Dietary Folic Acid Supplementation Attenuates Maternal High-Fat Diet-Induced Fetal Intrauterine Growth Retarded via Ameliorating Placental Inflammation and Oxidative Stress in Rats
Abstract
1. Introduction
2. Materials and Methods
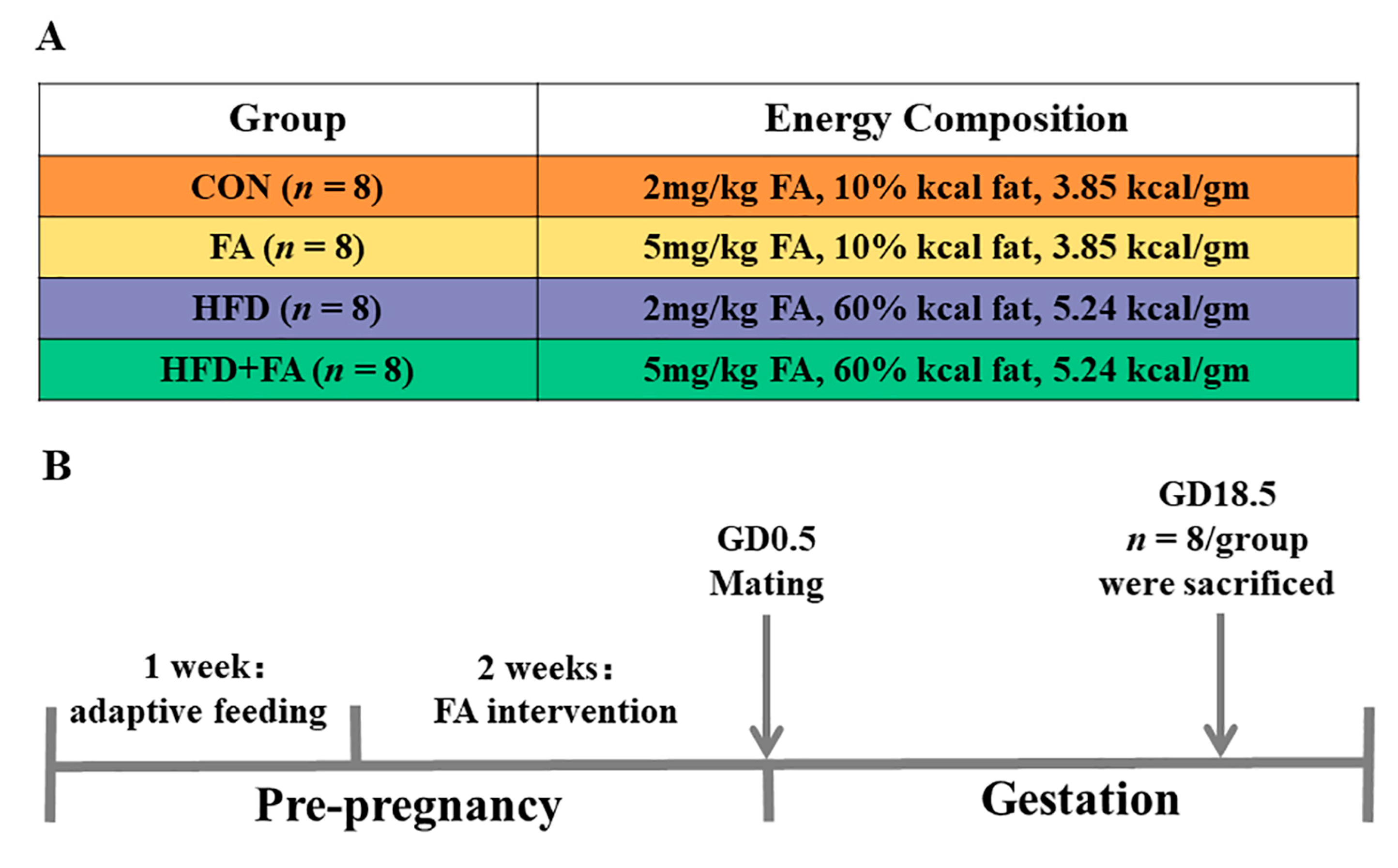
2.1. Animals and Experimental Design
2.2. Histological Analysis of Placenta
2.3. Maternal Serum Biochemical Assays
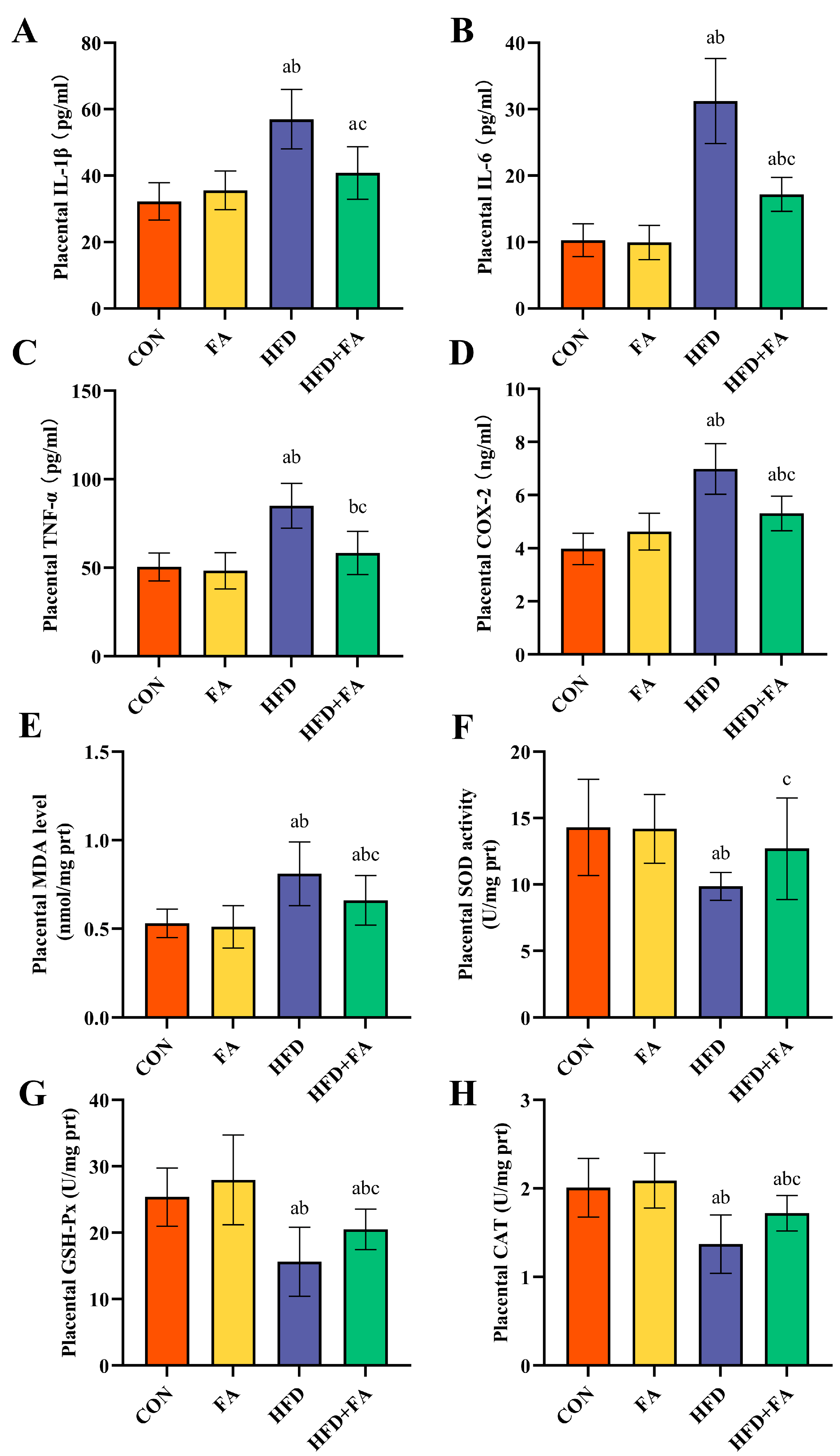
2.4. Placental Biochemical Assays
2.5. Western Blotting Analysis
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
3.1. Maternal Body Weight, Liver Weight, and Adipose Tissue Weight at GD18.5
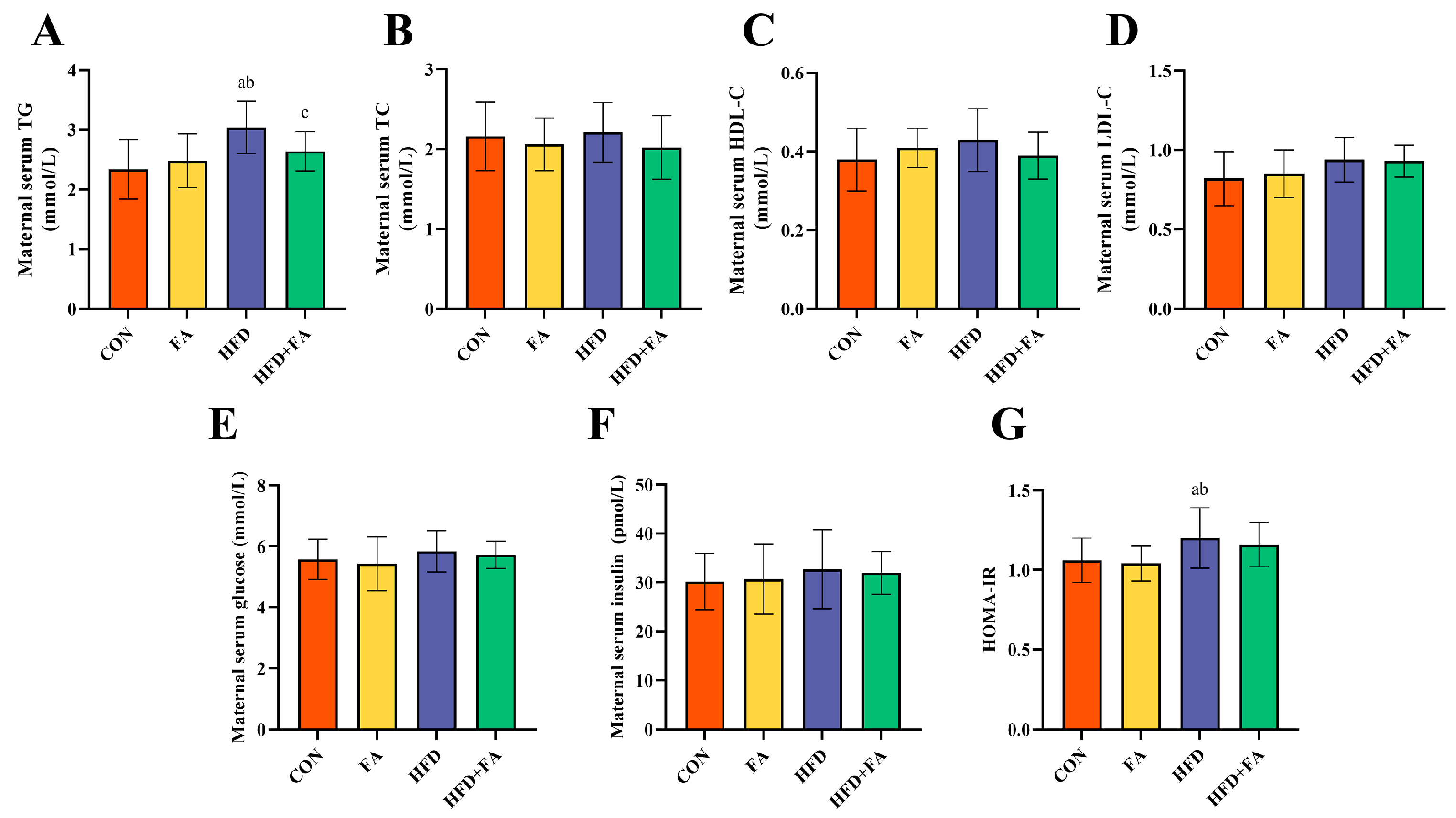
3.2. Maternal Serum Biochemical Indexes at GD18.5
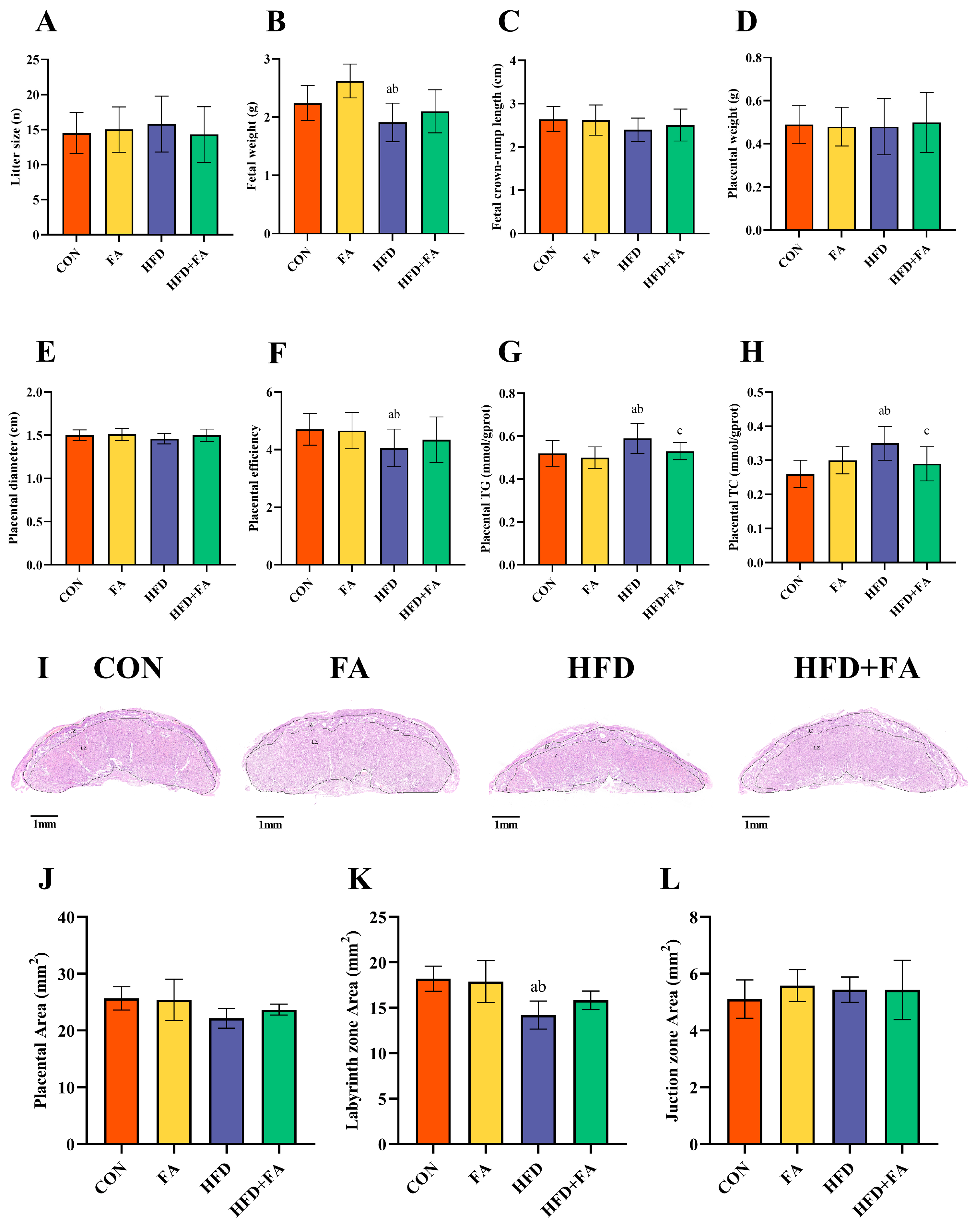
3.3. Maternal FA Supplementation Ameliorates Fetal and Placental Development at GD18.5
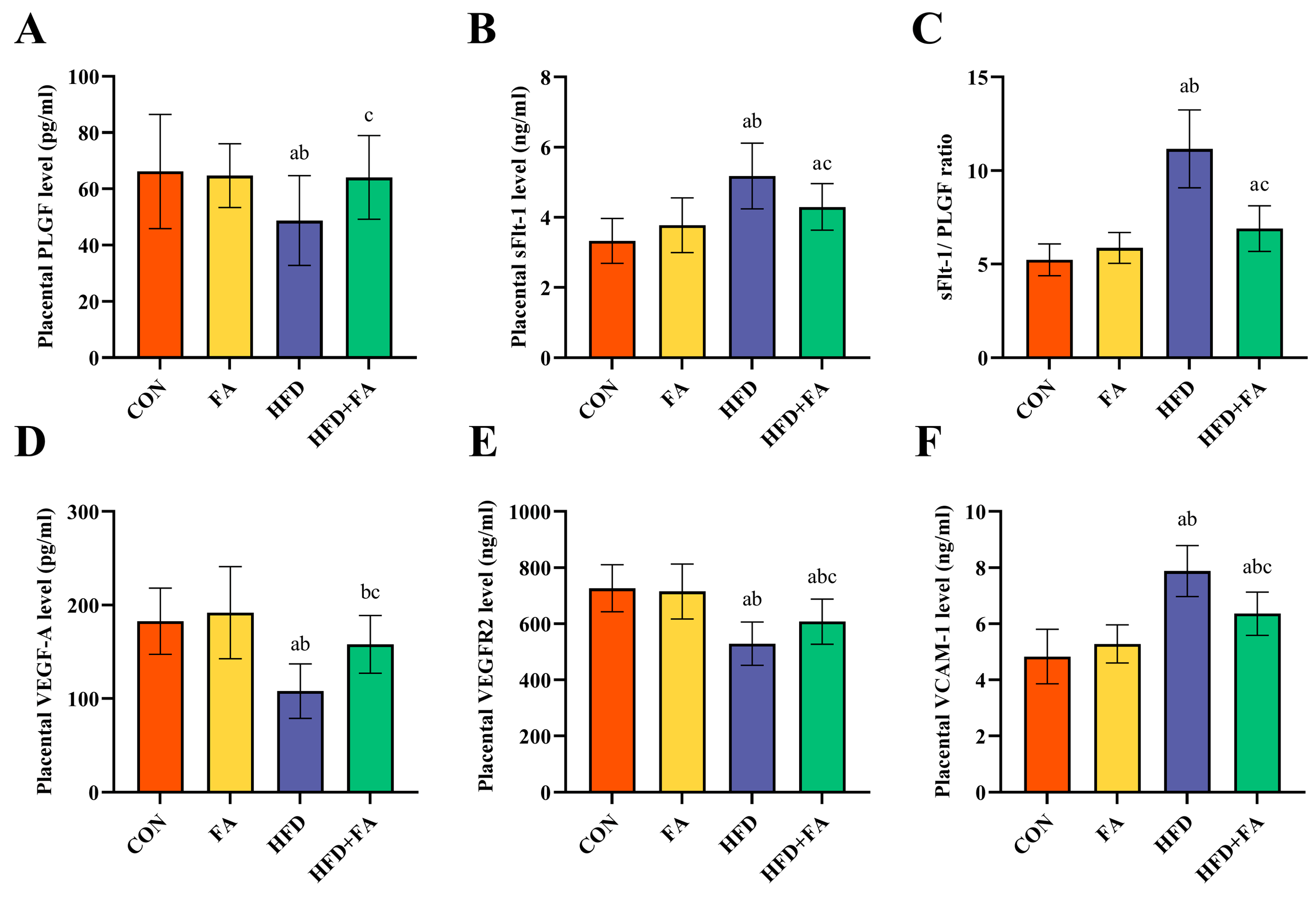
3.4. Maternal FA Supplementation Ameliorates Placental Angiogenesis at GD18.5
3.5. Maternal FA Supplementation Ameliorates Placental Inflammation and Oxidative Stress
3.6. Effects of Maternal FA Supplementation on SIRT1-Mediated Inflammatory and Oxidative Stress Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Freitag, N.; Tirado-Gonzalez, I.; Barrientos, G.; Powell, K.L.; Boehm-Sturm, P.; Koch, S.P.; Hecher, K.; Staff, A.C.; Arck, P.C.; Diemert, A.; et al. Galectin-3 deficiency in pregnancy increases the risk of fetal growth restriction (FGR) via placental insufficiency. Cell Death Dis. 2020, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Berends, L.M.; Fernandez-Twinn, D.S.; Martin-Gronert, M.S.; Cripps, R.L.; Ozanne, S.E. Catch-up growth following intra-uterine growth-restriction programmes an insulin-resistant phenotype in adipose tissue. Int. J. Obes. 2013, 37, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bernal, C.L.; Rebagliato, M.; Iñiguez, C.; Vioque, J.; Navarrete-Muñoz, E.M.; Murcia, M.; Bolumar, F.; Marco, A.; Ballester, F. Diet quality in early pregnancy and its effects on fetal growth outcomes: The Infancia y Medio Ambiente (Childhood and Environment) Mother and Child Cohort Study in Spain. Am. J. Clin. Nutr. 2010, 91, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, N.; Nagata, C.; Yasuo, S.; Morokuma, S.; Kato, K.; Sanefuji, M.; Shibata, E.; Tsuji, M.; Senju, A.; Kawamoto, T.; et al. Optimal protein intake during pregnancy for reducing the risk of fetal growth restriction: The Japan Environment and Children’s Study. Br. J. Nutr. 2018, 120, 1432–1440. [Google Scholar] [CrossRef]
