Association of Dietary Intake of Zinc and Selenium with Breast Cancer Risk: A Case-Control Study in Chinese Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Measurement of Dietary Exposure
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Cases and Controls
3.2. Dietary Intake of Energy and Nutrients among Cases and Controls
3.3. The Linear Relationship between Dietary Zn, Se and Breast Cancer Risk
3.4. The Non-Linear Relationship between Dietary Zn, Se, and Breast Cancer Risk
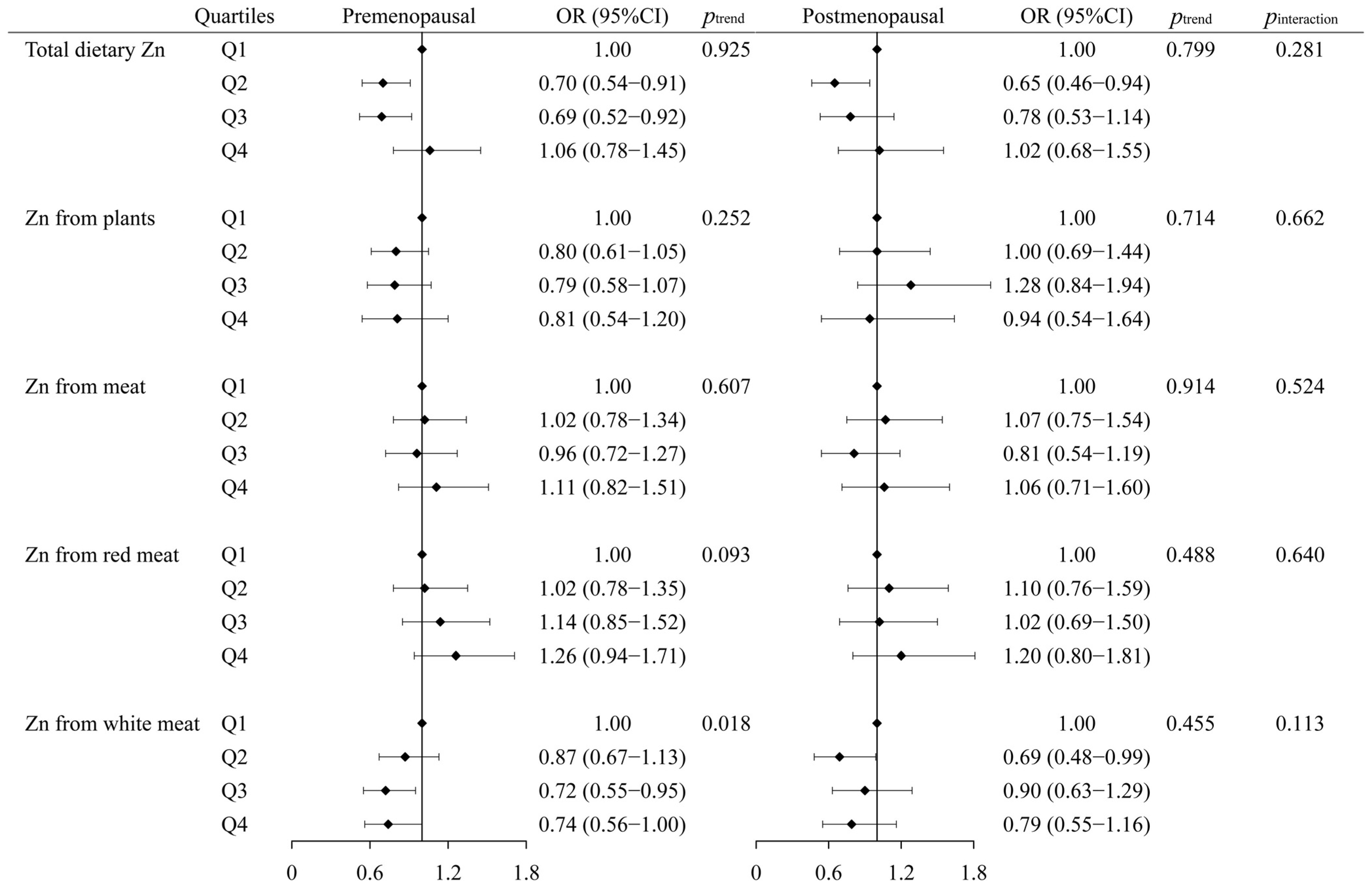
3.5. The Interaction between Zn, Se Intake, and Menopausal Status in Breast Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Wu, C.; Li, M.; Meng, H.; Liu, Y.; Niu, W.; Zhou, Y.; Zhao, R.; Duan, Y.; Zeng, Z.; Li, X.; et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci. China Life Sci. 2019, 62, 640–647. [Google Scholar] [CrossRef]
- Key, T.J.; Allen, N.E.; Spencer, E.A.; Travis, R.C. The effect of diet on risk of cancer. Lancet 2002, 360, 861–868. [Google Scholar] [CrossRef]
- Tas, F.; Hansel, H.; Belce, A.; Ilvan, S.; Argon, A.; Camlica, H.; Topuz, E. Oxidative stress in breast cancer. Med. Oncol. 2005, 22, 11–15. [Google Scholar] [CrossRef]
- Hecht, F.; Pessoa, C.F.; Gentile, L.B.; Rosenthal, D.; Carvalho, D.P.; Fortunato, R.S. The role of oxidative stress on breast cancer development and therapy. Tumour. Biol. 2016, 37, 4281–4291. [Google Scholar] [CrossRef]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Ntoupa, P.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef]
- Baltaci, S.B.; Mogulkoc, R.; Baltaci, A.K.; Emsen, A.; Artac, H. The effect of zinc and melatonin supplementation on immunity parameters in breast cancer induced by DMBA in rats. Arch. Physiol. Biochem. 2018, 124, 247–252. [Google Scholar] [CrossRef]
- Bobrowska-Korczak, B.; Gątarek, P.; Skrajnowska, D.; Bielecki, W.; Wyrebiak, R.; Kovalczuk, T.; Wrzesień, R.; Kałużna-Czaplińska, J. Effect of Zinc Supplementation on the Serum Metabolites Profile at the Early Stage of Breast Cancer in Rats. Nutrients 2020, 12, 3457. [Google Scholar] [CrossRef]
- Gulbahce-Mutlu, E.; Baltaci, S.B.; Menevse, E.; Mogulkoc, R.; Baltaci, A.K. The Effect of Zinc and Melatonin Administration on Lipid Peroxidation, IL-6 Levels, and Element Metabolism in DMBA-Induced Breast Cancer in Rats. Biol. Trace Elem. Res. 2021, 199, 1044–1051. [Google Scholar] [CrossRef]
- Iqbal, S.; Ali, I. Dietary Trace Element Intake and Risk of Breast Cancer: A Mini Review. Biol. Trace Elem. Res. 2022, 200, 4936–4948. [Google Scholar] [CrossRef]
- Adzersen, K.H.; Jess, P.; Freivogel, K.W.; Gerhard, I.; Bastert, G. Raw and cooked vegetables, fruits, selected micronutrients, and breast cancer risk: A case-control study in Germany. Nutr. Cancer 2003, 46, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Zhou, J.; Gibbons, L.; Morrison, H.; Wen, S.W. Antioxidants and breast cancer risk- a population-based case-control study in Canada. BMC Cancer 2011, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Pasche, C.; Lucchini, F.; La Vecchia, C. Dietary intake of selected micronutrients and breast-cancer risk. Int. J. Cancer 2001, 91, 260–263. [Google Scholar] [CrossRef]
