Abstract
A Mediterranean diet (MedDiet)-based intervention reduces the rate of immediate postpartum maternal metabolic disorders. Whether these effects persist long-term remains to be determined. A total of 2526 normoglycemic women were randomized before the 12th gestational week (GW). IG women followed a MedDiet with extra virgin olive oil (EVOO) (>40 mL/day) and a handful of nuts daily, whereas CG women had to restrict all kinds of dietary fat. At 3 months postpartum, a motivational lifestyle interview was held. The endpoint of the study evaluated the rate of abnormal glucose regulation (AGR) and metabolic syndrome (MetS) at 3 years postpartum in women of the San Carlos cohort. A total of 369/625 (59%) CG women and 1031/1603 (64.3%) IG women were finally analyzed. At 3 months and 3 years postdelivery, the IG women showed higher adherence to the MedDiet, which was associated with lower values of body mass index (BMI) and lipid and glycemic profiles. Body weight change and waist circumference were lower in the IG women. After applying multiple regression analysis, the ORs (95%CI) resulted in AGR (3.18 (2.48–4.08); p < 0.001)/MetS (3.79 (1.81–7.95); p = 0.001) for women with GDM and higher OR for development of MetS in CG women (3.73 (1.77–7.87); p = 0.001). A MedDiet-based intervention early in pregnancy demonstrated persistent beneficial effects on AGR and MetS rates at 3 years postpartum.
1. Introduction
Gestational diabetes mellitus (GDM) increases the risk of occurrence of type 2 diabetes mellitus (T2DM) later in life [1,2]. Women with a prior history of GDM have an almost 10-times higher risk of developing T2DM than those with normal glucose tolerance (NGT) during pregnancy, especially within the first five years postpartum [3,4]. Nevertheless, different rates of T2DM are found, depending on ethnicity and the diagnostic criteria employed [5]. The importance of a diagnosis of T2DM after delivery lies in its relationship with an increased risk of cardiovascular disease (CVD) [6,7].
The pathogenesis of GDM is complex and multifactorial [8,9,10,11]. However, different factors have been found to be related to its increasing incidence. Obesity, advanced maternal age, smoking habit, and a prior history of GDM seem to contribute to its risk, as well as that of subsequent abnormal glucose regulation (AGR) [8,12,13,14,15]. Ethnicity seems to be another risk factor in the case of certain ethnic groups, as Hispanics are more prone to the development of GDM [16]. Likewise, GDM can increase the predisposition for metabolic syndrome (MetS) [17]. Development of the latter, in turn, increases the risk of T2DM. In addition, other components of MetS, such as lipid disorders and hypertension, are found more frequently in women with a history of GDM [18]. Furthermore, the presence of dyslipidemia in women with a prior diagnosis of GDM is associated with an increased risk of future development of T2DM [19].
Healthy lifestyle and nutritional habits have been found to be factors that can lead to a decrease in the rate of occurrence of GDM. The Mediterranean diet (MedDiet) is one of the most widely studied dietary patterns [20,21]. Prior research conducted by our group on this same cohort of women (the San Carlos cohort) evaluated participants with NGT or GDM after an intervention with MedDiet early in pregnancy and during the immediate postpartum period. The results revealed a 26% decrease in the risk of developing MetS, being more marked in normoglycemic women of the IG compared with those of the control standard-care group [22]. In another study, women with a prior history of GDM were evaluated during pregnancy and within the following 3 years in the context of a lifestyle program. Results indicated a decrease in the risk of developing T2DM [23]. Other studies have also evaluated the relationship between diet and the development of T2DM later in life in women with a prior history of GDM [24]. In fact, several studies have detected that a nutritional intervention early in pregnancy can reduce the risk of GDM, especially in high-risk women [25,26,27].
Thus, the promotion of healthy nutritional habits during pregnancy seems to be decisive, as an early nutritional intervention can decrease health complications in the mother [28]. However, whether these benefits can be sustained has yet to be elucidated.
The aim of the current study is the assessment of the impact of a nutritional intervention with MedDiet early in pregnancy, reinforced during the postpartum period on the rates of AGR and MetS at 3 years postpartum in women from the San Carlos cohort.
2. Materials and Methods
2.1. Study Design
This is a prospective unicentric interventional analysis of the “San Carlos GDM prevention study”. This study began in 2015 and its follow-up is ongoing. The study has been undertaken at the Hospital Clínico San Carlos, providing medical assistance to 463.833 patients in Madrid, Spain. During pregnancy, women are followed up from the 8th to 12th gestational week (GW) when the first ultrasound is performed. Between the 24th and 28th GW, the 75 g oral glucose tolerance test (OGTT) is carried out for detection of GDM. Both the nursing staff and the obstetricians give medical recommendations and nutritional advice to pregnant women from the onset of pregnancy. Since 2012, IADPSG criteria have been applied for the diagnosis of GDM [29].
