Abstract
Mental health problems and obesity are two common complications during pregnancy and postpartum. The preconception period is considered an appropriate period for prevention. Therefore, insights into interpregnancy mental health and the impact on weight and body composition are of interest to developing effective weight management strategies. The primary aim of this study is to assess the difference in women’s mental health during the interpregnancy period and the association with pre-pregnancy body mass index (BMI) and body composition. The secondary aim is to study whether this association is affected by socio-demographic factors, interpregnancy interval and sleep. The study is a secondary analysis of the INTER-ACT e-health-supported lifestyle trial. Women were eligible if they had a subsequent pregnancy and mental health measurements at 6 weeks after childbirth and at the start of the next pregnancy (n = 276). We used univariate analyses to assess differences in mental health and performed regression analysis to assess their association with pre-pregnancy BMI and body composition at the start of the next pregnancy. Our results show a statistically significant increase in anxiety and depressive symptoms between 6 weeks after childbirth and the start of the next pregnancy (sSTAI-6 ≥ 40: +13%, p =≤ 0.001; GMDS ≥ 13: +9%, p = 0.01). Of the women who were not anxious at 6 weeks after childbirth (sSTAI < 40), more than one-third (39%) developed anxiety at the start of the next pregnancy (p =≤ 0.001). Regression analysis showed that sense of coherence (SOC-13) at the start of the next pregnancy was independently associated with women’s pre-pregnancy BMI and fat percentage. We believe that the development of preconception lifestyle interventions that focus on both weight reduction and support in understanding, managing and giving meaning to stressful events (sense of coherence) may be of added value in optimizing women’s preconception health.
1. Introduction
In high-income countries, it is estimated that 1 in 10 mothers experience perinatal mental health problems [1]. Nevertheless, perinatal depression is the most underdiagnosed obstetric complication [2,3,4], and, also, prenatal anxiety remains often undetected [5]. It is estimated that 70% of women hide or minimize their perinatal mental problems [6].
Negative maternal mental health is associated with women’s weight, body composition and sleep. Women with excessive GWG reported higher levels of maternal anxiety 2 days after childbirth compared to women with adequate GWG [7]. Women with pre-pregnancy overweight, whether or not in combination with excessive gestational weight gain (GWG), are at high risk for postpartum depression compared to their counterparts with a healthy pre-pregnancy weight [8,9,10]. Postpartum weight retention (PPWR) at 6 months is associated with higher levels of depression and anxiety from 6 months onwards [11]. Similarly, prospective cohort studies suggest that perinatal depression and anxiety are predictors of excessive GWG and PPWR [12,13,14]. Higher levels of depressive symptoms are also related to sleep disruption, and women with poor sleep are more vulnerable to experiencing perinatal depressive symptoms [15]. In the long term, negative mental health during the peripartum period can result in the development of chronic mental disorders, in both mother and child.
Prenatal lifestyle interventions focusing on diet and physical activity showed a positive impact on reducing GWG; however, the effect on the reduction in perinatal complications (e.g., diabetes and pregnancy-induced hypertension) is limited [16,17,18]. Previous research showed that a period between 2 and 8 months should be taken into account to build new behavior into daily routines [19], so behavioral changes take time. This may indicate that lifestyle interventions may create more impact if they start during the preconception period. Another possible reason for low lifestyle intervention effects may be the lack of attention to women’s mental health [20,21]. Mental health outcomes such as depression, anxiety, sense of coherence (SOC) and quality of life are hardly evaluated in lifestyle interventions [16,17,20,22,23]. As poor sleep is also significantly associated with gestational diabetes and pregnancy-induced hypertension, addressing women’s sleep behavior in lifestyle interventions can have a mediating effect on outcomes as well [15]. These findings suggest that lifestyle interventions focusing on weight management also need to address other related relevant health care behaviors including maternal mental health.
The interpregnancy period, which starts after childbirth and ends at the start of the next pregnancy, is an opportune time to address maternal mental health problems, as a history of depression and anxiety is a significant risk factor for postpartum depression [10,24]. Furthermore, the interpregnancy period offers an innovative window of opportunity to achieve behavioral change.
To the best of our knowledge, no studies have investigated the changes in mental health during the interpregnancy period in women with excessive GWG. Insight into the changes in interpregnancy mental health and the impact on weight and body composition can assist in the development of effective and timely weight management strategies in women at risk. The primary aim of this study is to assess the difference in mental health between 6 weeks after childbirth and the start of the next pregnancy and investigate its association with pre-pregnancy weight and body composition. The secondary aim is to study whether this association is affected by socio-demographic factors, interpregnancy interval and sleep.