- Bengtsson, Y.; Sandsveden, M.; Borgquist, S.; Manjer, J. Serum zinc and dietary intake of zinc in relation to risk of different breast cancer subgroups and serum levels as a marker of intake: A prospective nested case-control study. Breast Cancer Res. Treat. 2021, 189, 571–583. [Google Scholar] [CrossRef]
- Fernandez-Lazaro, C.I.; Martínez-González, M.; Aguilera-Buenosvinos, I.; Gea, A.; Ruiz-Canela, M.; Romanos-Nanclares, A.; Toledo, E. Dietary Antioxidant Vitamins and Minerals and Breast Cancer Risk: Prospective Results from the SUN Cohort. Antioxidants 2021, 10, 340. [Google Scholar] [CrossRef]
- Hu, W.; Zhao, C.; Hu, H.; Yin, S. Food Sources of Selenium and Its Relationship with Chronic Diseases. Nutrients 2021, 13, 1739. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium⁻Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, M.; Szczyglowska, M.; Konieczka, P.; Namiesnik, J. Methods of Selenium Supplementation: Bioavailability and Determination of Selenium Compounds. Crit. Rev. Food Sci. Nutr. 2016, 56, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Valdiglesias, V.; Pásaro, E.; Méndez, J.; Laffon, B. In vitro evaluation of selenium genotoxic, cytotoxic, and protective effects: A review. Arch. Toxicol. 2010, 84, 337–351. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, H.; Cheng, W.-H. Beneficial and paradoxical roles of selenium at nutritional levels of intake in healthspan and longevity. Free Radic. Biol. Med. 2018, 127, 3–13. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Flowers, B.; Poles, A.; Kastrati, I. Selenium and breast cancer—An update of clinical and epidemiological data. Arch. Biochem. Biophys. 2022, 732, 109465. [Google Scholar] [CrossRef]
- Pantavos, A.; Ruiter, R.; Feskens, E.F.; de Keyser, C.E.; Hofman, A.; Stricker, B.H.; Franco, O.H.; Kiefte-de Jong, J.C. Total dietary antioxidant capacity, individual antioxidant intake and breast cancer risk: The Rotterdam Study. Int. J. Cancer 2015, 136, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Hendryx, M.; Liang, X.; Manson, J.E.; He, K.; Vitolins, M.Z.; Li, Y.; Luo, J. Association between selenium intake and breast cancer risk: Results from the Women’s Health Initiative. Breast Cancer Res. Treat. 2020, 183, 217–226. [Google Scholar] [CrossRef]
- Bengtsson, Y.; Sandsveden, M.; Manjer, J. Risk of breast cancer in relation to dietary intake of selenium and serum selenium as a marker of dietary intake: A prospective cohort study within The Malmö Diet and Cancer Study. Cancer Causes Control 2021, 32, 815–826. [Google Scholar] [CrossRef]
- van’t Veer, P.; van der Wielen, R.P.; Kok, F.J.; Hermus, R.J.; Sturmans, F. Selenium in diet, blood, and toenails in relation to breast cancer: A case-control study. Am. J. Epidemiol. 1990, 131, 987–994. [Google Scholar] [CrossRef]
- Sharhar, S.; Normah, H.; Fatimah, A.; Fadilah, R.N.; Rohi, G.A.; Amin, I.; Cham, B.G.; Rizal, R.M.; Fairulnizal, M.N. Antioxidant intake and status, and oxidative stress in relation to breast cancer risk: A case-control study. Asian Pac. J. Cancer Prev. 2008, 9, 343–349. [Google Scholar]
- Suzana, S.; Cham, B.G.; Ahmad Rohi, G.; Mohd Rizal, R.; Fairulnizal, M.N.; Normah, H.; Fatimah, A. Relationship between selenium and breast cancer: A case-control study in the Klang Valley. Singap. Med. J. 2009, 50, 265–269. [Google Scholar]
