Green Tea Polyphenol (Epigallocatechin-3-Gallate) Improves Gut Dysbiosis and Serum Bile Acids Dysregulation in High-Fat Diet-Fed Mice. J. Clin. Biochem. Nutr. 2019, 65, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wu, Y.; Cheng, W.; Wang, D.; Zeng, L.; Wang, Y.; Li, T.-T.; Zhang, L.; Yang, J.; Sun, L.; et al. Amelioration of Cognitive Impairment Using Epigallocatechin-3-Gallate in Ovariectomized Mice Fed a High-Fat Diet Involves Remodeling with Prevotella and Bifidobacteriales. Front. Pharmacol. 2023, 13, 1079313. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, P.; Capobianco, D.; Campagna, G.; Laforgia, N.; Drimaco, P.; Dileone, A.; Baldassarre, M.E. Correlation between Lactoferrin and Beneficial Microbiota in Breast Milk and Infant’s Feces. Biometals 2014, 27, 1077–1086. [Google Scholar] [CrossRef]
- Savignac, H.M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria Exert Strain-Specific Effects on Stress-Related Behavior and Physiology in BALB/c Mice. Neurogastroenterol. Motil. 2014, 26, 1615–1627. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; Martínez-Gonzlez, A.E. Una Propuesta de Probiótico Basada En El Bifidobacterium Para Autismo. Rev. Española Nutr. Humana Y Dietética 2022, 26 (Suppl. S1), e1429. [Google Scholar] [CrossRef]
- Unno, T.; Sakuma, M.; Mitsuhashi, S. Effect of Dietary Supplementation of (-)-Epigallocatechin Gallate on Gut Microbiota and Biomarkers of Colonic Fermentation in Rats. J. Nutr. Sci. Vitaminol. 2014, 60, 213–219. [Google Scholar] [CrossRef]
- Tarek El-Banna; El-Aziz, A. A.; EL-Mahdy, N.; Samy, Y. Sherris Medical Microbiology, 4th ed.; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Pérez de Rozas Ruiz de Gauna, A.M.; Badiola Sáiz, I.; Castellà Gómez, G.; Universitat Autònoma de Barcelona; Departament de Sanitat i d’Anatomia Animals; Centre de Recerca de Sanitat Animal. Institut de Recerca i Tecnologia Agroalimentàries. Utilización de Cepas de Bacteroides Spp. Como Probiótico En Conejos. TDX (Tesis Dr. en Xarxa). Ph.D. Thesis, Universitat Autònoma de Barcelona, Catalonia, Spain, 2014. [Google Scholar]
- Jiang, C.C.; Lin, L.S.; Long, S.; Ke, X.Y.; Fukunaga, K.; Lu, Y.M.; Han, F. Signalling Pathways in Autism Spectrum Disorder: Mechanisms and Therapeutic Implications. Signal Transduct. Target. Ther. 2022, 7, 229. [Google Scholar] [CrossRef]
- Xu, X.J.; Lang, J.D.; Yang, J.; Long, B.; Liu, X.D.; Zeng, X.F.; Tian, G.; You, X. Differences of Gut Microbiota and Behavioral Symptoms between Two Subgroups of Autistic Children Based on ΓδT Cells-Derived IFN-γ Levels: A Preliminary Study. Front. Immunol. 2023, 14, 1100816. [Google Scholar] [CrossRef]
- Saitoh, S.; Noda, S.; Aiba, Y.; Takagi, A.; Sakamoto, M.; Benno, Y.; Koga, Y. Bacteroides Ovatus as the Predominant Commensal Intestinal Microbe Causing a Systemic Antibody Response in Inflammatory Bowel Disease. Clin. Diagn. Lab. Immunol. 2002, 9, 54–59. [Google Scholar] [CrossRef]
- Srikantha, P.; Hasan Mohajeri, M. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef]
- Tett, A.; Huang, K.D.; Asnicar, F.; Fehlner-Peach, H.; Pasolli, E.; Karcher, N.; Armanini, F.; Manghi, P.; Bonham, K.; Zolfo, M.; et al. The Prevotella Copri Complex Comprises Four Distinct Clades Underrepresented in Westernized Populations. Cell Host Microbe 2019, 26, 666–679.e7. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, J.; O’Connor, E.M.; Shanahan, F. Review Article: Dietary Fibre in the Era of Microbiome Science. Aliment. Pharmacol. Ther. 2019, 49, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Matusheski, N.V.; Caffrey, A.; Christensen, L.; Mezgec, S.; Surendran, S.; Hjorth, M.F.; McNulty, H.; Pentieva, K.; Roager, H.M.; Seljak, B.K.; et al. Diets, Nutrients, Genes and the Microbiome: Recent Advances in Personalised Nutrition. Br. J. Nutr. 2021, 126, 1489–1497. [Google Scholar] [CrossRef]
