Association between Dietary Indices and Dietary Patterns and Mortality and Cancer Recurrence among Cancer Survivors: An Updated Systematic Review and Meta-Analysis of Cohort Studies
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Certainty of Evidence
2.6. Statistical Analysis
3. Results
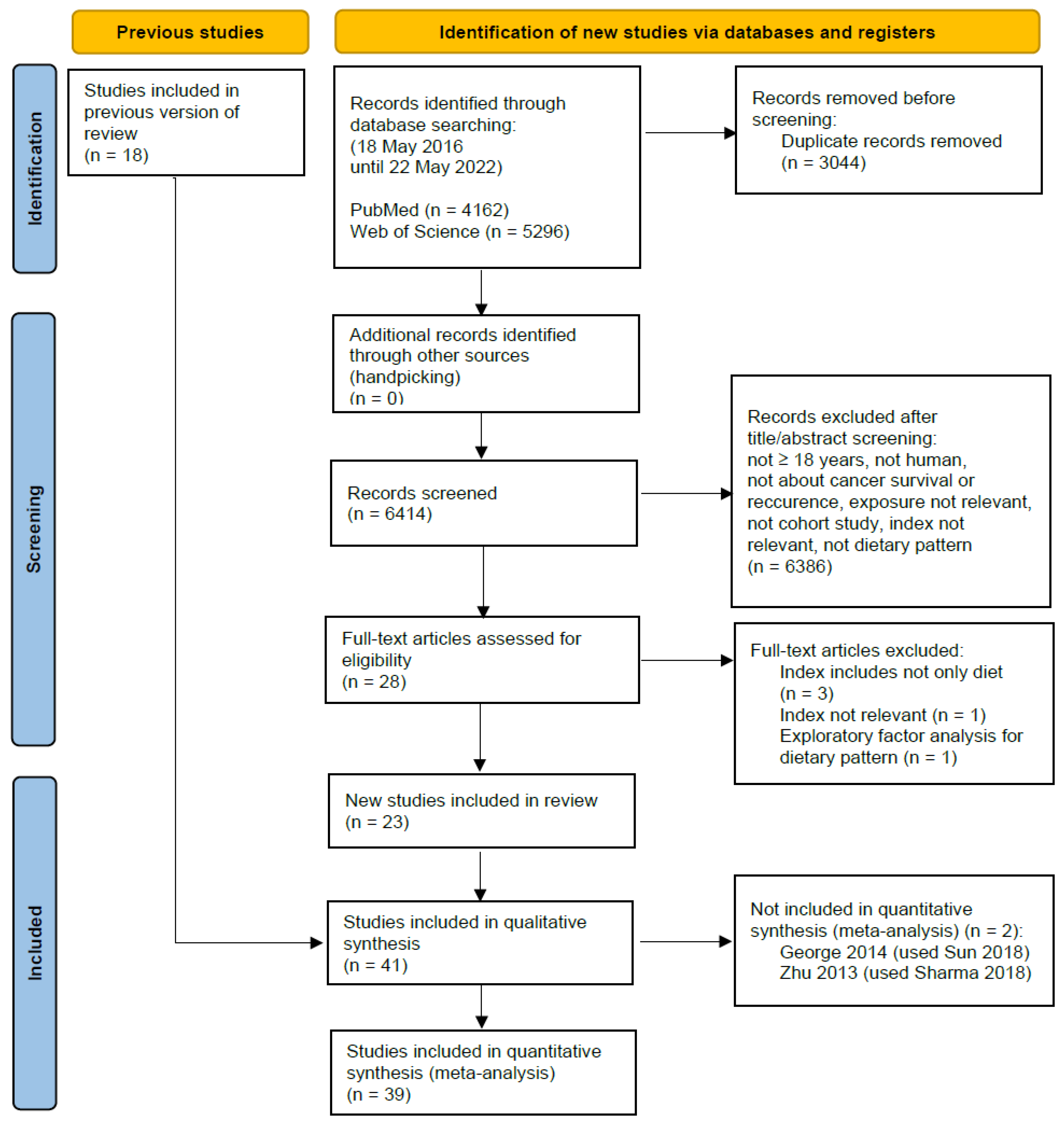
3.1. Literature Search and Study Characteristics
3.2. Risk of Bias Assessment
3.3. Main Outcomes
3.4. Subgroup Analyses–Cancer Types
3.5. Subgroup Analyses–Type of Diet Quality Index
3.6. Small Study Effects and Publication Bias
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Niksic, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Esteve, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.1, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Available online: http://www.wcrf.org/int/cancer-facts-figures/data-cancer-frequency-country (accessed on 12 June 2023).