- Vahid, F.; Hatami, M.; Sadeghi, M.; Ameri, F.; Faghfoori, Z.; Davoodi, S.H. The association between the Index of Nutritional Quality (INQ) and breast cancer and the evaluation of nutrient intake of breast cancer patients: A case-control study. Nutrition 2018, 45, 11–16. [Google Scholar] [CrossRef]
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium Exposure and Cancer Risk: An Updated Meta-analysis and Meta-regression. Sci. Rep. 2016, 6, 19213. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Al-Alem, U.; Dabbous, F.; Ali, M.M.; Batai, K.; Shah, E.; Kittles, R.A. Zinc Intake and Risk of Prostate Cancer: Case-Control Study and Meta-Analysis. PLoS ONE 2016, 11, e0165956. [Google Scholar] [CrossRef]
- He, P.; Li, H.; Liu, M.; Zhang, Z.; Zhang, Y.; Zhou, C.; Ye, Z.; Wu, Q.; Liang, M.; Jiang, J.; et al. J-shaped association between dietary zinc intake and new-onset hypertension: A nationwide cohort study in China. Front. Med. 2023, 17, 156–164. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Li, H.; Liu, M.; Zhang, Z.; Zhang, Y.; Zhou, C.; Li, Q.; Liu, C.; Qin, X. U-shaped Association Between Dietary Zinc Intake and New-onset Diabetes: A Nationwide Cohort Study in China. J. Clin. Endocrinol. Metab. 2022, 107, e815–e824. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Zhang, F.; Cui, Y.; Zhang, D.; Shen, X. Threshold effects and interactive effects of total zinc and selenium intake on cognitive function in older adults. Clin. Nutr. ESPEN 2022, 47, 383–390. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hajishafiee, M.; Clark, C.C.T.; Borges do Nascimento, I.J.; Milajerdi, A.; Amini, M.R.; Esmaillzadeh, A. Clinical effectiveness of zinc supplementation on the biomarkers of oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 161, 105166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Ho, S.C.; Chen, Y.M.; Fu, J.H.; Cheng, S.Z.; Lin, F.Y. Greater vegetable and fruit intake is associated with a lower risk of breast cancer among Chinese women. Int. J. Cancer 2009, 125, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Pan, M.X.; Li, B.; Wang, L.; Mo, X.F.; Chen, Y.M.; Lin, F.Y.; Ho, S.C. Choline and betaine intake is inversely associated with breast cancer risk: A two-stage case-control study in China. Cancer Sci. 2013, 104, 250–258. [Google Scholar] [CrossRef]
- Liu, K.Y.; Feng, X.L.; Mo, X.F.; Lin, F.Y.; Zhang, X.; Huang, C.Y.; Abulimiti, A.; Li, L.; Zhang, C.X. Iron intake with the risk of breast cancer among Chinese women: A case-control study. Public Health Nutr. 2021, 24, 5743–5755. [Google Scholar] [CrossRef]
- Nimptsch, K.; Zhang, X.; Cassidy, A.; Song, M.; O’Reilly, É.J.; Lin, J.H.; Pischon, T.; Rimm, E.B.; Willett, W.C.; Fuchs, C.S.; et al. Habitual intake of flavonoid subclasses and risk of colorectal cancer in 2 large prospective cohorts. Am. J. Clin. Nutr. 2016, 103, 184–191. [Google Scholar] [CrossRef]
- Zhang, C.X.; Ho, S.C. Validity and reproducibility of a food frequency Questionnaire among Chinese women in Guangdong province. Asia Pac. J. Clin. Nutr. 2009, 18, 240–250. [Google Scholar]
- Yang, Y.X.; Wang, G.Y.; Pan, X.C. China Food Composition 2002; Peking University Medical Press: Beijing, China, 2002; p. 393. [Google Scholar]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S; discussion 1229S–1231S. [Google Scholar] [CrossRef] [PubMed]