- Ortega-Santos, C.P.; Whisner, C.M. The Key to Successful Weight Loss on a High-Fiber Diet May Be in Gut Microbiome Prevotella Abundance. J. Nutr. 2019, 149, 2083–2084. [Google Scholar] [CrossRef]
- Hill, A.P.; Zuckerman, K.E.; Fombonne, E. Obesity and Autism. Pediatrics 2015, 136, 1051–1061. [Google Scholar] [CrossRef]
- Sharp, W.G.; Postorino, V.; McCracken, C.E.; Berry, R.C.; Criado, K.K.; Burrell, T.L.; Scahill, L. Dietary Intake, Nutrient Status, and Growth Parameters in Children with Autism Spectrum Disorder and Severe Food Selectivity: An Electronic Medical Record Review. J. Acad. Nutr. Diet. 2018, 118, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Dhir, S.; Tarasenko, M.; Napoli, E.; Giulivi, C. Neurological, Psychiatric, and Biochemical Aspects of Thiamine Deficiency in Children and Adults. Front. Psychiatry 2019, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Dandachi, I.; Anani, H.; Hadjadj, L.; Brahimi, S.; Lagier, J.C.; Daoud, Z.; Rolain, J.M. Genome Analysis of Lachnoclostridium Phocaeense Isolated from a Patient after Kidney Transplantation in Marseille. New Microbes New Infect. 2021, 41, 100863. [Google Scholar] [CrossRef] [PubMed]
- McNabney, S.M.; Henagan, T.M. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut Microbiota from Green Tea Polyphenol-Dosed Mice Improves Intestinal Epithelial Homeostasis and Ameliorates Experimental Colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, S.; Pang, J.; Wang, S.; Zhang, G.; Wang, J. Oral, but Not Rectal Delivery of Epigallocatechin-3-Gallate Alleviates Colitis by Regulating the Gut Microbiota, Oxidative Stress, Inflammation, and Barrier Integrity. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Amaral Montesino, C.; Abrego Sánchez, A.; Díaz Granados, M.A.; González Ponce, R.; Salinas Flores, A.; Rojas García, O.C. Akkermansia Muciniphila, Una Ventana de Investigación Para La Regulación Del Metabolismo y Enfermedades Relacionadas. Nutr. Hosp. 2021, 38, 675–676. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, Y.; Xu, N.; Mu, H.; Zhang, H.; Duan, J. Improved Viability of Akkermansia Muciniphila by Encapsulation in Spray Dried Succinate-Grafted Alginate Doped with Epigallocatechin-3-Gallate. Int. J. Biol. Macromol. 2020, 159, 373–382. [Google Scholar] [CrossRef]
- Sheng, L.; Jena, P.K.; Hui-Xin, L.; Hu, Y.; Nagar, N.; Bronner, D.N.; Settles, M.L.; Bäumler, A.J.; Wan, Y.J.Y. Obesity Treatment by Epigallocatechin-3-Gallate−regulated Bile Acid Signaling and Its Enriched Akkermansia Muciniphila. FASEB J. 2018, 32, 6371–6384. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, K.; Jing, N.; Zhao, Y.; Yang, X. EGCG Regulates Fatty Acid Metabolism of High-Fat Diet-Fed Mice in Association with Enrichment of Gut Akkermansia Muciniphila. J. Funct. Foods 2020, 75, 104261. [Google Scholar] [CrossRef]
- Manrique, D.; Elie, V. Ácidos Grasos de Cadena Corta (Ácido Butírico) y Patologías Intestinales. Nutr. Hosp. 2017, 34, 58–61. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, M.K.; AlKhulaifi, M.M.; Al Farraj, D.A.; Somily, A.M.; Albarrag, A.M. Incidence of Clostridium Perfringens and Its Toxin Genes in the Gut of Children with Autism Spectrum Disorder. Anaerobe 2020, 61, 102114. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Z.; Liu, C.; Liu, T.; Gao, J.; Cai, Y.; Fan, X. Alteration of Gut Microbiota: New Strategy for Treating Autism Spectrum Disorder. Front. Cell Dev. Biol. 2022, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Sandler, R.H.; Finegold, S.M.; Bolte, E.R.; Buchanan, C.P.; Maxwell, A.P.; Väisänen, M.L.; Nelson, M.N.; Wexler, H.M. Short-Term Benefit From Oral Vancomycin Treatment of Regressive-Onset Autism. J. Child Neurol. 2000, 15, 429–435. [Google Scholar] [CrossRef]
- Enterobacterias—EcuRed. Available online: https://www.ecured.cu/Enterobacterias (accessed on 11 October 2022).