- Connor, K.L.; Kibschull, M.; Matysiak-Zablocki, E.; Nguyen, T.T.-T.N.; Matthews, S.G.; Lye, S.J.; Bloise, E. Maternal malnutrition impacts placental morphology and transporter expression: An origin for poor offspring growth. J. Nutr. Biochem. 2020, 78, 108329. [Google Scholar] [CrossRef]
- Kretschmer, T.; Turnwald, E.-M.; Janoschek, R.; Zentis, P.; Bae-Gartz, I.; Beers, T.; Handwerk, M.; Wohlfarth, M.; Ghilav, M.; Bloch, W.; et al. Maternal high fat diet-induced obesity affects trophoblast differentiation and placental function in mice. Biol. Reprod. 2020, 103, 1260–1274. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef]
- Saben, J.; Lindsey, F.; Zhong, Y.; Thakali, K.; Badger, T.M.; Andres, A.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity is associated with a lipotoxic placental environment. Placenta 2014, 35, 171–177. [Google Scholar] [CrossRef]
- Sanches, A.P.V.; de Oliveira, J.L.; Ferreira, M.S.; Lima, B.d.S.; Miyamoto, J.É.; Simino, L.A.d.P.; Torsoni, M.A.; Torsoni, A.S.; Milanski, M.; Ignácio-Souza, L. Obesity phenotype induced by high-fat diet leads to maternal-fetal constraint, placental inefficiency, and fetal growth restriction in mice. J. Nutr. Biochem. 2022, 104, 108977. [Google Scholar] [CrossRef]
- Xu, F.; Ren, Z.X.; Zhong, X.M.; Zhang, Q.; Zhang, J.Y.; Yang, J. Intrauterine Inflammation Damages Placental Angiogenesis via Wnt5a-Flt1 Activation. Inflammation 2019, 42, 818–825. [Google Scholar] [CrossRef]
- Catov, J.M.; Bodnar, L.M.; Olsen, J.; Olsen, S.; Nohr, E.A. Periconceptional multivitamin use and risk of preterm or small-for-gestational-age births in the Danish National Birth Cohort. Am. J. Clin. Nutr. 2011, 94, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, X.; Zheng, X.; Gao, J.; Shang, M.; Xu, J.; Liang, H. Folic Acid Protects against Hyperuricemia in C57BL/6J Mice via Ameliorating Gut-Kidney Axis Dysfunction. J. Agric. Food Chem. 2022, 70, 15787–15803. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, P.; Zuo, Y.; Wang, Y.; Zhao, H.; Lan, T.; Xue, M.; Zhang, H.; Liang, H. Folic acid intervention changes liver Foxp3 methylation and ameliorates the damage caused by Th17/Treg imbalance after long-term alcohol exposure. Food Funct. 2022, 13, 5262–5274. [Google Scholar] [CrossRef]
- Zhang, H.; Zuo, Y.; Zhao, H.; Zhao, H.; Wang, Y.; Zhang, X.; Zhang, J.; Wang, P.; Sun, L.; Zhang, H.; et al. Folic acid ameliorates alcohol-induced liver injury via gut-liver axis homeostasis. Front. Nutr. 2022, 9, 989311. [Google Scholar] [CrossRef]
- Berry, R.J.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.Y.; Gindler, J.; et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med. 1999, 341, 1485–1490. [Google Scholar] [CrossRef]