- Durrleman, S.; Simon, R. Flexible regression models with cubic splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef]
- Nriagu, J. Zinc Toxicity in Humans. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Burlington, NJ, USA, 2011; pp. 801–807. [Google Scholar]
- de Oliveira Otto, M.C.; Alonso, A.; Lee, D.-H.; Delclos, G.L.; Bertoni, A.G.; Jiang, R.; Lima, J.A.; Symanski, E.; Jacobs, D.R.; Nettleton, J.A. Dietary Intakes of Zinc and Heme Iron from Red Meat, but Not from Other Sources, Are Associated with Greater Risk of Metabolic Syndrome and Cardiovascular Disease123. J. Nutr. 2012, 142, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.W.; Zhang, S.L.; Hao, Q.Y.; Huang, F.F.; Liu, Z.Y.; Zhang, H.F.; Yan, L.; Wang, J.F.; Liu, P.M. Association of dietary zinc intake with coronary artery calcium progression: The Multi-Ethnic Study of Atherosclerosis (MESA). Eur. J. Nutr. 2021, 60, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Sinha, R.; Gierach, G.L.; Ward, M.H. Red and processed meat, nitrite, and heme iron intakes and postmenopausal breast cancer risk in the NIH-AARP Diet and Health Study. Int. J. Cancer 2016, 138, 1609–1618. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In Red Meat and Processed Meat; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Kazemi, A.; Barati-Boldaji, R.; Soltani, S.; Mohammadipoor, N.; Esmaeilinezhad, Z.; Clark, C.C.T.; Babajafari, S.; Akbarzadeh, M. Intake of Various Food Groups and Risk of Breast Cancer: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2021, 12, 809–849. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, K.N.; Lee, J.S.; Yoon, M.O.; Lee, H.S. Dietary zinc intake and sources among Koreans: Findings from the Korea National Health and Nutrition Examination Survey 2016–2019. Nutr. Res. Pract. 2023, 17, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, X.F.; Zhang, B.; Wang, Z.H.; Zhang, J.G.; Huang, F.F.; Su, C.; Ouyang, Y.F.; Zhao, J.; Du, W.W.; et al. Dietary Zinc Intake and Its Association with Metabolic Syndrome Indicators among Chinese Adults: An Analysis of the China Nutritional Transition Cohort Survey 2015. Nutrients 2018, 10, 572. [Google Scholar] [CrossRef] [PubMed]
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, Á. Reported Dietary Intake and Food Sources of Zinc, Selenium, and Vitamins A, E and C in the Spanish Population: Findings from the ANIBES Study. Nutrients 2017, 9, 697. [Google Scholar] [CrossRef]
- Winkel, L.H.; Johnson, C.A.; Lenz, M.; Grundl, T.; Leupin, O.X.; Amini, M.; Charlet, L. Environmental selenium research: From microscopic processes to global understanding. Environ. Sci. Technol. 2012, 46, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Winther, K.H.; Pastor-Barriuso, R.; Cold, F.; Thvilum, M.; Stranges, S.; Guallar, E.; Cold, S. Effect of long-term selenium supplementation on mortality: Results from a multiple-dose, randomised controlled trial. Free Radic. Biol. Med. 2018, 127, 46–54. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Interactive effects of selenium and cadmium on mammary tumor development and growth in MMTV-infected female mice. A model study on the roles of cadmium and selenium in human breast cancer. Biol. Trace Elem. Res. 2008, 123, 27–34. [Google Scholar] [CrossRef]