- Mohammad, F.K.; Palukuri, M.V.; Shivakumar, S.; Rengaswamy, R.; Sahoo, S. A Computational Framework for Studying Gut-Brain Axis in Autism Spectrum Disorder. Front. Physiol. 2022, 13, 760753. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef]
- Matelski, L.; Van de Water, J. Risk Factors in Autism: Thinking Outside the Brain. J. Autoimmun. 2016, 67, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McAllister, A.K. Immune Contributions to Cause and Effect in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, A.; Van De Water, J. The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology 2017, 42, 284–298. [Google Scholar] [CrossRef]
- Leviton, A.; Joseph, R.M.; Allred, E.N.; Fichorova, R.N.; O’Shea, T.M.; Kuban, K.K.C.; Dammann, O. The Risk of Neurodevelopmental Disorders at Age 10 Years Associated with Blood Concentrations of Interleukins 4 and 10 during the First Postnatal Month of Children Born Extremely Preterm. Cytokine 2018, 110, 181–188. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Y.; Shi, L.; Liu, M.; Wu, R.; Xia, K.; Zhang, F.; Ou, J.; Zhao, J. Autism Spectrum Disorder and Severe Social Impairment Associated with Elevated Plasma Interleukin-8. Pediatr. Res. 2021, 89, 591–597. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Geng, L.; Davidow, A.L. Cytokine Profiles by Peripheral Blood Monocytes Are Associated with Changes in Behavioral Symptoms Following Immune Insults in a Subset of ASD Subjects: An Inflammatory Subtype? J. Neuroinflamm. 2014, 11, 187. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Geng, L.; Streck, D.L.; Dermody, J.J.; Toruner, G.A. MicroRNA Expression Changes in Association with Changes in Interleukin-1ß/Interleukin10 Ratios Produced by Monocytes in Autism Spectrum Disorders: Their Association with Neuropsychiatric Symptoms and Comorbid Conditions (Observational Study). J. Neuroinflamm. 2017, 14, 229. [Google Scholar] [CrossRef]
- Masi, A.; Quintana, D.S.; Glozier, N.; Lloyd, A.R.; Hickie, I.B.; Guastella, A.J. Cytokine Aberrations in Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2015, 20, 440–446. [Google Scholar] [CrossRef]
- Ricci, S.; Businaro, R.; Ippoliti, F.; Lo Vasco, V.R.; Massoni, F.; Onofri, E.; Troili, G.M.; Pontecorvi, V.; Morelli, M.; Rapp Ricciardi, M.; et al. Altered Cytokine and BDNF Levels in Autism Spectrum Disorder. Neurotox. Res. 2013, 24, 491–501. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-Derived Neurotrophic Factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Katche, C.; Slipczuk, L.; Rossato, J.I.; Goldin, A.; Izquierdo, I.; Medina, J.H. BDNF Is Essential to Promote Persistence of Long-Term Memory Storage. Proc. Natl. Acad. Sci. USA 2008, 105, 2711–2716. [Google Scholar] [CrossRef]
- Yamada, K.; Nabeshima, T. Brain-Derived Neurotrophic Factor/TrkB Signaling in Memory Processes. J. Pharmacol. Sci. 2003, 91, 267–270. [Google Scholar] [CrossRef]
- Hu, T.; Dong, Y.; He, C.; Zhao, M.; He, Q. The Gut Microbiota and Oxidative Stress in Autism Spectrum Disorders (ASD). Oxid. Med. Cell. Longev. 2020, 2020, 8396708. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C.K. Short-Chain Fatty Acids in Control of Energy Metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef]
- Codina-Solà, M.; Rodríguez-Santiago, B.; Homs, A.; Santoyo, J.; Rigau, M.; Aznar-Laín, G.; Del Campo, M.; Gener, B.; Gabau, E.; Botella, M.P.; et al. Integrated Analysis of Whole-Exome Sequencing and Transcriptome Profiling in Males with Autism Spectrum Disorders. Mol. Autism 2015, 6, 21. [Google Scholar] [CrossRef]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative Stress in Autism Spectrum Disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Attia, S.M.; AL-Ayadhi, L.Y.; Al-Harbi, N.O.; Bakheet, S.A. Dysregulated Enzymatic Antioxidant Network in Peripheral Neutrophils and Monocytes in Children with Autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 352–359. [Google Scholar] [CrossRef]