- Luan, Y.; Leclerc, D.; Cosín-Tomás, M.; Malysheva, O.V.; Wasek, B.; Bottiglieri, T.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Altered Methyl Metabolism and in Sex-Specific Placental Transcription Changes. Mol. Nutr. Food Res. 2021, 65, e2100197. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.W.; Zhou, J.; Yang, Q.; Fraser, W.; Olatunbosun, O.; Walker, M. Maternal exposure to folic acid antagonists and placenta-mediated adverse pregnancy outcomes. CMAJ 2008, 179, 1263–1268. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, Y.-H.; Dong, X.-T.; Zhou, J.; Chen, X.; Wang, H.; Wu, S.-X.; Xia, M.-Z.; Zhang, C.; Xu, D.-X. Folic acid protects against lipopolysaccharide-induced preterm delivery and intrauterine growth restriction through its anti-inflammatory effect in mice. PLoS ONE 2013, 8, e82713. [Google Scholar] [CrossRef]
- Xin, F.-Z.; Zhao, Z.-H.; Zhang, R.-N.; Pan, Q.; Gong, Z.-Z.; Sun, C.; Fan, J.-G. Folic acid attenuates high-fat diet-induced steatohepatitis deacetylase SIRT1-dependent restoration of PPARα. World J. Gastroenterol. 2020, 26, 2203–2220. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Fu, B.; Xiong, Y.; Xu, S.; Liu, J.; Zaky, M.Y.; Qiu, D.; Wu, H. olic acid Ameliorates the Declining Quality of Sodium Fluoride-Exposed Mouse Oocytes through the Sirt1/Sod2 Pathway. Aging Dis. 2022, 13, 1471–1487. [Google Scholar] [CrossRef]
- Peng, J.; Zhou, Y.; Hong, Z.; Wu, Y.; Cai, A.; Xia, M.; Deng, Z.; Yang, Y.; Song, T.; Xiong, J.; et al. Maternal eicosapentaenoic acid feeding promotes placental angiogenesis through a Sirtuin-1 independent inflammatory pathway. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Brien, M.-E.; Duval, C.; Palacios, J.; Boufaied, I.; Hudon-Thibeault, A.-A.; Nadeau-Vallée, M.; Vaillancourt, C.; Sibley, C.P.; Abrahams, V.M.; Jones, R.L.; et al. Uric Acid Crystals Induce Placental Inflammation and Alter Trophoblast Function via an IL-1-Dependent Pathway: Implications for Fetal Growth Restriction. J. Immunol. 2017, 198, 443–451. [Google Scholar] [CrossRef]
- Cotechini, T.; Komisarenko, M.; Sperou, A.; Macdonald-Goodfellow, S.; Adams, M.A.; Graham, C.H. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J. Exp. Med. 2014, 211, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Patruno, A.; Costantini, E.; Ferrone, A.; Pesce, M.; Diomede, F.; Trubiani, O.; Reale, M. Short ELF-EMF Exposure Targets SIRT1/Nrf2/HO-1 Signaling in THP-1 Cells. Int. J. Mol. Sci. 2020, 21, 7284. [Google Scholar] [CrossRef] [PubMed]