- Liang, Z.Z.; Zhang, Y.X.; Zhu, R.M.; Li, Y.L.; Jiang, H.M.; Li, R.B.; Chen, Q.X.; Wang, Q.; Tang, L.Y.; Ren, Z.F. Identification of epigenetic modifications mediating the antagonistic effect of selenium against cadmium-induced breast carcinogenesis. Environ. Sci. Pollut. Res. Int. 2022, 29, 22056–22068. [Google Scholar] [CrossRef]
- Ganash, M.A. Anticancer potential of ascorbic acid and inorganic selenium on human breast cancer cell line MCF-7 and colon carcinoma HCT-116. J. Cancer Res. Ther. 2021, 17, 122–129. [Google Scholar] [CrossRef]
- Manjer, J.; Sandsveden, M.; Borgquist, S. Serum Iodine and Breast Cancer Risk: A Prospective Nested Case-Control Study Stratified for Selenium Levels. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1335–1340. [Google Scholar] [CrossRef]
- Yildiz, A.; Kaya, Y.; Tanriverdi, O. Effect of the Interaction Between Selenium and Zinc on DNA Repair in Association With Cancer Prevention. J. Cancer Prev. 2019, 24, 146–154. [Google Scholar] [CrossRef]
- Farbood, Y.; Sarkaki, A.; Mahdavinia, M.; Ghadiri, A.; Teimoori, A.; Seif, F.; Dehghani, M.A.; Navabi, S.P. Protective Effects of Co-administration of Zinc and Selenium Against Streptozotocin-Induced Alzheimer’s Disease: Behavioral, Mitochondrial Oxidative Stress, and GPR39 Expression Alterations in Rats. Neurotox Res. 2020, 38, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.L.; Sandhir, R.; Adenuga, G.A. Protective roles of selenium and zinc against postnatal protein-undernutrition-induced alterations in Ca(2+)-homeostasis leading to cognitive deficits in Wistar rats. Int. J. Dev. Neurosci. 2015, 43, 1–7. [Google Scholar] [CrossRef]
- Hidaka, B.H.; Carlson, S.E.; Kimler, B.F.; Fabian, C.J. Dietary Associations with a Breast Cancer Risk Biomarker Depend on Menopause Status. Nutr. Cancer 2016, 68, 1115–1122. [Google Scholar] [CrossRef]
- Miquel, J.; Ramírez-Boscá, A.; Ramírez-Bosca, J.V.; Alperi, J.D. Menopause: A review on the role of oxygen stress and favorable effects of dietary antioxidants. Arch. Gerontol. Geriatr. 2006, 42, 289–306. [Google Scholar] [CrossRef]
- Larouche, D.; Hanna, M.; Chang, S.L.; Jacob, S.; Têtu, B.; Diorio, C. Evaluation of Antioxidant Intakes in Relation to Inflammatory Markers Expression Within the Normal Breast Tissue of Breast Cancer Patients. Integr. Cancer Ther. 2017, 16, 485–495. [Google Scholar] [CrossRef]
- Bagnardi, V.; Zambon, A.; Quatto, P.; Corrao, G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am. J. Epidemiol. 2004, 159, 1077–1086. [Google Scholar] [CrossRef]
- Smith, A.D.; Crippa, A.; Woodcock, J.; Brage, S. Physical activity and incident type 2 diabetes mellitus: A systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia 2016, 59, 2527–2545. [Google Scholar] [CrossRef]
- Chinese Nutrition Society; National Institute for Nutrition and Health Chinese Center for Disease Control and Prevention; Harbin Medical University; Tianjin Medical University; Henan Center for Disease Control and Prevention; People’s Liberation Army Bethune Medical Officer School of China. Chinese Dietary Reference Intakes—Part 3: Trace Element; WS/T 578.3-2017; National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2017.