- Messina, A.; Monda, V.; Sessa, F.; Valenzano, A.; Salerno, M.; Bitetti, I.; Precenzano, F.; Marotta, R.; Lavano, F.; Lavano, S.M.; et al. Sympathetic, Metabolic Adaptations, and Oxidative Stress in Autism Spectrum Disorders: How Far from Physiology? Front. Physiol. 2018, 9, 261. [Google Scholar] [CrossRef]
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of Peroxisomes in ROS/RNS-Metabolism: Implications for Human Disease. Biochim. Biophys. Acta 2012, 1822, 1363–1373. [Google Scholar] [CrossRef]
- Cipolla, C.M.; Lodhi, I.J. Peroxisomal Dysfunction in Age-Related Diseases. Trends Endocrinol. Metab. 2017, 28, 297–308. [Google Scholar] [CrossRef]
- James, S.J.; Melnyk, S.; Jernigan, S.; Cleves, M.A.; Halsted, C.H.; Wong, D.H.; Cutler, P.; Bock, K.; Boris, M.; Bradstreet, J.J.; et al. Metabolic Endophenotype and Related Genotypes Are Associated with Oxidative Stress in Children with Autism. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2006, 141B, 947–956. [Google Scholar] [CrossRef]
- Rose, S.; Bennuri, S.C.; Wynne, R.; Melnyk, S.; James, S.J.; Frye, R.E. Mitochondrial and Redox Abnormalities in Autism Lymphoblastoid Cells: A Sibling Control Study. FASEB J. 2017, 31, 904–909. [Google Scholar] [CrossRef]
- Ray, B.; Long, J.M.; Sokol, D.K.; Lahiri, D.K. Increased Secreted Amyloid Precursor Protein-α (SAPPα) in Severe Autism: Proposal of a Specific, Anabolic Pathway and Putative Biomarker. PLoS ONE 2011, 6, e20405. [Google Scholar] [CrossRef]
- Sokol, D.K.; Maloney, B.; Long, J.M.; Ray, B.; Lahiri, D.K. Autism, Alzheimer Disease, and Fragile X: APP, FMRP, and MGluR5 Are Molecular Links. Neurology 2011, 76, 1344–1352. [Google Scholar] [CrossRef]
- Lahiri, D.K.; Sokol, D.K.; Erickson, C.; Ray, B.; Ho, C.Y.; Maloney, B. Autism as Early Neurodevelopmental Disorder: Evidence for an SAPPα-Mediated Anabolic Pathway. Front. Cell. Neurosci. 2013, 7, 94. [Google Scholar] [CrossRef]
- Valenti, D.; de Bari, L.; de Rasmo, D.; Signorile, A.; Henrion-Caude, A.; Contestabile, A.; Vacca, R.A. The Polyphenols Resveratrol and Epigallocatechin-3-Gallate Restore the Severe Impairment of Mitochondria in Hippocampal Progenitor Cells from a Down Syndrome Mouse Model. Biochim. Biophys. Acta 2016, 1862, 1093–1104. [Google Scholar] [CrossRef]
- Serra, D.; Paixão, J.; Nunes, C.; Dinis, T.C.P.; Almeida, L.M. Cyanidin-3-Glucoside Suppresses Cytokine-Induced Inflammatory Response in Human Intestinal Cells: Comparison with 5-Aminosalicylic Acid. PLoS ONE 2013, 8, 73001. [Google Scholar] [CrossRef]
- Serra, D.; Rufino, A.T.; Mendes, A.F.; Almeida, L.M.; Dinis, T.C.P. Resveratrol Modulates Cytokine-Induced JAK/STAT Activation More Efficiently than 5-Aminosalicylic Acid: An In Vitro Approach. PLoS ONE 2014, 9, e109048. [Google Scholar] [CrossRef]
- Sunand, K.; Mohan, G.K.; Bakshi, V. Synergetic Potential of Combination Probiotic Complex with Phytopharmaceuticals in Valproic Acid Induced Autism: Prenatal Model. Int. J. Appl. Pharm. Sci. Res. 2021, 6, 33–43. [Google Scholar] [CrossRef]
- Avramovich-Tirosh, Y.; Reznichenko, L.; Amit, T.; Zheng, H.; Fridkin, M.; Weinreb, O.; Mandel, S.; Youdim, M. Neurorescue Activity, APP Regulation and Amyloid-Beta Peptide Reduction by Novel Multi-Functional Brain Permeable Iron- Chelating- Antioxidants, M-30 and Green Tea Polyphenol, EGCG. Curr. Alzheimer Res. 2007, 4, 403–411. [Google Scholar] [CrossRef]
- Pogačnik, L.; Pirc, K.; Palmela, I.; Skrt, M.; Kwang, K.S.; Brites, D.; Brito, M.A.; Ulrih, N.P.; Silva, R.F.M. Potential for Brain Accessibility and Analysis of Stability of Selected Flavonoids in Relation to Neuroprotection in Vitro. Brain Res. 2016, 1651, 17–26. [Google Scholar] [CrossRef]
- Porath, D.; Riegger, C.; Drewe, J.; Schwager, J. Epigallocatechin-3-Gallate Impairs Chemokine Production in Human Colon Epithelial Cell Lines. J. Pharmacol. Exp. Ther. 2005, 315, 1172–1180. [Google Scholar] [CrossRef]