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 563–591. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; AICR: Washington, DC, USA, 2007. [Google Scholar]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Freisling, H.; Leitzmann, M.F.; Taljaard-Krugell, C.; Jacobs, I.; Kruger, H.S.; Smuts, C.M.; Pieters, M. Diet and sedentary behaviour in relation to cancer survival. A report from the national health and nutrition examination survey linked to the U.S. mortality registry. Clin. Nutr. 2020, 39, 3489–3496. [Google Scholar] [CrossRef]
- Hurtado-Barroso, S.; Trius-Soler, M.; Lamuela-Raventós, R.M.; Zamora-Ros, R. Vegetable and Fruit Consumption and Prognosis Among Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Adv. Nutr. 2020, 11, 1569–1582. [Google Scholar] [CrossRef]
- Ernest, D.K.; Lemus, H.; Hsu, F.C.; Pierce, J.P.; Wu, T. The Independent and Joint Associations of Whole Grain and Refined Grain with Total Mortality among Breast Cancer Survivors: A Prospective Cohort Study. Nutrients 2022, 14, 3333. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; Du, M.; Wang, Y.; Vallis, J.; Parfrey, P.S.; McLaughlin, J.R.; Qi, X.; Wang, P.P. Association between Dietary Fiber Intake and Mortality among Colorectal Cancer Survivors: Results from the Newfoundland Familial Colorectal Cancer Cohort Study and a Meta-Analysis of Prospective Studies. Cancers 2022, 14, 3801. [Google Scholar] [CrossRef]
- Reedy, J.; Subar, A.F. 90th Anniversary Commentary: Diet Quality Indexes in Nutritional Epidemiology Inform Dietary Guidance and Public Health. J. Nutr. 2018, 148, 1695–1697. [Google Scholar] [CrossRef]
- Schwedhelm, C.; Boeing, H.; Hoffmann, G.; Aleksandrova, K.; Schwingshackl, L. Effect of diet on mortality and cancer recurrence among cancer survivors: A systematic review and meta-analysis of cohort studies. Nutr. Rev. 2016, 74, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; Group, P.-S. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Schunemann, H.J.; Cuello, C.; Akl, E.A.; Mustafa, R.A.; Meerpohl, J.J.; Thayer, K.; Morgan, R.L.; Gartlehner, G.; Kunz, R.; Katikireddi, S.V.; et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J. Clin. Epidemiol. 2019, 111, 105–114. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Heitz, A.E.; Baumgartner, R.N.; Baumgartner, K.B.; Boone, S.D. Healthy lifestyle impact on breast cancer-specific and all-cause mortality. Breast Cancer Res. Treat. 2018, 167, 171–181. [Google Scholar] [CrossRef]
- Parada, H., Jr.; Sun, X.; Tse, C.K.; Olshan, A.F.; Troester, M.A. Lifestyle Patterns and Survival Following Breast Cancer in the Carolina Breast Cancer Study. Epidemiology 2019, 30, 83–92. [Google Scholar] [CrossRef]
- Tabung, F.K.; Noonan, A.; Lee, D.H.; Song, M.; Clinton, S.K.; Spakowicz, D.; Wu, K.; Cheng, E.; Meyerhardt, J.A.; Fuchs, C.S.; et al. Post-diagnosis dietary insulinemic potential and survival outcomes among colorectal cancer patients. BMC Cancer 2020, 20, 817. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Fuchs, C.S.; Niedzwiecki, D.; Zhang, S.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Association of Survival with Adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors After Colon Cancer Diagnosis: The CALGB 89803/Alliance Trial. JAMA Oncol. 2018, 4, 783–790. [Google Scholar] [CrossRef]
- Westhoff, E.; Wu, X.; Kiemeney, L.A.; Lerner, S.P.; Ye, Y.; Huang, M.; Dinney, C.P.; Vrieling, A.; Tu, H. Dietary patterns and risk of recurrence and progression in non-muscle-invasive bladder cancer. Int. J. Cancer 2018, 142, 1797–1804. [Google Scholar] [CrossRef]
- George, S.M.; Alfano, C.M.; Neuhouser, M.L.; Smith, A.W.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L.; Ballard-Barbash, R. Better postdiagnosis diet quality is associated with less cancer-related fatigue in breast cancer survivors. J. Cancer Surviv. 2014, 8, 680–687. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, H.; Wang, P.P.; Savas, S.; Woodrow, J.; Wish, T.; Jin, R.; Green, R.; Woods, M.; Roebothan, B.; et al. Dietary patterns and colorectal cancer recurrence and survival: A cohort study. BMJ Open 2013, 3, e002270. [Google Scholar] [CrossRef] [PubMed]
- Izano, M.A.; Fung, T.T.; Chiuve, S.S.; Hu, F.B.; Holmes, M.D. Are diet quality scores after breast cancer diagnosis associated with improved breast cancer survival? Nutr. Cancer 2013, 65, 820–826. [Google Scholar] [CrossRef]