The first study (Study 1), whose participants have been included in the current one, was a randomized controlled trial (RCT), with women allocated before the 12th GW into the control group (CG) and the intervention group (IG). A MedDiet pattern was recommended: women of the IG were urged to increase the consumption of extra virgin olive oil (EVOO) >40 mL/day and to consume a handful of pistachios daily, with both foods provided at no cost. Conversely, CG women were encouraged to consume less than 40 mL/day of EVOO and to not eat nuts more than three times a week. The second study (Study 2) consisted in evaluating the effects of the MedDiet implemented early in pregnancy on a single group based on routine clinical practice (real world). Women from Study 2 were encouraged to follow the same nutritional indications as the IG from the RCT of Study 1 but were not provided EVOO and pistachios at no cost and were free to choose the nuts they preferred. The third study (Study 3) was an RCT in which women with a body mass index (BMI) ≥ 25 kg/m2 were randomized into CG and IG, the latter receiving a recommendation of increasing the consumption of pistachios and EVOO. However, the pistachios were not provided free-of-cost. The IG from both RCTs, as well as the group belonging to the study based on real practice, were analyzed as IG for this study, since these groups were urged to follow a MedDiet pattern along with a specific dietary recommendation in relation to nuts and EVOO. They were compared with the 2 CGs belonging to the RCTs. Women diagnosed with GDM were followed by the Endocrinology Department and received identical treatment per department protocols, regardless of whether they had been allocated to the IG or CG. This information is displayed in Figure 1.
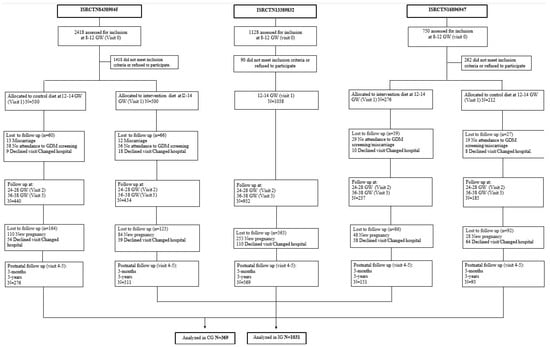
Figure 1.
Flowchart of the cohorts included in the analyses. Abbreviations: GDM—gestational diabetes mellitus; GW—gestational weeks.
After randomization of participants at the 8th–10th GW, three visits during gestation were undertaken. The first visit was made between the 12th and 14th GW, the second between the 20th and 24th, and the third between the 36th and 38th GW. After delivery, a 3-month visit was made, in which a 60 min motivational interview was carried out to encourage the subjects to continue with the nutritional and lifestyle habits acquired during pregnancy. Detailed information on each visit during and after childbirth is displayed in Figure 2.
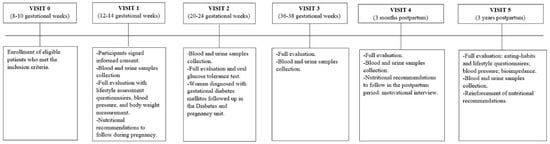
Figure 2.
Description of the intervention performed at each visit during pregnancy and postpartum.
All women belonging to previous studies were invited to attend a follow-up visit 3 years postpartum. Participants made an appointment by phone as well as by email/letter. They were informed of the timing of the follow-up as well as the volunteer character of the analysis. Participants who could not attend were invited to complete questionnaires via email or telephone. However, within this 3-year time period, several women were unable to participate in the monitoring for different reasons (such as having a new gestation during the follow-up, or any health condition or treatment that could result in modifications of analytic values and/or in body composition).
2.2. Study Population
All the participants analyzed in this study belong to the cohort of the “San Carlos GDM prevention study”. The trials they were included in were previously registered as ISRCTN84389045, ISRCTN13389832, and ISRCTN16896947, were approved by the Ethics Committee of Hospital Clínico San Carlos (ethic codes CI 13/296-E, CI 16/442-E, and CI 16/316), and conducted according to the Helsinki Declaration. All women signed a letter of informed consent. The three studies were carried out consecutively. A total of 2526 normoglycemic women (fasting serum glucose (FSG) < 92 mg/dL) were allocated between the 8th and 12th GW into 2 groups. A total of 2228 women were evaluated at the end of pregnancy (625 women in the CG and 1603 in the IG). Participants were followed up during gestation and postpartum from 2015 to 2018. Throughout the years 2017–2020, women belonging to all groups were followed up at a 3-year postpartum visit. Hence, a total of 369/625 (59%) CG and 1031/1603(64.3%) IG women completed all the visits and were finally included in the analysis at both 3 months and 3 years postpartum. Table 1 shows baseline characteristics.

Table 1.
Baseline characteristics of analyzed women.