- Frye, R.E.; Rossignol, D.A. Mitochondrial Dysfunction Can Connect the Diverse Medical Symptoms Associated with Autism Spectrum Disorders. Pediatr. Res. 2011, 69 Pt 2, 41–47. [Google Scholar] [CrossRef]
- Ming, X.; Stein, T.P.; Barnes, V.; Rhodes, N.; Guo, L. Metabolic Perturbance in Autism Spectrum Disorders: A Metabolomics Study. J. Proteome Res. 2012, 11, 5856–5862. [Google Scholar] [CrossRef]
- Frye, R.E.; Rose, S.; Slattery, J.; MacFabe, D.F. Gastrointestinal Dysfunction in Autism Spectrum Disorder: The Role of the Mitochondria and the Enteric Microbiome. Microb. Ecol. Health Dis. 2015, 26, 27458. [Google Scholar] [CrossRef]
- Sergent, T.; Piront, N.; Meurice, J.; Toussaint, O.; Schneider, Y.J. Anti-Inflammatory Effects of Dietary Phenolic Compounds in an in Vitro Model of Inflamed Human Intestinal Epithelium. Chem. Biol. Interact. 2010, 188, 659–667. [Google Scholar] [CrossRef]
- Agarwala, S.; Naik, B.; Ramachandra, N.B. Mucosa-Associated Specific Bacterial Species Disrupt the Intestinal Epithelial Barrier in the Autism Phenome. Brain Behav. Immun.-Health 2021, 15, 100269. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S RRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered Gut Microbiota and Short Chain Fatty Acids in Chinese Children with Autism Spectrum Disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, W.; Zhang, J.; He, Y.; Wang, J. Analysis of Gut Microbiota Profiles and Microbe-Disease Associations in Children with Autism Spectrum Disorders in China. Sci. Rep. 2018, 8, 13981. [Google Scholar] [CrossRef]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut Microbiome Metagenomics Analysis Suggests a Functional Model for the Development of Autoimmunity for Type 1 Diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef]
- Rose, S.; Bennuri, S.C.; Murray, K.F.; Buie, T.; Winter, H.; Frye, R.E. Mitochondrial Dysfunction in the Gastrointestinal Mucosa of Children with Autism: A Blinded Case-Control Study. PLoS ONE 2017, 12, e0186377. [Google Scholar] [CrossRef]
- Rose, S.; Bennuri, S.C.; Davis, J.E.; Wynne, R.; Slattery, J.C.; Tippett, M.; Delhey, L.; Melnyk, S.; Kahler, S.G.; MacFabe, D.F.; et al. Butyrate Enhances Mitochondrial Function during Oxidative Stress in Cell Lines from Boys with Autism. Transl. Psychiatry 2018, 8, 42. [Google Scholar] [CrossRef]
- Cristiano, C.; Hoxha, E.; Lippiello, P.; Balbo, I.; Russo, R.; Tempia, F.; Miniaci, M.C. Maternal Treatment with Sodium Butyrate Reduces the Development of Autism-like Traits in Mice Offspring. Biomed. Pharmacother. 2022, 156, 113870. [Google Scholar] [CrossRef]
- Kang, D.W.; Ilhan, Z.E.; Isern, N.G.; Hoyt, D.W.; Howsmon, D.P.; Shaffer, M.; Lozupone, C.A.; Hahn, J.; Adams, J.B.; Krajmalnik-Brown, R. Differences in Fecal Microbial Metabolites and Microbiota of Children with Autism Spectrum Disorders. Anaerobe 2018, 49, 121–131. [Google Scholar] [CrossRef]
- Andriamihaja, M.; Lan, A.; Beaumont, M.; Audebert, M.; Wong, X.; Yamada, K.; Yin, Y.; Tomé, D.; Carrasco-Pozo, C.; Gotteland, M.; et al. The Deleterious Metabolic and Genotoxic Effects of the Bacterial Metabolite P-Cresol on Colonic Epithelial Cells. Free Radic. Biol. Med. 2015, 85, 219–227. [Google Scholar] [CrossRef]
- Unno, T.; Ichitani, M. Epigallocatechin-3-Gallate Decreases Plasma and Urinary Levels of p-Cresol by Modulating Gut Microbiota in Mice. ACS Omega 2022, 7, 40034–40041. [Google Scholar] [CrossRef]
- Unno, K.; Pervin, M.; Nakagawa, A.; Iguchi, K.; Hara, A.; Takagaki, A.; Nanjo, F.; Minami, A.; Nakamura, Y. Blood–Brain Barrier Permeability of Green Tea Catechin Metabolites and Their Neuritogenic Activity in Human Neuroblastoma SH-SY5Y Cells. Mol. Nutr. Food Res. 2017, 61, 1700294. [Google Scholar] [CrossRef]