- Di Maso, M.; Augustin, L.S.A.; Toffolutti, F.; Stocco, C.; Dal Maso, L.; Jenkins, D.J.A.; Fleshner, N.E.; Serraino, D.; Polesel, J. Adherence to Mediterranean Diet, Physical Activity and Survival after Prostate Cancer Diagnosis. Nutrients 2021, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Irwin, M.L.; Smith, A.W.; Neuhouser, M.L.; Reedy, J.; McTiernan, A.; Alfano, C.M.; Bernstein, L.; Ulrich, C.M.; Baumgartner, K.B.; et al. Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early-stage breast cancer. Cancer Causes Control 2011, 22, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Meyerhardt, J.A.; Niedzwiecki, D.; Hollis, D.; Saltz, L.B.; Hu, F.B.; Mayer, R.J.; Nelson, H.; Whittom, R.; Hantel, A.; Thomas, J.; et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA 2007, 298, 754–764. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Zhang, S.; Ou, F.S.; Venlo, A.; Ng, K.; Atreya, C.; Van Loon, K.; Niedzwiecki, D.; Giovannucci, E.; Wolfe, E.G.; et al. Association of Diet Quality With Survival Among People With Metastatic Colorectal Cancer in the Cancer and Leukemia B and Southwest Oncology Group 80405 Trial. JAMA Netw. Open 2020, 3, e2023500. [Google Scholar] [CrossRef]
- Al Ramadhani, R.M.; Nagle, C.M.; Ibiebele, T.I.; Grant, P.; Friedlander, M.; DeFazio, A.; Webb, P.M. Pre- and Post-Diagnosis Diet Quality and Ovarian Cancer Survival. Cancer Epidemiol. Biomark. Prev. 2021, 30, 229–232. [Google Scholar] [CrossRef]
- Anyene, I.C.; Ergas, I.J.; Kwan, M.L.; Roh, J.M.; Ambrosone, C.B.; Kushi, L.H.; Cespedes Feliciano, E.M. Plant-Based Dietary Patterns and Breast Cancer Recurrence and Survival in the Pathways Study. Nutrients 2021, 13, 3374. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.E.; Peterson, K.E.; Rozek, L.S.; Taylor, J.M.; Light, E.; Chepeha, D.B.; Hébert, J.R.; Terrell, J.E.; Wolf, G.T.; Duffy, S.A. Pretreatment dietary patterns, weight status, and head and neck squamous cell carcinoma prognosis. Am. J. Clin. Nutr. 2013, 97, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.A.; Shirvani, S.M.; Likhacheva, A.; Chhatwal, J.; Chiao, E.Y.; Sonawane, K. The Association Between Dietary Quality and Overall and Cancer-Specific Mortality Among Cancer Survivors, NHANES III. JNCI Cancer Spectr. 2018, 2, pky022. [Google Scholar] [CrossRef] [PubMed]
- Di Maso, M.; Dal Maso, L.; Augustin, L.S.A.; Puppo, A.; Falcini, F.; Stocco, C.; Mattioli, V.; Serraino, D.; Polesel, J. Adherence to the Mediterranean Diet and Mortality after Breast Cancer. Nutrients 2020, 12, 3649. [Google Scholar] [CrossRef]
- Ergas, I.J.; Cespedes Feliciano, E.M.; Bradshaw, P.T.; Roh, J.M.; Kwan, M.L.; Cadenhead, J.; Santiago-Torres, M.; Troeschel, A.N.; Laraia, B.; Madsen, K.; et al. Diet Quality and Breast Cancer Recurrence and Survival: The Pathways Study. JNCI Cancer Spectr. 2021, 5, pkab019. [Google Scholar] [CrossRef]
- Ferronha, I.; Castro, C.; Carreira, H.; Bento, M.J.; Carvalho, I.; Peleteiro, B.; Lunet, N. Prediagnosis lifestyle exposures and survival of gastric cancer patients: A cohort study from Portugal. Br. J. Cancer 2012, 107, 537–543. [Google Scholar] [CrossRef]
- Fung, T.T.; Kashambwa, R.; Sato, K.; Chiuve, S.E.; Fuchs, C.S.; Wu, K.; Giovannucci, E.; Ogino, S.; Hu, F.B.; Meyerhardt, J.A. Post diagnosis diet quality and colorectal cancer survival in women. PLoS ONE 2014, 9, e115377. [Google Scholar] [CrossRef]
- Guinter, M.A.; McCullough, M.L.; Gapstur, S.M.; Campbell, P.T. Associations of Pre- and Postdiagnosis Diet Quality with Risk of Mortality Among Men and Women with Colorectal Cancer. J. Clin. Oncol. 2018, 36, 3404–3410. [Google Scholar] [CrossRef]
- Inoue-Choi, M.; Robien, K.; Lazovich, D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol. Biomark. Prev. 2013, 22, 792–802. [Google Scholar] [CrossRef]
- Jacobs, S.; Harmon, B.E.; Ollberding, N.J.; Wilkens, L.R.; Monroe, K.R.; Kolonel, L.N.; Le Marchand, L.; Boushey, C.J.; Maskarinec, G. Among 4 Diet Quality Indexes, Only the Alternate Mediterranean Diet Score Is Associated with Better Colorectal Cancer Survival and Only in African American Women in the Multiethnic Cohort. J. Nutr. 2016, 146, 1746–1755. [Google Scholar] [CrossRef]
- Karavasiloglou, N.; Pestoni, G.; Faeh, D.; Rohrmann, S. Post-Diagnostic Diet Quality and Mortality in Females with Self-Reported History of Breast or Gynecological Cancers: Results from the Third National Health and Nutrition Examination Survey (NHANES III). Nutrients 2019, 11, 2558. [Google Scholar] [CrossRef] [PubMed]