- Vinceti, M.; Filippini, T.; Wise, L.A. Environmental Selenium and Human Health: An Update. Curr. Environ. Health Rep. 2018, 5, 464–485. [Google Scholar] [CrossRef]
- Gorini, F.; Sabatino, L.; Pingitore, A.; Vassalle, C. Selenium: An Element of Life Essential for Thyroid Function. Molecules 2021, 26, 7084. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Medina, M.W.; Stammers, A.L.; Patel, S.; Souverein, O.W.; Dullemeijer, C.; Serra-Majem, L.; Nissensohn, M.; Hall Moran, V. The relationship between zinc intake and serum/plasma zinc concentration in adults: A systematic review and dose-response meta-analysis by the EURRECA Network. Br. J. Nutr. 2012, 108, 1962–1971. [Google Scholar] [CrossRef] [PubMed]



| Cases (n = 1591) | Controls (n = 1622) | p a | |
|---|---|---|---|
| Age (years), mean ± SD | 47.79 ± 9.57 | 47.73 ± 9.85 | 0.851 |
| Education, n (%) | 0.001 | ||
| Primary school or below | 395 (24.84) | 444 (27.39) | |
| Middle school | 454 (28.55) | 392 (24.18) | |
| High school/technical school | 389 (24.47) | 379 (23.38) | |
| Secondary technical school | 199 (12.52) | 187 (11.54) | |
| College or above | 153 (9.62) | 219 (13.51) | |
| Job, n (%) | 0.299 | ||
| White-collar | 634 (39.85) | 654 (40.32) | |
| Blue-collar | 398 (25.02) | 436 (26.88) | |
| Farmer/other | 559 (35.14) | 532 (32.80) | |
| Household income (Yuan/month), n (%) | 0.003 | ||
| <2000 | 306 (19.23) | 248 (15.29) | |
| 2001–5000 | 491 (30.86) | 467 (28.79) | |
| 5001–8000 | 433 (27.22) | 498 (30.70) | |
| ≥8001 | 361 (22.69) | 409 (25.22) | |
| Physical activity at work, n (%) | 0.027 | ||
| Nonworking | 505 (31.74) | 440 (27.13) | |
| Sedentary | 514 (32.31) | 545 (33.60) | |
| Low | 323 (20.30) | 374 (23.06) | |
| Moderate | 136 (8.55) | 159 (9.80) | |
| Heavy | 113 (7.10) | 104 (6.41) | |
| Ever smokers, n (%) | 18 (1.13) | 8 (0.49) | 0.044 |
| Second-hand smoke exposure, n (%) | 946 (59.46) | 831 (51.23) | <0.001 |
| Regular drinkers, n (%) | 195 (12.26) | 114 (7.03) | <0.001 |
| BMI (kg/m2), mean ± SD | 23.05 ± 3.38 | 22.56 ± 3.16 | <0.001 |
| Family history of cancer, n (%) | 238 (14.96) | 158 (9.74) | <0.001 |
| Age at menarche (years), mean ± SD | 14.52 ± 1.90 | 14.76 ± 1.83 | <0.001 |
| Age at first childbirth (years) b, mean ± SD | 25.56 ± 3.68 | 25.37 ± 3.59 | 0.146 |
| Breastfeeding b, n (%) | 1345 (88.49) | 1393 (90.16) | 0.149 |
| Previous benign breast disease, n (%) | 608 (38.21) | 371 (22.87) | <0.001 |
| Ever use of oral contraceptives, n (%) | 134 (8.43) | 100 (6.17) | 0.014 |
| Menopausal status, n (%) | 0.312 | ||
| Premenopausal | 1021 (64.17) | 1013 (62.45) | |
| Postmenopausal | 570 (35.83) | 609 (37.55) | |
| Number of births, n (%) | 0.323 | ||
| 0 | 51 (3.21) | 57 (3.51) | |
| 1–2 | 1021 (64.17) | 1075 (66.28) | |
| ≥3 | 519 (32.62) | 490 (30.21) |
| Cases (n = 1591) | Controls (n = 1622) | p b | |
|---|---|---|---|
| Energy (kcal/d) a | 1363.40 (1154.85, 1634.81) | 1355.62 (1164.49, 1615.31) | 0.455 |
| Dietary Zn intake (mg/d) a | |||
| Total dietary Zn | 9.92 (8.90, 11.18) | 10.21 (9.31, 11.27) | <0.001 |
| Zn from plants | 6.09 (5.45, 6.72) | 6.32 (5.68, 7.01) | <0.001 |