- Kohri, T.; Matsumoto, N.; Yamakawa, M.; Suzuki, M.; Nanjo, F.; Hara, Y.; Oku, N. Metabolic Fate of (−)-[4-3H]Epigallocatechin Gallate in Rats after Oral Administration. Am. Chem. Soc. 2001, 49, 4102–4112. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Nakagawa, A.; Takahashi, Y.; Iguchi, K.; Yamamoto, H.; Hoshino, M.; Hara, A.; Takagaki, A.; Nanjo, F.; et al. Blood Brain Barrier Permeability of (-)-Epigallocatechin Gallate, Its Proliferation-Enhancing Activity of Human Neuroblastoma SH-SY5Y Cells, and Its Preventive Effect on Age-Related Cognitive Dysfunction in Mice. Biochem. Biophys. Rep. 2017, 9, 180–186. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and Its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef]
- Lambert, J.D.; Rice, J.E.; Hong, J.; Hou, Z.; Yang, C.S. Synthesis and Biological Activity of the Tea Catechin Metabolites, M4 and M6 and Their Methoxy-Derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 873–876. [Google Scholar] [CrossRef]
- Kim, Y.H.; Won, Y.S.; Yang, X.; Kumazoe, M.; Yamashita, S.; Hara, A.; Takagaki, A.; Goto, K.; Nanjo, F.; Tachibana, H. Green Tea Catechin Metabolites Exert Immunoregulatory Effects on CD4(+) T Cell and Natural Killer Cell Activities. J. Agric. Food Chem. 2016, 64, 3591–3597. [Google Scholar] [CrossRef]
- Chen, L.; Lee, M.-J.; Li, H.; Yang, C.S. Absorption, Distribution, and Elimination of Tea Polyphenols in Rats. Drug Metab. Dispos. 1997, 25, 1045–1050. [Google Scholar]
- Kim, S.; Lee, M.J.; Hong, J.; Li, C.; Smith, T.J.; Yang, G.Y.; Seril, D.N.; Yang, C.S. Plasma and Tissue Levels of Tea Catechins in Rats and Mice during Chronic Consumption of Green Tea Polyphenols. Nutr. Cancer 2000, 37, 41–48. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-Drug Loaded Nanoparticles of Epigallocatechin-3-Gallate (EGCG)/Ascorbic Acid Enhance Therapeutic Efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s Disease Mice Model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Barro, L.; Tsai, S.T.; Feng, T.W.; Wu, X.Y.; Chao, C.W.; Yu, R.S.; Chin, T.Y.; Hsieh, M.F. Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection. Int. J. Mol. Sci. 2021, 22, 3037. [Google Scholar] [CrossRef]
- Abbasalipour, H.; Hajizadeh Moghaddam, A.; Ranjbar, M. Sumac and Gallic Acid-Loaded Nanophytosomes Ameliorate Hippocampal Oxidative Stress via Regulation of Nrf2/Keap1 Pathway in Autistic Rats. J. Biochem. Mol. Toxicol. 2022, 36, e23035. [Google Scholar] [CrossRef]
- Mindell, J.A.; Meltzer, L.J.; Carskadon, M.A.; Chervin, R.D. Developmental Aspects of Sleep Hygiene: Findings from the 2004 National Sleep Foundation Sleep in America Poll. Sleep Med. 2009, 10, 771–779. [Google Scholar] [CrossRef]
- Samanta, P.; Panigrahi, A.; Senapati, L.K.; Mishra, D.P.; Ravan, J.R.; Mishra, J. Maladaptive Behavior and Associated Factors among Young Children with Autism. Indian J. Pediatr. 2022, 89, 1134–1136. [Google Scholar] [CrossRef]
- Evans, M.O.; Starley, B.; Galagan, J.C.; Yabes, J.M.; Evans, S.; Salama, J.J. Tea and Recurrent Clostridium Difficile Infection. Gastroenterol. Res. Pract. 2016, 2016, 4514687. [Google Scholar] [CrossRef]
- Vossoughinia, H.; Salari, M.; Amirmajdi, E.M.; Saadatnia, H.; Abedini, S.; Shariati, A.; Shariati, M.; Khorashad, A.K. An Epidemiological Study of Gastroesophageal Reflux Disease and Related Risk Factors in Urban Population of Mashhad, Iran. Iran. Red Crescent Med. J. 2014, 16, e15832. [Google Scholar] [CrossRef]
- Buie, T.; Campbell, D.B.; Fuchs, G.J.; Furuta, G.T.; Levy, J.; Van De Water, J.; Whitaker, A.H.; Atkins, D.; Bauman, M.L.; Beaudet, A.L.; et al. Evaluation, Diagnosis, and Treatment of Gastrointestinal Disorders in Individuals with ASDs: A Consensus Report. Pediatrics 2010, 125 (Suppl. S1), S1–S18. [Google Scholar] [CrossRef]