- Kenfield, S.A.; DuPre, N.; Richman, E.L.; Stampfer, M.J.; Chan, J.M.; Giovannucci, E.L. Mediterranean diet and prostate cancer risk and mortality in the Health Professionals Follow-up Study. Eur. Urol. 2014, 65, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Willett, W.C.; Fung, T.; Rosner, B.; Holmes, M.D. Diet quality indices and postmenopausal breast cancer survival. Nutr. Cancer 2011, 63, 381–388. [Google Scholar] [CrossRef]
- Kroenke, C.H.; Fung, T.T.; Hu, F.B.; Holmes, M.D. Dietary patterns and survival after breast cancer diagnosis. J. Clin. Oncol. 2005, 23, 9295–9303. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Weltzien, E.; Kushi, L.H.; Castillo, A.; Slattery, M.L.; Caan, B.J. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J. Clin. Oncol. 2009, 27, 919–926. [Google Scholar] [CrossRef]
- Lee, D.H.; Fung, T.T.; Tabung, F.K.; Marinac, C.R.; Devore, E.E.; Rosner, B.A.; Ghobrial, I.M.; Colditz, G.A.; Giovannucci, E.L.; Birmann, B.M. Prediagnosis dietary pattern and survival in patients with multiple myeloma. Int. J. Cancer 2020, 147, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Ho, S.C.; Kwok, C.; Cheng, A.C.; Cheung, K.L.; Lee, R.; Yeo, W. Dietary Pattern at 18-Month Post-Diagnosis and Outcomes of Breast Cancer Among Chinese Women with Early-Stage Breast Cancer. Cancer Manag. Res. 2021, 13, 4553–4565. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Y.J.; Zhang, D.M.; Yishake, D.; Liu, Z.Y.; Chen, M.S.; Wang, F.; Zhou, Z.G.; Long, J.A.; Zhong, R.H.; et al. Association between dietary patterns and prognosis of hepatocellular carcinoma in the Guangdong liver cancer cohort study. Hepatol. Res. 2020, 50, 1164–1175. [Google Scholar] [CrossRef]
- McCullough, M.L.; Gapstur, S.M.; Shah, R.; Campbell, P.T.; Wang, Y.; Doyle, C.; Gaudet, M.M. Pre- and postdiagnostic diet in relation to mortality among breast cancer survivors in the CPS-II Nutrition Cohort. Cancer Causes Control 2016, 27, 1303–1314. [Google Scholar] [CrossRef]
- Ollberding, N.J.; Aschebrook-Kilfoy, B.; Caces, D.B.; Smith, S.M.; Weisenburger, D.D.; Chiu, B.C. Dietary intake of fruits and vegetables and overall survival in non-Hodgkin lymphoma. Leuk. Lymphoma 2013, 54, 2613–2619. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, M.; Shvetsov, Y.B.; Setiawan, V.W.; Boushey, C.J.; Haiman, C.A.; Wilkens, L.R.; Le Marchand, L. Diet quality and all-cause and cancer-specific mortality in cancer survivors and non-cancer individuals: The Multiethnic Cohort Study. Eur. J. Nutr. 2022, 61, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Pelser, C.; Arem, H.; Pfeiffer, R.M.; Elena, J.W.; Alfano, C.M.; Hollenbeck, A.R.; Park, Y. Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP Diet and Health Study. Cancer 2014, 120, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, I.; Schafmayer, C.; di Giuseppe, R.; Waniek, S.; Plachta-Danielzik, S.; Koch, M.; Nöthlings, U.; Hampe, J.; Schlesinger, S.; Lieb, W. Postdiagnostic Mediterranean and Healthy Nordic Dietary Patterns Are Inversely Associated with All-Cause Mortality in Long-Term Colorectal Cancer Survivors. J. Nutr. 2017, 147, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, I.; Enderle, J.; Burmeister, G.; Koch, M.; Nöthlings, U.; Hampe, J.; Lieb, W. Post-diagnostic reliance on plant-compared with animal-based foods and all-cause mortality in omnivorous long-term colorectal cancer survivors. Am. J. Clin. Nutr. 2021, 114, 441–449. [Google Scholar] [CrossRef]
- Sharma, I.; Roebothan, B.; Zhu, Y.; Woodrow, J.; Parfrey, P.S.; McLaughlin, J.R.; Wang, P.P. Hypothesis and data-driven dietary patterns and colorectal Cancer survival: Findings from Newfoundland and Labrador colorectal Cancer cohort. Nutr. J. 2018, 17, 55. [Google Scholar] [CrossRef]
- Song, R.; Petimar, J.; Wang, M.; Tabung, F.K.; Song, M.; Liu, L.; Lee, D.H.; Giovannucci, E.L.; Zhang, X.; Smith-Warner, S.A. Adherence to the World Cancer Research Fund/American Institute for Cancer Research Cancer Prevention Recommendations and Colorectal Cancer Survival. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1816–1825. [Google Scholar] [CrossRef]
- Sun, Y.; Bao, W.; Liu, B.; Caan, B.J.; Lane, D.S.; Millen, A.E.; Simon, M.S.; Thomson, C.A.; Tinker, L.F.; Van Horn, L.V.; et al. Changes in Overall Diet Quality in Relation to Survival in Postmenopausal Women with Breast Cancer: Results from the Women’s Health Initiative. J. Acad. Nutr. Diet. 2018, 118, 1855–1863.e1856. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A.; Crane, T.E.; Wertheim, B.C.; Neuhouser, M.L.; Li, W.; Snetselaar, L.G.; Basen-Engquist, K.M.; Zhou, Y.; Irwin, M.L. Diet quality and survival after ovarian cancer: Results from the Women’s Health Initiative. J. Natl. Cancer Inst. 2014, 106, dju314. [Google Scholar] [CrossRef]