- Onda, K.; Tong, S.; Nakahara, A.; Kondo, M.; Monchusho, H.; Hirano, T.; Kaitu’u-Lino, T.u.; Beard, S.; Binder, N.; Tuohey, L.; et al. Sofalcone upregulates the nuclear factor (erythroid-derived 2)-like 2/heme oxygenase-1 pathway, reduces soluble fms-like tyrosine kinase-1, and quenches endothelial dysfunction: Potential therapeutic for preeclampsia. Hypertension 2015, 65, 855–862. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, S.; Zhao, H.; Liu, Y.; Xue, M.; Zhang, H.; Qiu, X.; Sun, Z.; Liang, H. Protective effects of fucoidan against ethanol-induced liver injury through maintaining mitochondrial function and mitophagy balance in rats. Food Funct. 2021, 12, 3842–3854. [Google Scholar] [CrossRef]
- Wang, P.; Guo, P.; Wang, Y.; Teng, X.; Zhang, H.; Sun, L.; Xue, M.; Liang, H. Propolis Ameliorates Alcohol-Induced Depressive Symptoms in C57BL/6J Mice by Regulating Intestinal Mucosal Barrier Function and Inflammatory Reaction. Nutrients 2022, 14, 1213. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, Y.; Zuo, Y.; Zhang, X.; Gao, Y.; Wang, P.; Sun, L.; Zhang, H.; Liang, H. Nicotinamide riboside ameliorates high-fructose-induced lipid metabolism disorder in mice via improving FGF21 resistance in the liver and white adipose tissue. Food Funct. 2022, 13, 12400–12411. [Google Scholar] [CrossRef]
- Rao, L.G. Intrauterine growth retardation. Lancet 1979, 1, 976. [Google Scholar] [CrossRef]
- Kretschmer, T.; Schulze-Edinghausen, M.; Turnwald, E.-M.; Janoschek, R.; Bae-Gartz, I.; Zentis, P.; Handwerk, M.; Wohlfarth, M.; Schauss, A.; Hucklenbruch-Rother, E.; et al. Effect of Maternal Obesity in Mice on IL-6 Levels and Placental Endothelial Cell Homeostasis. Nutrients 2020, 12, 296. [Google Scholar] [CrossRef]
- Hayes, E.K.; Lechowicz, A.; Petrik, J.J.; Storozhuk, Y.; Paez-Parent, S.; Dai, Q.; Samjoo, I.A.; Mansell, M.; Gruslin, A.; Holloway, A.C.; et al. Adverse fetal and neonatal outcomes associated with a life-long high fat diet: Role of altered development of the placental vasculature. PLoS ONE 2012, 7, e33370. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Yu, H.-R.; Tiao, M.-M.; Tain, Y.-L.; Lin, I.C.; Sheen, J.-M.; Lin, Y.-J.; Chang, K.-A.; Chen, C.-C.; Tsai, C.-C.; et al. Maternal Obesity Related to High Fat Diet Induces Placenta Remodeling and Gut Microbiome Shaping That Are Responsible for Fetal Liver Lipid Dysmetabolism. Front. Nutr. 2021, 8, 736944. [Google Scholar] [CrossRef]
- Stuart, T.J.; O’Neill, K.; Condon, D.; Sasson, I.; Sen, P.; Xia, Y.; Simmons, R.A. Diet-induced obesity alters the maternal metabolome and early placenta transcriptome and decreases placenta vascularity in the mouse. Biol. Reprod. 2018, 98, 795–809. [Google Scholar] [CrossRef]