2.3. Sociodemographic, Anthropometric, and Clinical Data
Information on age, ethnicity (Caucasian/Hispanics and others), family history of T2DM and/or MetS, history of previous GDM and/or miscarriages, educational status (holding a university degree), being primiparous, and employment status was collected at the first visit. Pre-pregnancy body weight (BW) and pre-pregnancy BMI was also registered at the first visit. Fat mass (FM) (kg), BMI (kg/m2), and BW (kg), measured without shoes and with lightweight clothes, were provided by electrical bioimpedance analysis (SECA mBCA 514) and registered at 3 years postpartum. Waist circumference (WC) was taken using a non-extendable measuring tape. It was measured according to ISAK guidelines [30,31] between the last rib and the iliac crest, slightly above the navel. Blood pressure (BP) was measured using a digital sphygmomanometer with an adequate armlet after a rest period of 10 min in a sitting position (Omron 705IT, Omron Global, Kyoto, Japan). Current diagnoses and medication were registered at the first visit, as well as at the 3-month and 3-year postpartum visits, as was smoking habit (never or current smoker).
2.4. Biochemical Analysis
Blood and urine samples were collected early in the morning after a minimum 8 h fast.
The following data were determined: Total cholesterol (T-Chol) was quantitatively determined by the colorimetric cholesterol enzymatic test method (CHOD-PAP). Serum levels of HDL-cholesterol were measured by the enzymatic immunoinhibiting method in an Olympus 5800 (Beckman-Coulter, Brea, CA, USA). LDL-cholesterol was calculated with the Friedewald formula. Serum triglycerides were measured with a colorimetric enzymatic method using glycerol phosphate oxidase p-amino phenazone (GPO-PAP). Dimension Vista (Siemens Healthcare Diagnostics, Munich, Germany) was used to measure apolipoprotein B and C-RP by immunonephelometry and nephelometry, respectively. Fasting serum insulin (FSI) was measured by a chemiluminescence immunoassay in an IMMULITE 2000 Xpi (Siemens, Healthcare Diagnostics, Munich, Germany), with an inter-assay accuracy in concentrations of 11 uIU/mL of 6.3% and for insulin concentration of 21 uIU/mL of 5.91. HOMA-IR was calculated as glucose (mmol/L) × insulin (µUI/mL)/22.7. FSG (glucose oxidase) and HbA1c (%) levels were standardized by the International Federation of Clinical Chemistry and Laboratory Medicine, using ion-exchange high-performance liquid chromatography in gradient, with a Tosoh G8 analyzer (Tosoh Co., Tokyo, Japan). Inter-assay imprecision of HbA1c for levels of 5.1% had standard deviation (SD) of 0.06 and coefficient of variation (CV) of 1.23%; for levels of HbA1c 10.39%, SD was 0.11 and CV was 1.04. An external quality guarantee program of the SEQC (Sociedad Española de Química Clínica) evaluated the quality of the methods monthly.
2.5. Dietary Assessment
Lifestyle and MedDiet adherences were evaluated by means of two semiquantitative validated questionnaires. The Mediterranean diet adherence screener (MEDAS) was used to assess adherence to MedDiet. This questionnaire, derived from the PREDIMED study [32], was composed of 14 points. However, only 12 points were applied, as alcohol and juices were excluded from the diet during pregnancy. The compliance with each item was provided as +1. Although a score >5 indicated adequate compliance with the MedDiet, a score >7 was preferable. The second questionnaire applied was the diabetes nutrition and complications trial (DNCT). It collected information on the consumption of specific food groups and physical activity habits. The DNCT questionnaire was made up of 15 items; the first 3 collected information on physical activity and the remaining 12 pertained to the frequency of intake of specified foods. The answers from participants were valued as follows: A (value +1), B (value 0), and C (value −1). Obtention of an A was considered to be a preventive factor for T2DM, whereas obtaining a C reflected an increased risk. Additional information on the MEDAS and DNCT questionnaires used is available in previous publications [25,33].
2.6. Outcome Analysis
The endpoint of this study was to evaluate the rates of AGR and MetS at 3 years postpartum.
AGR was defined as impaired fasting serum glucose (IFG) ≥ 100 (mg/dL) and/or impaired glucose tolerance (IGT) by 2 h serum glucose during 75-g OGTT ≥ 140 (mg/dL) and/or impaired HbA1C when rates were ≥5.7%, according to the ADA criteria [34]. Insulin resistance (IR) was diagnosed when the homeostasis assessment model for insulin resistance (HOMA-IR) was ≥3.5, according to cut-off values described in the Spanish population [35,36].
MetS diagnosis and each of its components were evaluated by applying the international harmonized criteria for the diagnosis of MetS [37]. MetS diagnosis was considered when patients showed three or more of the following: a waist circumference (WC) (cm) ≥ 89.5, an FSG (mg/dL) ≥ 100 and/or a HbA1C (%) ≥ 5.7, systolic blood pressure (sBP) (mmHg) ≥ 130 mmHg and/or diastolic blood pressure (dBP) (mmHg) ≥ 85 mmHg, HDL-cholesterol (mg/dL) < 50, and triglycerides (g/L) ≥ 150.
2.7. Statistical Analysis
Data were presented as the median and interquartile range (IQR) or numbers (%). Comparisons between groups for categorical variables were evaluated using the chi 2 test; the Mann–Whitney-U test was applied for continuous variables expressed as median (IQR). The magnitude of the association between study groups (CG vs. IG, using CG as the reference group, or GDM group vs. NGT group, using NGT as the reference group) and binary outcomes (IR, AGR, and each MetS component) was evaluated using the relative risk (RR) and 95% confidence interval (CI).