| Zn from meat | 3.10 (2.21, 4.29) | 3.11 (2.20, 4.20) | 0.555 |
| Zn from red meat | 2.23 (1.45, 3.32) | 2.15 (1.36, 3.21) | 0.050 |
| Zn from white meat | 0.68 (0.39, 1.05) | 0.72 (0.45, 1.12) | 0.006 |
| Dietary Se intake (μg/d) a | |||
| Total dietary Se | 48.00 (39.41, 58.05) | 49.12 (40.43, 59.95) | 0.004 |
| Se from plants | 19.28 (14.63, 24.15) | 20.45 (15.64, 24.91) | <0.001 |
| Se from meat | 22.21 (14.95, 30.65) | 22.16 (15.20, 30.85) | 0.795 |
| Se from red meat | 9.42 (6.10, 13.82) | 8.97 (5.75, 12.99) | 0.003 |
| Se from white meat | 10.41 (5.46, 18.36) | 11.01 (6.25, 19.38) | 0.023 |
| Total fat (g/d) a | 28.91 (22.32, 36.13) | 28.95 (22.92, 35.22) | 0.550 |
| Dietary fiber (g/d) a | 8.25 (6.83, 9.87) | 9.07 (7.52, 10.84) | <0.001 |
| Vitamin A (μgRE/d) a | 128.69 (94.15, 171.40) | 149.23 (113.72, 193.35) | <0.001 |
| Vitamin C (mg/d) a | 706.58 (527.42, 938.73) | 810.71 (623.94, 1026.11) | <0.001 |
| Vitamin E (mg/d) a | 9.33 (7.52, 11.91) | 10.38 (8.26, 13.08) | <0.001 |
| Q1 | Q2 | Q3 | Q4 | ptrend c | |
|---|---|---|---|---|---|
| Total dietary Zn | |||||
| N (cases/controls) | 567/405 | 340/406 | 303/406 | 381/405 | |
| Median (mg/d) | 8.65 | 9.77 | 10.72 | 12.14 | |
| cOR (95%CI) | 1.00 | 0.60 (0.49–0.73) | 0.53 (0.44–0.65) | 0.67 (0.56–0.81) | <0.001 |
| aOR (95%CI) a | 1.00 | 0.53 (0.43–0.65) | 0.71 (0.58–0.88) | 1.00 (0.99–1.01) | <0.001 |
| aOR (95%CI) b | 1.00 | 0.68 (0.55–0.84) | 0.71 (0.57–0.90) | 1.06 (0.83–1.35) | 0.786 |
| Zn from plants | |||||
| N (cases/controls) | 526/405 | 414/406 | 383/405 | 268/406 | |
| Median (mg/d) | 5.18 | 5.99 | 6.62 | 7.48 | |
| cOR (95%CI) | 1.00 | 0.79 (0.65–0.95) | 0.73 (0.60–0.88) | 0.51 (0.42–0.62) | <0.001 |
| aOR (95%CI) a | 1.00 | 0.82 (0.67–0.99) | 0.77 (0.63–0.95) | 0.53 (0.43–0.65) | <0.001 |
| aOR (95%CI) b | 1.00 | 0.91 (0.73–1.13) | 0.96 (0.75–1.22) | 0.86 (0.62–1.18) | 0.473 |
| Zn from meat | |||||
| N (cases/controls) | 395/405 | 402/406 | 383/406 | 411/405 | |
| Median (mg/d) | 1.64 | 2.67 | 3.56 | 5.20 | |
| cOR (95%CI) | 1.00 | 1.02 (0.84–1.23) | 0.97 (0.79–1.18) | 1.04 (0.86–1.27) | 0.818 |
| aOR (95%CI) a | 1.00 | 1.00 (0.81–1.22) | 0.95 (0.77–1.17) | 1.12 (0.91–1.38) | 0.401 |
| aOR (95%CI) b | 1.00 | 0.98 (0.79–1.22) | 0.94 (0.75–1.18) | 1.11 (0.87–1.41) | 0.496 |
| Zn from red meat | |||||
| N (cases/controls) | 355/405 | 405/406 | 402/406 | 429/405 | |
| Median (mg/d) | 0.90 | 1.78 | 2.61 | 4.25 | |
| cOR (95%CI) | 1.00 | 1.14 (0.93–1.39) | 1.13 (0.93–1.38) | 1.21 (0.99–1.47) | 0.080 |
| aOR (95%CI) a | 1.00 | 1.16 (0.94–1.43) | 1.17 (0.95–1.45) | 1.40 (1.14–1.73) | 0.003 |
| aOR (95%CI) b | 1.00 | 1.07 (0.86–1.34) | 1.07 (0.85–1.34) | 1.26 (0.99–1.60) | 0.073 |
| Zn from white meat | |||||
| N (cases/controls) | 475/406 | 382/405 | 379/406 | 355/405 | |
| Median (mg/d) | 0.27 | 0.58 | 0.88 | 1.52 | |
| cOR (95%CI) | 1.00 | 0.81 (0.67–0.98) | 0.80 (0.66–0.97) | 0.75 (0.62–0.91) | 0.004 |
| aOR (95%CI) a | 1.00 | 0.76 (0.62–0.93) | 0.75 (0.61–0.92) | 0.64 (0.52–0.78) | <0.001 |
| aOR (95%CI) b | 1.00 | 0.78 (0.64–0.97) | 0.79 (0.64–0.97) | 0.76 (0.61–0.95) | 0.020 |