- Sun, S.; Cao, C.; Li, J.; Meng, Q.; Cheng, B.; Shi, B.; Shan, A. Lycopene Modulates Placental Health and Fetal Development Under High-Fat Diet During Pregnancy of Rats. Mol. Nutr. Food Res. 2021, 65, e2001148. [Google Scholar] [CrossRef]
- Xue, Q.; Chen, F.; Zhang, H.; Liu, Y.; Chen, P.; Patterson, A.J.; Luo, J. Maternal high-fat diet alters angiotensin II receptors and causes changes in fetal and neonatal rats†. Biol. Reprod. 2019, 100, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Sasson, I.E.; Vitins, A.P.; Mainigi, M.A.; Moley, K.H.; Simmons, R.A. Pre-gestational vs gestational exposure to maternal obesity differentially programs the offspring in mice. Diabetologia 2015, 58, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Yu, Z.; Fu, L.; Wang, H.; Chen, X.; Zhang, C.; Lv, Z.-M.; Xu, D.-X. Vitamin D3 inhibits lipopolysaccharide-induced placental inflammation through reinforcing interaction between vitamin D receptor and nuclear factor kappa B p65 subunit. Sci. Rep. 2015, 5, 10871. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Jaddoe, V.W.V.; Hofman, A.; Steegers-Theunissen, R.P.M.; Steegers, E.A.P. Periconception folic acid supplementation, fetal growth and the risks of low birth weight and preterm birth: The Generation R Study. Br. J. Nutr. 2009, 102, 777–785. [Google Scholar] [CrossRef]
- Li, N.; Li, Z.; Ye, R.; Liu, J.; Ren, A. Impact of Periconceptional Folic Acid Supplementation on Low Birth Weight and Small-for-Gestational-Age Infants in China: A Large Prospective Cohort Study. J. Pediatr. 2017, 187, 105–110. [Google Scholar] [CrossRef]
- Chmurzynska, A.; Stachowiak, M.; Gawecki, J.; Pruszynska-Oszmalek, E.; Tubacka, M. Protein and folic acid content in the maternal diet determine lipid metabolism and response to high-fat feeding in rat progeny in an age-dependent manner. Genes. Nutr. 2012, 7, 223–234. [Google Scholar] [CrossRef]
- Yang, X.; Huang, Y.; Sun, C.; Li, J. Maternal Prenatal Folic Acid Supplementation Programs Offspring Lipid Metabolism by Aberrant DNA Methylation in Hepatic ATGL and Adipose LPL in Rats. Nutrients 2017, 9, 935. [Google Scholar] [CrossRef] [PubMed]