2. Materials and Methods
The interpregnancy coaching for a healthy future (INTER-ACT) (ClinicalTrials.gov; NCT02989142) intervention is a combined e-health-supported and face-to-face coaching program, from childbirth to the end of the next pregnancy, in women with excessive gestational weight gain in the previous pregnancy. The primary aim of the study was to assess the effectiveness of the INTER-ACT intervention on pregnancy and birth-related complications (a composite outcome: gestational diabetes, pregnancy-induced hypertension, cesarean section and large-gestational-age babies) in the subsequent pregnancy. Secondary outcomes (postpartum maternal mental health, postpartum weight retention and body composition and postpartum lifestyle behaviors such as eating behavior and physical activity) have already been analyzed [25,26,27,28,29]. The current secondary analyses focus on maternal mental health, pre-pregnancy weight and body composition during the interpregnancy period. Details of the INTER-ACT randomized controlled trial (RCT) are available elsewhere [30].
2.1. Study Design
The INTER-ACT RCT was a multicenter RCT with a longitudinal study design. Participants received the first study visit 6 weeks after childbirth. The intervention group received the next study visit on week 12 and months 6, 12, 18, 24 and 30 after childbirth and the control group at months 6, 12, 18, 24 and 30. Once participants were pregnant, the visits on previous dates were discontinued and replaced with a pregnancy visit in the first, second and third trimesters. The intervention group received 4 e-health-supported face-to-face coaching sessions during the first 6 months after childbirth in addition to usual care (weeks 6, 8 and 12 and month 6). The control group received usual care only.
To address the current research question, we focused on the group of women who started a subsequent pregnancy and who completed mental health questionnaires at 6 weeks after childbirth and at the start of the next pregnancy.
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved on 9 March 2017 by the Clinical Trial Centre/Ethical Committee UZ Leuven (protocol code B322201730956/S59889). All participants confirmed their participation by written informed consent.
2.2. Participants
Participants were enrolled in 6 Flemish hospitals between May 2017 and April 2019. Women were informed and recruited by trained study nurses 2 to 3 days after childbirth if they had excessive GWG according to the 2009 National Academy of Medicine (NAM) guideline [31], were aged ≥18 years and were Dutch-speaking. Women who had a twin birth, pre-pregnancy underweight (body mass index (BMI) < 18.5), had previous or planned bariatric surgery or suffered from diabetes mellitus, kidney disease, a mental disorder or stillbirth were excluded.
A total of 1450 women were enrolled and registered in the electronic case report form (eCRF) system ‘Castor’ (https://data.castoredc.com/, accessed on 20 June 2023). Randomization to the intervention or control group was performed by the biostatistician within the first week after childbirth, using an allocation ratio of 1:1 supported by block randomization (block sizes 4, 6 and 8) and stratification by hospital. Finally, 1450 women were recruited, and 1047 women were assessed at baseline (6 weeks after childbirth) (i.e., completed mental health questionnaire and body measurements). Subsequently, 403 women were registered in the medical records of the participating hospitals with a subsequent pregnancy. Of these, 6 women were excluded because of twin pregnancies, and 121 women were excluded because of missing data at the start of the next pregnancy (no mental health measurement at the start of the next pregnancy or no pre-pregnancy weight reported). A total of 276 women reported pre-pregnancy weight and completed at least 2 mental health questionnaires, 1 at 6 weeks after childbirth (start interpregnancy) and 1 at the start of the next pregnancy (end of interpregnancy) The start of the next pregnancy is defined as the timepoint nearest to the start of the next pregnancy and at least within 1 year preconception. These women constituted the study population of the current analyses. More details are shown in Figure 1.
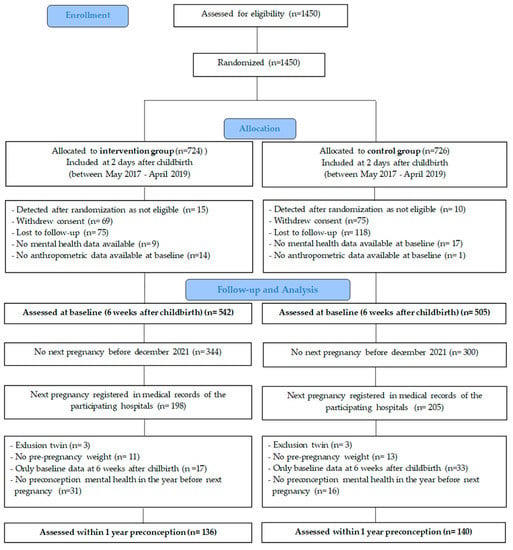
Figure 1.