Multivariate logistic regression analyses were conducted to estimate the odds ratios (ORs) and 95% confidence intervals (95% CIs) for AGR and MetS at 3 years postpartum. They were adjusted for maternal age and pregestational BMI. Continuous variables were categorized as ≥35, <35 and >30 kg/m2, and <30 kg/m2, respectively. ORs were estimated for GDM, CG, and Hispanic ethnicity, pregestational obesity (BMI > 30 kg/m2), and 3-years BW > pregestational BW. All analyses were carried out using SPSS, version 21 (SPSS, IBM, Chicago, IL, USA).
3. Results
Table 2 displays the biochemical, anthropometric, and clinical data of the women belonging to the IG (n = 1031) and CG (n = 369) at 3 months and 3 years postpartum.

Table 2.
Postnatal biochemical, anthropometric, and clinical data at 3 months and 3 years postdelivery by groups.
At 3 months postpartum, adherence to the MedDiet given by the MEDAS score was higher in the IG. A higher MEDAS score was associated with significantly lower BMIs, total cholesterol, LDL-cholesterol, APO-B levels, and CPR. In relation to the glycemic profile, FSI and FSG levels, as well as HOMA-IR, were also lower in the IG. At 3 years postpartum, women in the IG presented a significant reduction in the same parameters as at 3 months postpartum, with the exception of FSG. Additionally, they showed significantly lower values of changes in BW in relation to pregestational values, WC, dBP, HDL-cholesterol, 2H-OGTT, and physical activity score.
Table 3 shows the RR for developing MetS and each component of the MetS in relation to the nutritional intervention.

Table 3.
Comparison of postdelivery rate of metabolic syndrome (MetS) components between women from the intervention group (IG) vs. the control group (CG) and women with gestational diabetes mellitus (GDM) vs. normal glucose tolerance (NGT). Panel A, 3 months postpartum. Panel B, 3 years postpartum.
When analyzing each component of the MetS at 3 months postpartum, no significant differences were found, except for IFG, where belonging to the CG showed a higher risk. When evaluating at 3 years postpartum, the RR of developing prediabetes was higher for women in the CG, who also showed an increased risk of elevated WC, dBP, triglycerides, decreased HDL-cholesterol, and having >2 MetS components.
The RR of developing MetS and each of its components was assessed in women with a diagnosis of GDM. At 3 months postpartum, the risk of developing each MetS component was higher in women with GDM, with the exception of raised triglyceride levels, decreased HDL-cholesterol levels, and elevated HOMA-IR, where no significant differences were observed. At 3 years postpartum, each MetS component in women with a diagnosis of GDM was significantly higher, except for triglycerides, HDL-cholesterol levels, and dBP. The risk of developing >2 components of MetS was higher in women with a diagnosis of GDM in both postnatal periods.
After applying multivariate logistic regression, the OR (95% CI) to present AGR and/or MetS, respectively, was: (1.82 (1.30–2.54), p = 0.000 and 2.75 (1.22–6.18), p = 0.04) for Hispanic ethnicity, (1.99 (1.19–3.32), p = 0.009 and 4.06 (1.58–10.40) p= 0.009) for women with pre-pregnancy obesity, and (0.99 (0.98–1.01) p= 0.284 and 1.50 (1.09–2.06), p = 0.007) for women >35 years of age at the 12th GW. The OR for developing AGR and/or MetS in women with a diagnosis of GDM was (3.18 (2.48–4.08), p < 0.001) and (3.79 (1.81–7.95), p = 0.001), respectively. The statistically significant OR in women belonging to the CG was solely in relation to the development of MetS (3.73 (1.77–7.87), p = 0.001). This information can be found in Figure 3.

Figure 3.
Multiple logistic regression adjusted by maternal age and body mass index at 3 years postdelivery. Abbreviations: OR—odds ratios; CI—confidence intervals; AGR—abnormal glucose regulation (p > 0.05).
4. Discussion
A MedDiet-based nutritional intervention enriched with EVOO and nuts implemented early in pregnancy (before the 12th GW) and maintained throughout the postpartum period in the IG women was accompanied by a higher MEDAS score, which was in turn associated with lower rates of AGR and MetS at 3 years postdelivery compared with the CG women who initiated the intervention after delivery. Women in the IG presented more risk factors for the development of AGR and MetS, such as older age and a more frequent history of gestational adverse events (miscarriages and/or diagnosis of GDM). Despite these factors, the rates of AGR and/or MetS were lower. This fact reinforces the importance of starting the nutritional intervention as early as possible during pregnancy to reduce the possibility of an unfavorable postnatal metabolic impact. The response rate to postnatal follow-up was about 60% of the participants, as expected, indicating the difficulty in carrying out postnatal follow-up as recommended by all scientific societies.