- Lacko, L.A.; Hurtado, R.; Hinds, S.; Poulos, M.G.; Butler, J.M.; Stuhlmann, H. Altered feto-placental vascularization, feto-placental malperfusion and fetal growth restriction in mice with loss of function. Development 2017, 144, 2469–2479. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Jiang, J.; Yu, G.; Zhang, X.; Qi, X.; Zhang, J.; Zhang, L.; Wang, T. Dietary curcumin supplementation ameliorates placental inflammation in rats with intra-uterine growth retardation by inhibiting the NF-κB signaling pathway. J. Nutr. Biochem. 2022, 104, 108973. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zou, Y.; Liu, Y.; Yuan, L.; Garfield, R.E.; Liu, H. Nicotine protects fetus against LPS-induced fetal growth restriction through ameliorating placental inflammation and vascular development in late pregnancy in rats. Biosci. Rep. 2019, 39, BSR20190386. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Sun, B.; Boersma, G.J.; Cordner, Z.A.; Yan, J.; Moran, T.H.; Tamashiro, K.L.K. Prenatal high-fat diet alters placental morphology, nutrient transporter expression, and mtorc1 signaling in rat. Obesity 2017, 25, 909–919. [Google Scholar] [CrossRef]
- Furukawa, S.; Tsuji, N.; Sugiyama, A. Morphology and physiology of rat placenta for toxicological evaluation. J. Toxicol. Pathol. 2019, 32, 1–17. [Google Scholar] [CrossRef]
- Napso, T.; Lean, S.C.; Lu, M.; Mort, E.J.; Desforges, M.; Moghimi, A.; Bartels, B.; El-Bacha, T.; Fowden, A.L.; Camm, E.J.; et al. Diet-induced maternal obesity impacts feto-placental growth and induces sex-specific alterations in placental morphology, mitochondrial bioenergetics, dynamics, lipid metabolism and oxidative stress in mice. Acta Physiol. 2022, 234, e13795. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Miao, Y.; Wei, H.; Peng, J.; Zhou, Y. Diallyl Trisulfide Promotes Placental Angiogenesis by Regulating Lipid Metabolism and Alleviating Inflammatory Responses in Obese Pregnant Mice. Nutrients 2022, 14, 2230. [Google Scholar] [CrossRef]
- Kang, M.-C.; Park, S.J.; Kim, H.J.; Lee, J.; Yu, D.H.; Bae, K.B.; Ji, Y.R.; Park, S.J.; Jeong, J.; Jang, W.Y.; et al. Gestational loss and growth restriction by angiogenic defects in placental growth factor transgenic mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2276–2282. [Google Scholar] [CrossRef]
- Almeida-Toledano, L.; Andreu-Fernández, V.; Aras-López, R.; García-Algar, Ó.; Martínez, L.; Gómez-Roig, M.D. Epigallocatechin Gallate Ameliorates the Effects of Prenatal Alcohol Exposure in a Fetal Alcohol Spectrum Disorder-Like Mouse Model. Int. J. Mol. Sci. 2021, 22, 715. [Google Scholar] [CrossRef]