- Kucera, O.; Mezera, V.; Moravcova, A.; Endlicher, R.; Lotkova, H.; Drahota, Z.; Cervinkova, Z. In Vitro Toxicity of Epigallocatechin Gallate in Rat Liver Mitochondria and Hepatocytes. Oxid. Med. Cell. Longev. 2015, 2015, 476180. [Google Scholar] [CrossRef]
- Galati, G.; Lin, A.; Sultan, A.M.; O’Brien, P.J. Cellular and in Vivo Hepatotoxicity Caused by Green Tea Phenolic Acids and Catechins. Free Radic. Biol. Med. 2006, 40, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Mezera, V.; Kucera, O.; Moravcova, A.; Peterova, E.; Cervinkova, Z. The Effect of Epigallocatechin Gallate on Hepatocytes Isolated from Normal and Partially Hepatectomized Rats. Can. J. Physiol. Pharmacol. 2014, 92, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.-H.S.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and Safety of Green Tea Polyphenols after Multiple-Dose Administration of Epigallocatechin Gallate and Polyphenon E in Healthy Individuals1. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar] [PubMed]
- Abe, S.K.; Inoue, M. Green Tea and Cancer and Cardiometabolic Diseases: A Review of the Current Epidemiological Evidence. Eur. J. Clin. Nutr. 2021, 75, 865–876. [Google Scholar] [CrossRef] [PubMed]
| Study | Study Type | Population/Sample Size | Intervention | Results | Ref. |
|---|---|---|---|---|---|
| Ankolekar C et al., 2011 | Analytical Longitudinal Experimental | Cultures of Helicobacter pylori Lactic acid bacteria strains such as B. longum, L. acidophilus, and L. plantarum | They were exposed to tea preparations with rest times of 2 and 5 min | The 4 types of tea inhibited H. pylori | [47] |
| Shin JS et al., 2007 | Analytical Cross-sectional Experimental | Cultures of bacteria like S. aureus and E. coli | The antibacterial activities of the main phenolic components of Camellia sinensis | (-)-epicatechin is more toxic against S. aureus and E. coli | [48] |
| Nakayama M et al., 2012 | Analytical Longitudinal Experimental | Different cultures: E. coli O157:H7 producing Stx 1 and Stx 2 S. aureus NBRC 13276 | Green tea extract concentration was 0.0625 mg/mL for S. aureus and 1.0 mg/mL for E. coli O157:H7. | Green tea extract (GTE) inhibits the absorption and secretion of substrates and inhibits enzyme activity in both species | [49] |
| Bancirova M et al., 2010 | Analytical Cross-sectional Experimental | Cultures of standard strains of Enterococcus faecalis 4224, S. aureus 3953 and 4223, P. aeruginosa 3955 and E. coli 3954 and 3988 | Tea infusion (2 g/100 mL) | E. faecalis was the most resistant bacterial strain and P. aeruginosa was the least resistant bacterial strain. But the predominant antimicrobial activity of non-fermented tea infusions was not confirmed. | [52] |
| Liu Z et al., 2020 | Analytical Cross-sectional Experimental | Cultures of fecal material from four human volunteers | Treatment with catechin concentration of 0.1 mmol/L during 48 h. | EGCG increases the beneficial bacteria Bacteroides, Christensenellaceae, and Bifidobacterium. And inhibited the pathogenic bacteria Fusobacterium varium, Bilophila, and Enterobacteriaceae. | [65] |
| Trovò L et al., 2020 | Analytical Longitudinal Experimental | In vivo study with Cdkl5-KO mice N = 5–6 | Primary hippocampal neurons were treated daily with EGCG [1 μM, 0.5 μM, 1 μM, and 3 μM] or harmine | Treatment with EGCG efficiently restores defects in the dendritic and synaptic development of Cdkl5-KO hippocampal neurons. | [57] |
| Kumaravel P et al., 2017 | Analytical Longitudinal Experimental | In vivo study with Wistar rats N = 6 per group | Rats were treated with EGCG in doses of 1, 2, and 5 mg/kg body weight via oral administration. | EGCG ameliorates and reverses autistic attributes possibly due to its neuroprotective activity. | [58] |
| Ushiroda C et al., 2019 | Analytical Longitudinal Experimental | In vivo study with male C57BL/6 N mice | EGCG at a concentration of 0.32% for 8 weeks | EGCG increases the abundance of Adlercreutzia, Akkermansia, and Allobaculum and a significantly lower abundance of Desulfovibrionaceae | [67] |