A recent systematic review also highlighted that lifestyle and nutritional interventions maintained in the postpartum period can induce changes in glucose regulation [38]. Especially relevant is when diet is started early in pregnancy, focusing on women at high risk [26,27]. Several trials have addressed the association between diet started in pregnancy and maintained postdelivery and the development of T2DM in women with a diagnosis of GDM [39,40,41]. Other studies have also assessed the influence of diet in the postpartum period on women with pre-pregnancy obesity and a previous history of GDM, obtaining successful outcomes [24,42]. Our results coincide with the aforementioned studies regarding the impact of diet in the reduction of the risk in developing postpartum prediabetes, especially regarding high-risk women. However, to our knowledge, this is the first study that has evaluated a MedDiet-based nutritional intervention early in pregnancy in a population study (both NGT and GDM women) over a long follow-up period in relation to glucose dysregulation at 3 years postpartum. In addition to showing greater adherence to the MedDiet, the women who received the nutritional intervention from the beginning of pregnancy compared with those who received it after childbirth had a lower BMI and waist circumference, gained less body weight in relation to the pre-pregnancy body weight, and had a lower value of HOMA-IR. These data suggest that adherence to the MedDiet is associated with an improvement in IR. In this regard, one of the factors involved in the progression towards MetS and/or AGR is IR [43]. The risk of developing these diseases can increase further if pancreatic insulin secretion is reduced as a consequence of impairment in beta-cell function [44]. In the current study, women presented a lower value in the 2 h-OGTT glucose levels, indicating the preservation of the beta cell and its secretory capacity.
Considering the specific cut-off points to determine each of the MetS and/or AGR components, GDM emerged as the main risk factor associated with MetS and AGR development at 3 years postpartum. Although the rates of MetS and AGR were substantially reduced when comparing prior studies [22,23,45] to the current analysis, they remained high. Furthermore, the risk of later development of T2DM seemed to increase within the first years after delivery [46]. The results of the current study indicate that in women with a diagnosis of GDM, the risk of having each component of MetS and an overall risk of developing MetS is higher than in women with NGT. Furthermore, an association is observed between a smaller weight gain and lower levels of HOMA in IG women. This could explain lower rates of AGR at 3 years postdelivery in the IG due to greater adherence to the MedDiet patterns both during pregnancy and after delivery. This reduction in HOMA levels could in turn explain the decrease in the risk of developing MetS and its components. The role of the MedDiet in the prevention of MetS and AGR may be due to its antioxidant characteristics and other anti-inflammatory aspects [47]. EVOO is a rich source of monounsaturated fatty acids and has been found to lower postprandial glucose levels as well as to improve the inflammatory profile; it could also limit weight gain by reducing the carbohydrate load of meals. Nut consumption may facilitate weight loss within energy-restricted diets, possibly due to enhanced satiety, increased thermogenesis, incomplete mastication, and fat malabsorption Nuts are rich in unsaturated fatty acids, fiber, magnesium, and other phytochemical constituents, with potential beneficial effects on insulin sensitivity, fasting glucose levels, and inflammation. Therefore, a reduction in the MetS and AGR rates could be expected with the MedDiet.
Other established risk factors, such as Hispanic ethnicity, maternal age, pregestational obesity, and a prior history of GDM or family history of T2DM, were also considered in the current study. In this analysis, only the Hispanic race was found to be associated with a higher rate of MetS and AGR at 3 years postpartum. Previous studies have shown a higher risk of the development of GDM in Hispanic women compared with Caucasian women, as well as postpartum AGR or MetS. Similarly, a family history of diabetes mellitus has also been found to increase the risk of postpartum prediabetes in women with a diagnosis of GDM [16,48,49]. In the current study, pre-pregnancy obesity rates (≥30 kg/m2) were probably insufficient to achieve statistical significance, but when we adjusted OR for pre-pregnancy BMI and age, as continuous variables, only GDM was associated with more than a 3-fold increased risk of developing AGR and/or MetS.
This study has certain limitations. First, the differences in relation to the MEDAS score between the IG and CG were small, since MedDiet-based nutritional recommendations were provided to both groups (similarly, supplementation or restriction in the consumption of nuts and EVOO). Thus, small or absent differences in the questionnaire scores were to be expected. Secondly, the IG was larger than the CG. Moreover, IG women were older and had more previous adverse events; thus, worse outcomes could be expected in the IG, whereas the results indicated the opposite, further supporting the benefits of the nutritional intervention. Lastly, semiquantitative questionnaires may not always be accurate in terms of the responses obtained. However, they are validated, are the instruments most frequently used to obtain a vision of eating habits, and are always applied by specialized professionals to minimize bias. The greatest strength of our study lies in the study of both populations, NGT women and those with GDM, since most studies only evaluate nutritional intervention in women who have developed GDM.