- van Zutphen, M.; Boshuizen, H.C.; Kenkhuis, M.F.; Wesselink, E.; Geijsen, A.; de Wilt, J.H.W.; van Halteren, H.K.; Spillenaar Bilgen, E.J.; Keulen, E.T.P.; Janssen-Heijnen, M.L.G.; et al. Lifestyle after colorectal cancer diagnosis in relation to recurrence and all-cause mortality. Am. J. Clin. Nutr. 2021, 113, 1447–1457. [Google Scholar] [CrossRef]
- Vrieling, A.; Buck, K.; Seibold, P.; Heinz, J.; Obi, N.; Flesch-Janys, D.; Chang-Claude, J. Dietary patterns and survival in German postmenopausal breast cancer survivors. Br. J. Cancer 2013, 108, 188–192. [Google Scholar] [CrossRef]
- Wang, F.; Cai, H.; Gu, K.; Shi, L.; Yu, D.; Zhang, M.; Zheng, W.; Zheng, Y.; Bao, P.; Shu, X.O. Adherence to Dietary Recommendations among Long-Term Breast Cancer Survivors and Cancer Outcome Associations. Cancer Epidemiol. Biomark. Prev. 2020, 29, 386–395. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Liu, C.; Liu, F.H.; Wei, Y.F.; Xu, H.L.; Wang, R.; Li, X.Y.; Li, Y.Z.; Yan, S.; Qin, X.; et al. Association between pre-diagnostic dietary pattern and survival of ovarian cancer: Evidence from a prospective cohort study. Clin. Nutr. 2022, 41, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Kenfield, S.A.; Van Blarigan, E.L.; Batista, J.L.; Sesso, H.D.; Ma, J.; Stampfer, M.J.; Chavarro, J.E. Dietary patterns after prostate cancer diagnosis in relation to disease-specific and total mortality. Cancer Prev. Res. 2015, 8, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kady, V.; Han, E.; Montan, K.; Normuminova, M.; Rovito, M.J. Healthy Eating and Mortality among Breast Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Int. J. Environ. Res. Public Health 2022, 19, 7579. [Google Scholar] [CrossRef] [PubMed]
- Morze, J.; Danielewicz, A.; Hoffmann, G.; Schwingshackl, L. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: A Second Update of a Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2020, 120, 1998–2031.e1915. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef]
- Steck, S.E.; Murphy, E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer 2020, 20, 125–138. [Google Scholar] [CrossRef]
- Liese, A.D.; Krebs-Smith, S.M.; Subar, A.F.; George, S.M.; Harmon, B.E.; Neuhouser, M.L.; Boushey, C.J.; Schap, T.E.; Reedy, J. The Dietary Patterns Methods Project: Synthesis of Findings across Cohorts and Relevance to Dietary Guidance. J. Nutr. 2015, 145, 393–402. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer. II. Mechanisms. Cancer Causes Control 1991, 2, 427–442. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, F.; Jiang, L.; Miao, Z.; Liang, X.; Yang, J.; Larsson, S.C.; Zheng, J.S. Circulating vitamin C concentration and risk of cancers: A Mendelian randomization study. BMC Med. 2021, 19, 171. [Google Scholar] [CrossRef]
- Arayici, M.E.; Mert-Ozupek, N.; Yalcin, F.; Basbinar, Y.; Ellidokuz, H. Soluble and Insoluble Dietary Fiber Consumption and Colorectal Cancer Risk: A Systematic Review and Meta-Analysis. Nutr. Cancer 2022, 74, 2412–2425. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L. Mechanisms for the impact of whole grain foods on cancer risk. J. Am. Coll. Nutr. 2000, 19, 300S–307S. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Vernia, F.; Longo, S.; Stefanelli, G.; Viscido, A.; Latella, G. Dietary Factors Modulating Colorectal Carcinogenesis. Nutrients 2021, 13, 143. [Google Scholar] [CrossRef]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Navarro Rosenblatt, D.A.; Chan, D.S.; Vieira, A.R.; Vieira, R.; Greenwood, D.C.; Vatten, L.J.; Norat, T. Dairy products, calcium, and prostate cancer risk: A systematic review and meta-analysis of cohort studies. Am. J. Clin. Nutr. 2015, 101, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R. Calcium and/or vitamin D supplementation: Could they affect your risks of colorectal cancer development or progression? Ann. Transl. Med. 2018, 6, S4. [Google Scholar] [CrossRef]
- Kim, H.; Hur, J.; Wu, K.; Song, M.; Wang, M.; Smith-Warner, S.A.; Zhang, X.; Giovannucci, E.L. Total calcium, dairy foods and risk of colorectal cancer: A prospective cohort study of younger US women. Int. J. Epidemiol. 2023, 52, 87–95. [Google Scholar] [CrossRef]
- Norat, T.; Riboli, E. Dairy products and colorectal cancer. A review of possible mechanisms and epidemiological evidence. Eur. J. Clin. Nutr. 2003, 57, 1–17. [Google Scholar] [CrossRef]