| Qu Y et al., 2022 | Analytical Longitudinal Experimental | In vivo study with female C57BL/6 mice (8–13 weeks old) | EGCG [45 mg/kg] was administered for 8 weeks. The mice were then subjected to behavioral and gut microbiota analysis | EGCG effectively increased Prevotella and decreased Bifidobacteriales but had no effect on Alloprevotella or Lactobacillaceae | [68] |
| Unno T et al., 2014 | Analytical Longitudinal Experimental | In vivo study with four-week-old male Wistar rats N= 7 per group | Rats were fed an assigned diet of either a control diet, a 0.3% (w/w) EGCG diet, or a 0.6% (w/w) EGCG diet for 4 weeks | Dietary EGCG affects the growth of certain species of gut microbiota which could be responsible for regulating energy metabolism in the body | [72] |
| Wu Z et al., 2021 | Analytical Longitudinal Experimental | In vivo study with seven- to eight-week-old specifc pathogen-free (SPF) female C57BL/6J mice N = 8 per group | Modulated the gut microbial community of mice by the EGCG [50 mg/kg] pre-supplementation | Oral EGCG suppressed DSS-induced oxidative stress in the intestinal mucosa, improved the barrier function, and regulated the composition and SCFAs production of gut microbiota | [88] |
| Wu Z et al., 2020 | Analytical Longitudinal Experimental | In vivo study with seven- to eight-week-old specific pathogen-free (SPF) female C57L/6J mice | Tested the effect of oral or rectal administration of EGCG [50 mg/kg] | EGCG enriched Akkermansia, Faecalibaculum, and Bifidobacterium and enhanced acetate, propionate, and butyrate production. | [89] |
| Sheng L et al., 2018 | Analytical Longitudinal Experimental | In vivo study with specific pathogen-free male C57BL/6 wild-type (WT) mice N = 16 | Mice received vehicle (PBS) or EGCG daily [100 mg/d per gram body weight, orally] for 2 mo. | Increased Verrucomicrobiaceae and Akkermansia muciniphila. | [92] |
| Liu X et al., 2020 | Analytical Longitudinal Experimental | In vivo study with C57BL/6J male mice N = 8 | C57BL/6J mice fed with HFD were administrated with 210 mg/kg EGCG for 12 weeks. | Enriched the Verrucomicrobia and decreased the Firmicutes and Saccharibacteria. | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Rubia Ortí, J.E.; Moneti, C.; Serrano-Ballesteros, P.; Castellano, G.; Bayona-Babiloni, R.; Carriquí-Suárez, A.B.; Motos-Muñoz, M.; Proaño, B.; Benlloch, M. Liposomal Epigallocatechin-3-Gallate for the Treatment of Intestinal Dysbiosis in Children with Autism Spectrum Disorder: A Comprehensive Review. Nutrients 2023, 15, 3265. https://doi.org/10.3390/nu15143265
de la Rubia Ortí JE, Moneti C, Serrano-Ballesteros P, Castellano G, Bayona-Babiloni R, Carriquí-Suárez AB, Motos-Muñoz M, Proaño B, Benlloch M. Liposomal Epigallocatechin-3-Gallate for the Treatment of Intestinal Dysbiosis in Children with Autism Spectrum Disorder: A Comprehensive Review. Nutrients. 2023; 15(14):3265. https://doi.org/10.3390/nu15143265
Chicago/Turabian Stylede la Rubia Ortí, Jose Enrique, Costanza Moneti, Pilar Serrano-Ballesteros, Gloria Castellano, Raquel Bayona-Babiloni, Ana Belén Carriquí-Suárez, María Motos-Muñoz, Belén Proaño, and María Benlloch. 2023. "Liposomal Epigallocatechin-3-Gallate for the Treatment of Intestinal Dysbiosis in Children with Autism Spectrum Disorder: A Comprehensive Review" Nutrients 15, no. 14: 3265. https://doi.org/10.3390/nu15143265
APA Stylede la Rubia Ortí, J. E., Moneti, C., Serrano-Ballesteros, P., Castellano, G., Bayona-Babiloni, R., Carriquí-Suárez, A. B., Motos-Muñoz, M., Proaño, B., & Benlloch, M. (2023). Liposomal Epigallocatechin-3-Gallate for the Treatment of Intestinal Dysbiosis in Children with Autism Spectrum Disorder: A Comprehensive Review. Nutrients, 15(14), 3265. https://doi.org/10.3390/nu15143265