5. Conclusions
In summary, our study shows that a nutritional intervention based on the principles of the MedDiet and supplemented with EVOO and nuts initiated early in pregnancy not only reduces the rate of GDM but also has metabolic benefits up to 3 years postpartum when compared with a nutritional intervention commenced after delivery. Therefore, easily adopted eating patterns, such as an increase in the consumption of EVOO and pistachios, maintained over time, improve clinical parameters and can act as a preventive factor for later development of T2DM. They can help to reduce the risk of GDM and in turn a later progression to T2DM. Postnatal MedDiet-based nutritional interventions should be intensified in women with GDM. Further studies will be needed to evaluate whether these benefits persist for more than three years.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15143252/s1, Table S1: Clinical and metabolic characteristics during pregnancy of analyzed women.
Author Contributions
Conceptualization, V.M., M.A., A.B., J.V., L.d.V., R.M.O., M.P.d.M., J.A.D., C.F., I.M., A.D., M.C., M.J.T., G.R. and M.P.; data curation, V.M., M.A., L.d.V. and M.A.R.; formal analysis, A.B., L.d.V., C.F., M.C., M.J.T., M.M.-N., M.M., G.R., M.A.R. and A.L.C.-P.; investigation, V.M., A.B., J.V., R.M.O., M.P.d.M., J.A.D., C.F., I.M., M.C., M.M.-N., I.R. and M.P.; methodology, M.A., A.B., J.V., R.M.O., A.D., M.J.T., M.M., I.R., M.A.R., P.M.-M. and A.L.C.-P.; validation, J.A.D., A.D., M.P. and A.L.C.-P.; writing—original draft, V.M., M.A., I.R., P.M.-M. and A.L.C.-P.; writing—review and editing, V.M., M.A., I.R., P.M.-M. and A.L.C.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the Instituto de Salud Carlos III/MICINN of Spain under grant number PI20/01758, and European Regional Development Fund (FEDER) ‘‘A way to build Europe’’ and Ministerio de Ciencia e Innovación, and Agencia Estatal de Investigación of Spain under grant number PREDIGES RTC2019-007406-1. The design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication are the responsibilities of the authors alone and independent of the funders.
Institutional Review Board Statement
All the participants analyzed in this study belong to the cohort of the “San Carlos GDM prevention study”. The trials registered as ISRCTN84389045, ISRCTN13389832, and ISRCTN16896947, were approved by the Ethics Committee of Hospital Clínico San Carlos (ethic codes CI 13/296-E, CI 16/442-E, and CI 16/316), and conducted according to the Helsinki Declaration.
Informed Consent Statement
All women signed a letter of informed consent.
Data Availability Statement
The data analyzed in this study are subject to the following licenses/restrictions: no restriction. Requests to access these datasets should be directed to acalle.edu@gmail.com.
Acknowledgments
We wish to acknowledge our deep appreciation for the administrative personnel and nurses and dieticians from the Laboratory Department (Marisol Sanchez Orta, María Dolores Hermoso Martín, María Victoria Saez de Parayuelo, and Jose Luis Espadas), the Pregnancy and Diabetes Unit, and to all members of the Endocrinology and Nutrition and Obstetrics and Gynecology departments of the San Carlos Clinical Hospital, Madrid, Spain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IDF Diabetes Atlas. Available online: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/159-idf-diabetes-atlas-ninth-edition-2019.html (accessed on 22 April 2023).
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef]
- Bengtson, A.M.; Ramos, S.Z.; Savitz, D.A.; Werner, E.F. Risk Factors for Progression From Gestational Diabetes to Postpartum Type 2 Diabetes: A Review. Clin. Obstet. Gynecol. 2021, 64, 234–243. [Google Scholar] [CrossRef]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef]
- Sun, J.; Kim, G.R.; Lee, S.J.; Kim, H.C. Gestational diabetes mellitus and the role of intercurrent type 2 diabetes on long-term risk of cardiovascular events. Sci. Rep. 2021, 11, 21140. [Google Scholar] [CrossRef]
- Shostrom, D.C.V.; Sun, Y.; Oleson, J.J.; Snetselaar, L.G.; Bao, W. History of Gestational Diabetes Mellitus in Relation to Cardiovascular Disease and Cardiovascular Risk Factors in US Women. Front. Endocrinol. 2017, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef]
- Sharma, A.K.; Singh, S.; Singh, H.; Mahajan, D.; Kolli, P.; Mandadapu, G.; Kumar, B.; Kumar, D.; Kumar, S.; Jena, M.K. Deep Insight of the Pathophysiology of Gestational Diabetes Mellitus. Cells 2022, 11, 2672. [Google Scholar] [CrossRef]
- Li, M.; Zhang, C.Y.; Yue, C.Y. Effects of pre-pregnancy BMI and gestational weight gain on adverse pregnancy outcomes and complications of GDM. J. Obstet. Gynaecol. 2022, 42, 630–635. [Google Scholar] [CrossRef]