- Parra-Soto, S.; Ahumada, D.; Petermann-Rocha, F.; Boonpoor, J.; Gallegos, J.L.; Anderson, J.; Sharp, L.; Malcomson, F.C.; Livingstone, K.M.; Mathers, J.C.; et al. Association of meat, vegetarian, pescatarian and fish-poultry diets with risk of 19 cancer sites and all cancer: Findings from the UK Biobank prospective cohort study and meta-analysis. BMC Med. 2022, 20, 79. [Google Scholar] [CrossRef]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef]
- Linhart, K.; Bartsch, H.; Seitz, H.K. The role of reactive oxygen species (ROS) and cytochrome P-450 2E1 in the generation of carcinogenic etheno-DNA adducts. Redox Biol. 2014, 3, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Millonig, G.; Wang, Y.; Homann, N.; Bernhardt, F.; Qin, H.; Mueller, S.; Bartsch, H.; Seitz, H.K. Ethanol-mediated carcinogenesis in the human esophagus implicates CYP2E1 induction and the generation of carcinogenic DNA-lesions. Int. J. Cancer 2011, 128, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Fedirko, V.; Tran, H.Q.; Gewirtz, A.T.; Stepien, M.; Trichopoulou, A.; Aleksandrova, K.; Olsen, A.; Tjonneland, A.; Overvad, K.; Carbonnel, F.; et al. Exposure to bacterial products lipopolysaccharide and flagellin and hepatocellular carcinoma: A nested case-control study. BMC Med. 2017, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Castelló, A.; Pollán, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Lope, V.; Antolín, S.; Ramos, M.; Muñoz, M.; et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case-control EpiGEICAM study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Hershey, M.S.; Zazpe, I.; Trichopoulou, A. Transferability of the Mediterranean Diet to Non-Mediterranean Countries. What Is and What Is Not the Mediterranean Diet. Nutrients 2017, 9, 1226. [Google Scholar] [CrossRef]
- van Zutphen, M.; Boshuizen, H.C.; Kok, D.E.; van Baar, H.; Geijsen, A.; Wesselink, E.; Winkels, R.M.; van Halteren, H.K.; de Wilt, J.H.W.; Kampman, E.; et al. Colorectal cancer survivors only marginally change their overall lifestyle in the first 2 years following diagnosis. J. Cancer Surviv. 2019, 13, 956–967. [Google Scholar] [CrossRef]
- Tan, S.Y.; Wong, H.Y.; Vardy, J.L. Do cancer survivors change their diet after cancer diagnosis? Support Care Cancer 2021, 29, 6921–6927. [Google Scholar] [CrossRef] [PubMed]
- Aldossari, A.; Sremanakova, J.; Sowerbutts, A.M.; Jones, D.; Hann, M.; Burden, S.T. Do people change their eating habits after a diagnosis of cancer? A systematic review. J. Hum. Nutr. Diet. 2023, 36, 566–579. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund. About the Global Cancer Update Programme. Available online: https://www.wcrf.org/diet-activity-and-cancer/global-cancer-update-programme/about-the-global-cancer-update-programme/ (accessed on 12 June 2023).
| Inclusion | Exclusion | |
|---|---|---|
| Populations/participants |
|
|
| Interventions/exposure |
|
|
| Comparators/Comparison |
| |
| Outcomes |
|
|
| Study designs |
|
|
| Study | Bias Due to Confounding | Bias Due to Selection of Participants | Bias Due to Exposure Assessment | Bias Due to Misclassification during Follow-Up | Bias Due to Missing Data | Bias Due to Measurement of the Outcome | Bias Due to Selective Reporting of the Results | Overall Judgment |
|---|---|---|---|---|---|---|---|---|
| Al Ramadhani et al. (2021) [32] | High | Low | Moderate | Moderate | Low | Low | Low | H |
| Anyene et al. (2021) [33] | High | Moderate | Moderate | Low | Low | Low | Low | H |
| Arthur et al. (2013) [34] | Moderate | Moderate | Moderate | Moderate | Low | Low | Low | M |
| Deshmukh et al. (2018) [35] | High | Low | High | Moderate | Low | Low | Low | H |
| Di Maso et al. (2020) [36] | High | Moderate | Moderate | Moderate | Low | Low | Low | H |
| Di Maso et al. (2021) [28] | High | Moderate | Moderate | Moderate | Low | Low | Low | H |
| Ergas et al. (2021) [37] | Moderate | Moderate | Moderate | Moderate | Low | Low | Low | M |
| Ferronha et al. (2012) [38] | Moderate | Moderate | Moderate | Moderate | Low | Low | Low | M |
| Fung et al. (2014) [39] | Moderate | Low | Moderate | Moderate | Low | Low | Low | M |
| George et al. (2011) [29] | N.I. | Low | Moderate | Moderate | Low | Low | Low | N.I. |
| Guinter et al. (2018) [40] | Moderate | Low | Moderate | Low | Low | Low | Low | M |
| Inoue-Choi et al. (2013) [41] | High | Low | Moderate | Moderate | Low | Low | Low | H |
| Izano et al. (2013) [27] | Moderate | Low | Moderate | Low | Low | Moderate | Low | M |
| Jacobs et al. (2016) [42] | Moderate | Moderate | Moderate | Low | Low | Low | Low | M |