| Q1 | Q2 | Q3 | Q4 | ptrend c | |
|---|---|---|---|---|---|
| Total dietary Se | |||||
| N (cases/controls) | 439/405 | 406/406 | 410/406 | 336/405 | |
| Median (μg/d) | 34.31 | 44.88 | 54.14 | 68.57 | |
| cOR (95%CI) | 1.00 | 0.92 (0.76–1.12) | 0.93 (0.77–1.13) | 0.77 (0.63–0.93) | 0.015 |
| aOR (95%CI) a | 1.00 | 0.91 (0.74–1.12) | 0.87 (0.70–1.07) | 0.71 (0.57–0.88) | 0.002 |
| aOR (95%CI) b | 1.00 | 1.00 (0.80–1.24) | 1.03 (0.82–1.30) | 0.86 (0.67–1.10) | 0.268 |
| Se from plants | |||||
| N (cases/controls) | 483/405 | 416/406 | 353/406 | 339/405 | |
| Median (μg/d) | 12.63 | 18.15 | 22.46 | 28.53 | |
| cOR (95%CI) | 1.00 | 0.86 (0.71–1.04) | 0.73 (0.60–0.89) | 0.70 (0.58–0.85) | <0.001 |
| aOR (95%CI) a | 1.00 | 0.87 (0.71–1.07) | 0.71 (0.58–0.88) | 0.67 (0.54–0.83) | <0.001 |
| aOR (95%CI) b | 1.00 | 1.01 (0.82–1.25) | 0.87 (0.70–1.08) | 0.88 (0.70–1.11) | 0.155 |
| Se from meat | |||||
| N (cases/controls) | 415/405 | 375/406 | 405/406 | 396/405 | |
| Median (μg/d) | 11.23 | 18.86 | 25.76 | 40.43 | |
| cOR (95%CI) | 1.00 | 0.90 (0.74–1.10) | 0.97 (0.80–1.18) | 0.95 (0.79–1.16) | 0.830 |
| aOR (95%CI) a | 1.00 | 0.86 (0.70–1.06) | 0.95 (0.77–1.17) | 0.92 (0.74–1.13) | 0.625 |
| aOR (95%CI) b | 1.00 | 0.84 (0.68–1.05) | 0.93 (0.74–1.18) | 0.93 (0.73–1.17) | 0.789 |
| Se from red meat | |||||
| N (cases/controls) | 357/405 | 373/406 | 400/406 | 461/405 | |
| Median (μg/d) | 3.78 | 7.40 | 10.66 | 16.55 | |
| cOR (95%CI) | 1.00 | 1.04 (0.85–1.27) | 1.12 (0.92–1.36) | 1.29 (1.06–1.57) | 0.007 |
| aOR (95%CI) a | 1.00 | 1.09 (0.88–1.34) | 1.16 (0.94–1.44) | 1.45 (1.18–1.79) | <0.001 |
| aOR (95%CI) b | 1.00 | 1.02 (0.82–1.27) | 1.08 (0.86–1.37) | 1.36 (1.04–1.77) | 0.026 |
| Se from white meat | |||||
| N (cases/controls) | 465/405 | 372/406 | 389/406 | 365/405 | |
| Median (μg/d) | 3.61 | 8.54 | 14.50 | 29.27 | |
| cOR (95%CI) | 1.00 | 0.80 (0.66–0.97) | 0.83 (0.69–1.01) | 0.79 (0.65–0.95) | 0.025 |
| aOR (95%CI) a | 1.00 | 0.78 (0.63–0.95) | 0.76 (0.62–0.94) | 0.70 (0.57–0.86) | 0.001 |
| aOR (95%CI) b | 1.00 | 0.82 (0.66–1.01) | 0.85 (0.69–1.05) | 0.82 (0.66–1.02) | 0.108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, K.; Liu, K.; Wang, Y.; Jiang, Y.; Zhang, C. Association of Dietary Intake of Zinc and Selenium with Breast Cancer Risk: A Case-Control Study in Chinese Women. Nutrients 2023, 15, 3253. https://doi.org/10.3390/nu15143253
Tu K, Liu K, Wang Y, Jiang Y, Zhang C. Association of Dietary Intake of Zinc and Selenium with Breast Cancer Risk: A Case-Control Study in Chinese Women. Nutrients. 2023; 15(14):3253. https://doi.org/10.3390/nu15143253
Chicago/Turabian StyleTu, Kexin, Kaiyan Liu, Yifan Wang, Yiling Jiang, and Caixia Zhang. 2023. "Association of Dietary Intake of Zinc and Selenium with Breast Cancer Risk: A Case-Control Study in Chinese Women" Nutrients 15, no. 14: 3253. https://doi.org/10.3390/nu15143253
APA StyleTu, K., Liu, K., Wang, Y., Jiang, Y., & Zhang, C. (2023). Association of Dietary Intake of Zinc and Selenium with Breast Cancer Risk: A Case-Control Study in Chinese Women. Nutrients, 15(14), 3253. https://doi.org/10.3390/nu15143253