- Zehravi, M.; Maqbool, M.; Ara, I. Correlation between obesity, gestational diabetes mellitus, and pregnancy outcomes: An overview. Int. J. Adolesc. Med. Health 2021, 33, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Teh, W.T.; Teede, H.J.; Paul, E.; Harrison, C.L.; Wallace, E.M.; Allan, C. Risk factors for gestational diabetes mellitus: Implications for the application of screening guidelines. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 26–30. [Google Scholar] [CrossRef]
- Ben-Haroush, A.; Yogev, Y.; Hod, M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet. Med. 2004, 21, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, S.S.; Linder, B.; Cowie, C.C. Prevalence of gestational diabetes and subsequent Type 2 diabetes among U.S. women. Diabetes Res. Clin. Pract. 2018, 141, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Nielsen, M.F.; Kallfa, E.; Dubietyte, G.; Lauszus, F.F. Metabolic syndrome in women with previous gestational diabetes. Sci. Rep. 2021, 11, 11558. [Google Scholar] [CrossRef]
- Shen, Y.; Li, W.; Leng, J.; Zhang, S.; Liu, H.; Li, W.; Wang, L.; Tian, H.; Chen, J.; Qi, L.; et al. High risk of metabolic syndrome after delivery in pregnancies complicated by gestational diabetes. Diabetes Res. Clin. Pract. 2019, 150, 219–226. [Google Scholar] [CrossRef]
- Lai, M.; Al Rijjal, D.; Röst, H.L.; Dai, F.F.; Gunderson, E.P.; Wheeler, M.B. Underlying dyslipidemia postpartum in women with a recent GDM pregnancy who develop type 2 diabetes. eLife 2020, 9, e59153. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic-Vukas, J.; Capling, L.; Cheng, S.; Stamatakis, E.; Louie, J.; Wah Cheung, N.; Markovic, T.; Ross, G.; Senior, A.; Brand-Miller, J.C.; et al. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 698. [Google Scholar] [CrossRef]
- Al Wattar, B.H.; Dodds, J.; Placzek, A.; Beresford, L.; Spyreli, E.; Moore, A.; Gonzalez Carreras, F.J.; Austin, F.; Murugesu, N.; Roseboom, T.J.; et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): A pragmatic multicentre randomised trial. PLoS Med. 2019, 16, e1002857. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; de la Torre, N.G.; Durán, A.; Bordiu, E.; del Valle, L.; Familiar, C.; Valerio, J.; Jimenez, I.; Herraiz, M.A.; Izquierdo, N.; et al. An Early, Universal Mediterranean Diet-Based Intervention in Pregnancy Reduces Cardiovascular Risk Factors in the “Fourth Trimester”. J. Clin. Med. 2019, 8, 1499. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ferre, N.; Del Valle, L.; Torrejón, M.J.; Barca, I.; Calvo, M.I.; Matía, P.; Rubio, M.A.; Calle-Pascual, A.L. Diabetes mellitus and abnormal glucose tolerance development after gestational diabetes: A three-year, prospective, randomized, clinical-based, Mediterranean lifestyle interventional study with parallel groups. Clin. Nutr. 2015, 34, 579–585. [Google Scholar] [CrossRef]
- Li, N.; Yang, Y.; Cui, D.; Li, C.; Ma, R.C.W.; Li, J.; Yang, X. Effects of lifestyle intervention on long-term risk of diabetes in women with prior gestational diabetes: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2021, 22, e13122. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; García De La Torre, N.; Durán, A.; Fuentes, M.; Bordiú, E.; Del Valle, L.; Familiar, C.; Ortolá, A.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef]
- Song, C.; Li, J.; Leng, J.; Ma, R.C.; Yang, X. Lifestyle intervention can reduce the risk of gestational diabetes: A meta-analysis of randomized controlled trials. Obes. Rev. 2016, 17, 960–969. [Google Scholar] [CrossRef]
- Guo, X.Y.; Shu, J.; Fu, X.H.; Chen, X.P.; Zhang, L.; Ji, M.X.; Liu, X.M.; Yu, T.T.; Sheng, J.Z.; Huang, H.F. Improving the effectiveness of lifestyle interventions for gestational diabetes prevention: A meta-analysis and meta-regression. BJOG 2019, 126, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Amati, F.; Hassounah, S.; Swaka, A. The Impact of Mediterranean Dietary Patterns During Pregnancy on Maternal and Offspring Health. Nutrients 2019, 11, 1098. [Google Scholar] [CrossRef]
- Duran, A.; Śaenz, S.; Torrejón, M.J.; Bordí, U.E.; Del Valle, L.; Galindo, M.; Perez, N.; Herraiz, M.A.; Izquierdo, N.; Rubio, M.A.; et al. Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: The St. Carlos Gestational Diabetes Study. Diabetes Care 2014, 37, 2442–2450. [Google Scholar] [CrossRef]
- da Silva, V.S.; Vieira, M.F.S. International Society for the Advancement of Kinanthropometry (ISAK) Global: International accreditation scheme of the competent anthropometrist. Rev. Bras. Cineantropom. Desempenho Hum. 2020, 22, e70517. [Google Scholar] [CrossRef]