| Karavasiloglou et al. (2019) [43] | High | Low | High | Moderate | Low | Low | Low | H |
| Kenfield et al. (2014) [44] | Moderate | Moderate | Moderate | Low | Low | Moderate | Low | M |
| Kim et al. (2011) [45] | Moderate | Low | Moderate | Moderate | Low | Moderate | Low | M |
| Kroenke et al. (2005) [46] | Moderate | Low | Moderate | Moderate | Low | Moderate | Low | M |
| Kwan et al. (2009) [47] | Moderate | Low | Moderate | Moderate | Low | Low | Low | M |
| Lee et al. (2020) [48] | High | Low | Moderate | Low | Low | Low | Low | H |
| Lei et al. (2021) [49] | High | Moderate | Moderate | Moderate | Low | Low | Low | H |
| Luo et al. (2020) [50] | Moderate | Moderate | Moderate | Moderate | Low | Low | Low | M |
| McCullough et al. (2016) [51] | Moderate | Low | Moderate | Moderate | Low | Low | Low | M |
| Meyerhardt et al. (2007) [30] | Moderate | Moderate | Moderate | Low | N.I. | N.I. | Low | N.I. |
| Ollberding et al. (2013) [52] | High | Moderate | Moderate | Moderate | Low | Low | Low | H |
| Park et al. (2022) [53] | Moderate | Low | Moderate | Low | Low | Low | Low | M |
| Pelser et al. (2014) [54] | Moderate | Moderate | Moderate | Moderate | Low | Low | Low | M |
| Ratjen et al. (2017) [55] | High | Low | Moderate | Moderate | Low | Low | Low | H |
| Ratjen et al. (2021) [56] | High | Low | Moderate | Moderate | Low | Low | Low | H |
| Sharma et al. (2018) [57] | High | Moderate | Moderate | Moderate | Low | Moderate | Low | H |
| Song et al. (2021) [58] | High | Low | Moderate | Low | Low | Low | Low | H |
| Sun et al. (2018) [59] | High | Moderate | Moderate | Low | Low | Low | Low | H |
| Thomson et al. (2014) [60] | High | Moderate | Moderate | Moderate | Low | Low | Low | H |
| Van Blarigan et al. (2020) [31] | High | Moderate | Moderate | Moderate | N.I. | N.I. | Low | N.I. |
| Van Zutphen et al. (2021) [61] | Moderate | Moderate | Moderate | Low | Low | Low | Low | M |
| Vrieling et al. (2013) [62] | Moderate | Moderate | Moderate | Moderate | Low | Moderate | Low | M |
| Wang et al. (2020) [63] | Moderate | Moderate | Moderate | Moderate | Low | Low | Low | M |
| Wen et al. (2022) [64] | High | Moderate | Moderate | Low | Low | Low | Low | H |
| Yang et al. (2015) [65] | High | Low | Moderate | Moderate | Low | Moderate | Low | H |
| Exposure | No. of Studies | Risk Ratio (95% CI) | I2 | Forest Plot |
|---|---|---|---|---|
| All diet-quality indices, Cancer subtypes | Figure S1 | |||
| Breast cancer | 10 | 0.83 [0.77, 0.91] | 58% | |
| Colorectal cancer | 10 | 0.85 [0.78, 0.93] | 56% | |
| All cancer types | 3 | 0.74 [0.65, 0.86] | 50% | |
| All diet-quality indices, Cancer subtypes, postdiagnosis only | Figure S2 | |||
| Breast cancer | 8 | 0.83 [0.75, 0.92] | 43% | |
| Colorectal cancer | 6 | 0.78 [0.67, 0.91] | 45% | |
| All cancer types | 3 | 0.74 [0.65, 0.86] | 50% | |
| Diet-quality indices | Figure S3 | |||
| HEI | 10 | 0.81 [0.74, 0.88] | 41% | |
| AHEI | 6 | 0.82 [0.72, 0.94] | 60% | |
| MED | 13 | 0.77 [0.72, 0.83] | 23% | |
| DASH | 9 | 0.86 [0.80, 0.93] | 28% | |
| ACS | 4 | 0.83 [0.69, 1.00] | 56% | |
| WCRF | 3 | 0.89 [0.77, 1.02] | 0% | |
| Diet-quality indices, postdiagnosis only | Figure S4 | |||
| HEI | 5 | 0.73 [0.64, 0.82] | 29% | |
| AHEI | 3 | 0.81 [0.73, 0.89] | 0% | |
| MED | 6 | 0.75 [0.68, 0.84] | 28% | |
| DASH | 5 | 0.81 [0.74, 0.88] | 0% | |
| ACS | 3 | 0.78 [0.58, 1.05] | 58% | |
| WCRF | 3 | 0.88 [0.75, 1.04] | 0% | |
| Healthy/prudent dietary pattern | Figure S5 | |||
| Breast cancer | 4 | 0.84 [0.61, 1.15] | 53% | |
| Colorectal cancer | 5 | 0.89 [0.77, 1.04] | 19% | |
| Healthy/prudent dietary pattern, postdiagnosis only | Figure S6 | |||
| Breast cancer | 3 | 0.84 [0.52, 1.34] | 68% | |
| Colorectal cancer | 3 | 0.93 [0.66, 1.30] | 66% | |
| Unhealthy/western dietary pattern | Figure S7 | |||
| Breast cancer | 4 | 1.36 [1.11, 1.66] | 4% | |
| Colorectal cancer | 5 | 1.28 [0.96, 1.70] | 76% | |
| Unhealthy/western dietary pattern, postdiagnosis only | Figure S8 | |||
| Breast cancer | 3 | 1.31 [0.91, 1.89] | 37% | |
| Colorectal cancer | 3 | 1.47 [1.05, 2.05] | 53% |
| Exposure | No. of Studies | Risk Ratio (95% CI) | I2 | Forest Plot |
|---|---|---|---|---|
| Cancer subtypes | Figure S9 | |||
| Breast cancer | 9 | 0.92 [0.79, 1.06] | 69% | |
| Colorectal cancer | 5 | 0.88 [0.77, 0.99] | 44% | |
| All cancer types | 3 | 0.67 [0.47, 0.96] | 72% | |
| Cancer subtypes, postdiagnosis only | Figure S10 | |||
| Breast cancer | 7 | 0.95 [0.76, 1.19] | 73% | |