- Hosni, A.; El-Twab, S.A.; Abdul-Hamid, M.; Prinsen, E.; AbdElgawad, H.; Abdel-Moneim, A.; Beemster, G.T.S. Cinnamaldehyde mitigates placental vascular dysfunction of gestational diabetes and protects from the associated fetal hypoxia by modulating placental angiogenesis, metabolic activity and oxidative stress. Pharmacol. Res. 2021, 165, 105426. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.M.; El-Agamy, D.S.; Elsaed, W.M.; Sirwi, A.; Asfour, H.Z.; Koshak, A.E.; Elhady, S.S. Cucurbitacin E glucoside alleviates concanavalin A-induced hepatitis through enhancing SIRT1/Nrf2/HO-1 and inhibiting NF-ĸB/NLRP3 signaling pathways. J. Ethnopharmacol. 2022, 292, 115223. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Mitton, A.; Lim, R.; Barker, G.; Riley, C.; Permezel, M. SIRT1 is a novel regulator of key pathways of human labor. Biol. Reprod. 2011, 84, 167–178. [Google Scholar] [CrossRef]
- Peng, H.; Wei, M.; Huang, L.; Yan, Y.; Li, Q.; Li, S.; Wu, Y.; Gao, Y.; Xing, Y.; Luo, X. Maternal obesity inhibits placental angiogenesis by down-regulating the SIRT1/PGC-1α pathway. Ann. Transl. Med. 2022, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Song, C.; Chen, J.; Zhou, L.; Jiang, X.; Cao, X.; Sun, Y.; Zhang, Q. Limonin ameliorates acetaminophen-induced hepatotoxicity by activating Nrf2 antioxidative pathway and inhibiting NF-κB inflammatory response via upregulating Sirt1. Phytomedicine 2020, 69, 153211. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wu, Z.; Huang, Z.; Hao, X.; Zhang, L.; Hu, C.; Wei, J.; Deng, J.; Tan, C. Maternal supply of cysteamine alleviates oxidative stress and enhances angiogenesis in porcine placenta. J. Anim. Sci. Biotechnol. 2021, 12, 91. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Zhao, M.; Chen, X.; Zhang, Y.; Wang, H.; Huang, Y.-Y.; Wang, Z.; Zhang, Z.-H.; Zhang, C.; Xu, D.-X. Zinc supplementation during pregnancy protects against lipopolysaccharide-induced fetal growth restriction and demise through its anti-inflammatory effect. J. Immunol. 2012, 189, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Bagherieh, M.; Kheirollahi, A.; Zamani-Garmsiri, F.; Emamgholipour, S.; Meshkani, R. Folic acid ameliorates palmitate-induced inflammation through decreasing homocysteine and inhibiting NF-κB pathway in HepG2 cells. Arch. Physiol. Biochem. 2023, 129, 893–900. [Google Scholar] [CrossRef]
- Jones, M.L.; Mark, P.J.; Mori, T.A.; Keelan, J.A.; Waddell, B.J. Maternal dietary omega-3 fatty acid supplementation reduces placental oxidative stress and increases fetal and placental growth in the rat. Biol. Reprod. 2013, 88, 37. [Google Scholar] [CrossRef]