- Mangla, A.G.; Dhamija, N.; Gupta, U.; Dhall, M. Anthropometric Markers as a Paradigm for Obesity Risk Assessment. J. Biosci. Med. 2020, 8, 1–16. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, N.G.; Assaf-Balut, C.; Varas, I.J.; Del Valle, L.; Durán, A.; Fuentes, M.; Del Prado, N.; Bordiú, E.; Valerio, J.J.; Herraiz, M.A.; et al. Effectiveness of following mediterranean diet recommendations in the real world in the incidence of gestational diabetes mellitus (Gdm) and adverse maternal-foetal outcomes: A prospective, universal, interventional study with a single group. the st carlos study. Nutrients 2019, 11, 1210. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Martínez-Larrad, M.T.; Fernández-Pérez, C.; Corbatón-Anchuelo, A.; Gabriel, R.; Lorenzo, C.; Serrano-Ríos, M. Revised waist circumference cut-off points for the criteria of abdominal obesity in the Spanish population: Multicenter nationwide Spanish population based study. Av. Diabetol. 2011, 27, 168–174. [Google Scholar] [CrossRef]
- Marcuello, C.; Calle-Pascual, A.L.; Fuentes, M.; Runkle, I.; Rubio, M.A.; Montañez, C.; Rojo-Martinez, G.; Soriguer, F.; Bordiu, E.; Goday, A.; et al. Prevalence of the metabolic syndrome in Spain using regional cutoff points for waist circumference: The di@bet.es study. Acta Diabetol. 2013, 50, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Magny-Normilus, C.; McMahon, E.; Whittemore, R. Systematic Review of Lifestyle Interventions for Gestational Diabetes Mellitus in Pregnancy and the Postpartum Period. J. Obstet. Gynecol. Neonatal Nurs. 2022, 51, 115–125. [Google Scholar] [CrossRef]
- Moore, A.P.; D’Amico, M.I.; Cooper, N.A.M.; Thangaratinam, S. Designing a lifestyle intervention to reduce risk of type 2 diabetes in postpartum mothers following gestational diabetes: An online survey with mothers and health professionals. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 220, 106–112. [Google Scholar] [CrossRef]
- Hedeager Momsen, A.M.; Høtoft, D.; Ørtenblad, L.; Friis Lauszus, F.; Krogh, R.H.A.; Lynggaard, V.; Juel Christiansen, J.; Terkildsen Maindal, H.; Vinther Nielsen, C. Diabetes prevention interventions for women after gestational diabetes mellitus: An overview of reviews. Endocrinol. Diabetes Metab. 2021, 4, e00230. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, M.Y.; Yang, J.H.; Park, S.Y.; Yim, C.H.; Han, K.O.; Yoon, H.K.; Park, S. Nutritional risk factors of early development of postpartum prediabetes and diabetes in women with gestational diabetes mellitus. Nutrition 2011, 27, 782–788. [Google Scholar] [CrossRef]
- Huvinen, E.; Koivusalo, S.B.; Meinilä, J.; Valkama, A.; Tiitinen, A.; Rönö, K.; Stach-Lempinen, B.; Eriksson, J.G. Effects of a Lifestyle Intervention During Pregnancy and First Postpartum Year: Findings From the RADIEL Study. J. Clin. Endocrinol. Metab. 2018, 103, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, L.; Liu, H.; Zhang, S.; Tian, H.; Shen, Y.; Tuomilehto, J.; Yu, Z.; Yang, X.; Hu, G.; et al. β-Cell function or insulin resistance was associated with the risk of type 2 diabetes among women with or without obesity and a history of gestational diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001060. [Google Scholar] [CrossRef]
- Miao, Z.; Wu, H.; Ren, L.; Bu, N.; Jiang, L.; Yang, H.; Zhang, J.; Guo, X. Long-Term Postpartum Outcomes of Insulin Resistance and β-cell Function in Women with Previous Gestational Diabetes Mellitus. Int. J. Endocrinol. 2020, 2020, 7417356. [Google Scholar] [CrossRef] [PubMed]
- Assaf-Balut, C.; Bordiú, E.; del Valle, L.; Lara, M.; Duran, A.; Rubio, M.A.; Familiar, C.; Herraiz, M.A.; Izquierdo, N.; Pérez, N.; et al. The impact of switching to the one-step method for GDM diagnosis on the rates of postpartum screening attendance and glucose disorder in women with prior GDM. The San Carlos Gestational Study. J. Diabetes Complicat. 2016, 30, 1360–1364. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, P.; Wang, L.; Zhang, S.; Liu, H.; Li, W.; Li, N.; Li, W.; Leng, J.; Wang, J.; et al. Gestational diabetes with diabetes and prediabetes risks: A large observational study. Eur. J. Endocrinol. 2018, 179, 51–58. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef]
- Diaz-Santana, M.V.; O’brien, K.M.; Park, Y.M.M.; Sandler, D.P.; Weinberg, C.R. Persistence of Risk for Type 2 Diabetes After Gestational Diabetes Mellitus. Diabetes Care 2022, 45, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; de Gennaro, G.; Brocchi, A.; Minaldi, E.; Del Prato, S.; Bertolotto, A. Risk factors associated with postpartum impaired glucose regulation in women with previous gestational diabetes. J. Diabetes Complicat. 2021, 35, 107854. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).