| Colorectal cancer | 3 | 0.74 [0.49, 1.13] | 68% | |
| All cancer types | 3 | 0.67 [0.47, 0.96] | 72% | |
| Diet-quality indices | Figure S11 | |||
| HEI | 11 | 0.86 [0.76, 0.98] | 53% | |
| AHEI | 6 | 0.88 [0.73, 1.06] | 69% | |
| MED | 9 | 0.84 [0.73, 0.96] | 45% | |
| DASH | 8 | 0.84 [0.76, 0.93] | 0% | |
| ACS | 3 | 0.85 [0.56, 1.28] | 66% | |
| Diet-quality indices, postdiagnosis only | Figure S12 | |||
| HEI | 6 | 0.79 [0.58, 1.10] | 72% | |
| AHEI | 4 | 0.93 [0.77, 1.13] | 27% | |
| MED | 4 | 0.90 [0.69, 1.17] | 64% | |
| DASH | 5 | 0.78 [0.68, 0.90] | 9% | |
| Healthy/prudent dietary pattern | Figure S13 | |||
| Breast cancer | 4 | 0.99 [0.77, 1.28] | 0% | |
| Healthy/prudent dietary pattern, postdiagnosis only | Figure S14 | |||
| Breast cancer | 3 | 1.06 [0.77, 1.46] | 0% | |
| Unhealthy/western dietary pattern | Figure S15 | |||
| Breast cancer | 4 | 1.01 [0.79, 1.30] | 0% | |
| Unhealthy/western dietary pattern, postdiagnosis only | 6 | 1.15 [0.84, 1.58] | 28% | Figure S16 |
| Breast cancer | 3 | 1.03 [0.72, 1.46] | 0% |
| Exposure | No. of Studies | Risk Ratio (95% CI) | I2 | Forest Plot |
|---|---|---|---|---|
| Healthy/prudent dietary pattern | Figure S17 | |||
| Breast cancer | 3 | 0.87 [0.68, 1.10] | 0% | |
| Unhealthy/western dietary pattern | Figure S18 | |||
| Breast cancer | 3 | 0.96 [0.74, 1.25] | 0% |
| N of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Relative Effect (95% CI) | Certainty |
|---|---|---|---|---|---|---|---|---|
| Diet-quality indices-Overall mortality | ||||||||
| 28 | observational studies | very serious a | not serious | not serious | not serious | none | RR 0.81 (0.77 to 0.86) | ⨁⨁◯◯ Low |
| Healthy/prudent dietary pattern-Overall mortality | ||||||||
| 14 | observational studies | very serious a | not serious | not serious | not serious | none | RR 0.80 (0.70 to 0.92) | ⨁⨁◯◯ Low |
| Unhealthy/western dietary pattern-Overall mortality | ||||||||
| 14 | observational studies | very serious a | not serious | not serious | not serious | none | RR 1.26 (1.08 to 1.47) | ⨁⨁◯◯ Low |
| Diet-quality indices-Cancer specific mortality | ||||||||
| 23 | observational studies | very serious a | not serious | not serious | not serious | none | RR 0.86 (0.79 to 0.94) | ⨁⨁◯◯ Low |
| Healthy/prudent dietary pattern-Cancer specific mortality | ||||||||
| 8 | observational studies | very serious a | not serious | not serious | not serious | none | RR 0.79 (0.64 to 0.97) | ⨁⨁◯◯ Low |
| Unhealthy/western dietary pattern-Cancer specific mortality | ||||||||
| 8 | observational studies | very serious a | not serious | not serious | serious b | none | RR 1.21 (0.96 to 1.53) | ⨁◯◯◯ Very low |
| Diet-quality indices-Cancer recurrence | ||||||||
| 3 | observational studies | very serious a | not serious | not serious | not serious | none | RR 1.10 (1.02 to 1.19) | ⨁⨁◯◯ Low |
| Healthy/prudent dietary pattern-Cancer recurrence | ||||||||
| 5 | observational studies | very serious a | not serious | not serious | serious c | none | RR 0.89 (0.73 to 1.09) | ⨁◯◯◯ Very low |
| Unhealthy/western dietary pattern-Cancer recurrence | ||||||||
| 5 | observational studies | very serious a | not serious | not serious | serious d | none | RR 1.17 (0.75 to 1.84) | ⨁◯◯◯ Very low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trauchburg, A.; Schwingshackl, L.; Hoffmann, G. Association between Dietary Indices and Dietary Patterns and Mortality and Cancer Recurrence among Cancer Survivors: An Updated Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2023, 15, 3151. https://doi.org/10.3390/nu15143151
Trauchburg A, Schwingshackl L, Hoffmann G. Association between Dietary Indices and Dietary Patterns and Mortality and Cancer Recurrence among Cancer Survivors: An Updated Systematic Review and Meta-Analysis of Cohort Studies. Nutrients. 2023; 15(14):3151. https://doi.org/10.3390/nu15143151
Chicago/Turabian StyleTrauchburg, Angela, Lukas Schwingshackl, and Georg Hoffmann. 2023. "Association between Dietary Indices and Dietary Patterns and Mortality and Cancer Recurrence among Cancer Survivors: An Updated Systematic Review and Meta-Analysis of Cohort Studies" Nutrients 15, no. 14: 3151. https://doi.org/10.3390/nu15143151
APA StyleTrauchburg, A., Schwingshackl, L., & Hoffmann, G. (2023). Association between Dietary Indices and Dietary Patterns and Mortality and Cancer Recurrence among Cancer Survivors: An Updated Systematic Review and Meta-Analysis of Cohort Studies. Nutrients, 15(14), 3151. https://doi.org/10.3390/nu15143151