- Reyes-Hernández, C.G.; Ramiro-Cortijo, D.; Rodríguez-Rodríguez, P.; Giambelluca, S.; Simonato, M.; González, M.D.C.; López de Pablo, A.L.; López-Giménez, M.D.R.; Cogo, P.; Sáenz de Pipaón, M.; et al. Effects of Arachidonic and Docosohexahenoic Acid Supplementation during Gestation in Rats. Implication of Placental Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 3863. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Yan, B.; Zhao, H.; Wang, Y.; Gao, T.; Liang, H. Maternal high-fructose consumption provokes placental oxidative stress resulting in asymmetrical fetal growth restriction in rats. J. Clin. Biochem. Nutr. 2021, 69, 68–76. [Google Scholar] [CrossRef]
- Nezu, M.; Souma, T.; Yu, L.; Sekine, H.; Takahashi, N.; Wei, A.Z.-S.; Ito, S.; Fukamizu, A.; Zsengeller, Z.K.; Nakamura, T.; et al. Nrf2 inactivation enhances placental angiogenesis in a preeclampsia mouse model and improves maternal and fetal outcomes. Sci. Signal 2017, 10, eaam5711. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Cosín-Tomás, M.; Leclerc, D.; Malysheva, O.V.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Altered Sex-Specific Gene Expression in Brain of Young Mice and Embryos. Nutrients 2022, 14, 1051. [Google Scholar] [CrossRef] [PubMed]
- Ihirwe, R.G.; Martel, J.; Rahimi, S.; Trasler, J. Protective and sex-specific effects of moderate dose folic acid supplementation on the placenta following assisted reproduction in mice. FASEB J. 2023, 37, e22677. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, X.; Wang, Y.; Zhao, X.; Zhang, L.; Li, J.; Zhang, Y.; Wang, P.; Liang, H. Dietary Folic Acid Supplementation Attenuates Maternal High-Fat Diet-Induced Fetal Intrauterine Growth Retarded via Ameliorating Placental Inflammation and Oxidative Stress in Rats. Nutrients 2023, 15, 3263. https://doi.org/10.3390/nu15143263
Zhang H, Zhang X, Wang Y, Zhao X, Zhang L, Li J, Zhang Y, Wang P, Liang H. Dietary Folic Acid Supplementation Attenuates Maternal High-Fat Diet-Induced Fetal Intrauterine Growth Retarded via Ameliorating Placental Inflammation and Oxidative Stress in Rats. Nutrients. 2023; 15(14):3263. https://doi.org/10.3390/nu15143263
Chicago/Turabian StyleZhang, Huaqi, Xinyu Zhang, Yutong Wang, Xuenuo Zhao, Li Zhang, Jing Li, Yabin Zhang, Peng Wang, and Hui Liang. 2023. "Dietary Folic Acid Supplementation Attenuates Maternal High-Fat Diet-Induced Fetal Intrauterine Growth Retarded via Ameliorating Placental Inflammation and Oxidative Stress in Rats" Nutrients 15, no. 14: 3263. https://doi.org/10.3390/nu15143263
APA StyleZhang, H., Zhang, X., Wang, Y., Zhao, X., Zhang, L., Li, J., Zhang, Y., Wang, P., & Liang, H. (2023). Dietary Folic Acid Supplementation Attenuates Maternal High-Fat Diet-Induced Fetal Intrauterine Growth Retarded via Ameliorating Placental Inflammation and Oxidative Stress in Rats. Nutrients, 15(14), 3263. https://doi.org/10.3390/nu15143263