Flow chart of participant follow-up.
2.3. Measurements
All participants were assessed at 6 weeks after childbirth and at the start of the next pregnancy by completing self-reported questionnaires. A personal link to the questionnaire was sent from the eCRF two weeks before the scheduled study visit.
Mental health was assessed by using the Edinburgh Postnatal Depression Scale (EPDS) and the Gotland Male Depression Scale (GMDS) for symptoms of depression, the Spielberger State Trait Anxiety Inventory 6-item (sSTAI-6) and the Edinburgh Depression Scale-3 Anxiety item (EDS-3A) for symptoms of anxiety, the Sense Of Coherence-13item (SOC-13) for measuring the sense of coherence and a Linear Analogue Scale (LAS) for measuring quality of life (QoL). Characteristics and cut-off scores of the mental health questionnaires are presented in Table 1.

Table 1.
Characteristics of the mental health questionnaires.
The EPDS is suitable for assessing symptoms of depression and anxiety during the postpartum period [32]. A cut-off score of ≥10 is recommended for the detection of mild to severe postpartum depression [33]. The questionnaire included one question about self-harming. The generation of an integrated alert in the eCRF system ‘Castor’ allowed the research staff to respond immediately if this question was completed in the affirmative.
The GMDS complemented the EPDS as it is a valid instrument for assessing non-typical suicidality-related symptoms of depression. The use of the GMDS as a supplement on the GMDS can increase the detection rate of depressive symptoms [34,35]. Cut-off scores for GMDS were defined in 3 classes: <13 = no signs of depression; 13–26 indicates possible major depression; and ≥27 clearly indicates depression [36]. The sSTAI-6 [37] is a validated, reliable measure of maternal postpartum anxiety [38]. We used the shortened validated 6-item version of the sSTAI to make it less time consuming for participants. A cut-off score of 40 is commonly used to predict postpartum anxiety and mood disorder with the original 20-item questionnaire [38,39]. Therefore, the numeric sum score of the 6 items was converted to a range between 20 and 80 by using the following formula [40]: 6-item sum score/6 × 20.
Previous research indicates that three questions from the EPDS questionnaire are also sensitive to the measurement of anxiety [41]. The three items (3–4–5) are brought together under the EDS-3A. For the EDS-3A, we used a cut-off score of 5 due to the specificity of 90% and less misclassification than with a lower cut-off [41].
The SOC is measured with the 13-item short scale (SOC-13), derived from the original 29-item Orientation to Life Questionnaire [42]. For the current analysis, the format of the Institute for Data Collection and Research (centERdata) was used [43]. A SOC score <70 indicates that participants are less able to understand, influence and make sense of situations, while a high sense of coherence (≥70) indicates a better ability to cope with stressful situations in life.
QoL was assessed using the Linear Analogue Scale (LAS). Participants were asked to score QoL on a scale from 0 to 100, with 0 representing a poor quality of life and 100 an extremely good quality of life. The LAS was one comprehensive score, summarizing physical, psychological and social aspects.
Age, level of education, ethnicity, employment status, family composition, family income, a history of depression and a history of anxiety were self-reported at baseline. Questions on breastfeeding and maternal sleep were based on previous research and self-reported at each time point [44,45,46,47]. Pregnancy- and birth-related data from the first pregnancy were extracted from the medical records by research nurses at the time of recruitment.
Interpregnancy mental health was calculated based on 2 measurement points: 6 weeks after childbirth (start interpregnancy) and at the start of the next pregnancy (end of interpregnancy, defined as the timepoint nearest to the start of the next pregnancy and at least within 1 year preconception).
The difference in mental health during the interpregnancy period was calculated as the difference between mental health at 6 weeks after childbirth vs. at the start of the next pregnancy for continuous variables and as a change of group according to the cut-off score (high/low) at 6 weeks after childbirth vs. the start of the next pregnancy for categorical variables.
Interpregnancy interval was determined by calculating the difference in months between the start date of the next pregnancy (day of childbirth minus gestational age) minus the date of the previous childbirth.
2.4. Outcomes
Study nurses collected the self-reported pre-pregnancy weight before the previous pregnancy from the medical records 2 to 3 days after childbirth, and the self-reported pre-pregnancy weight of the next pregnancy was questioned by the INTER-ACT coach at the first INTER-ACT pregnancy visit. Body composition was electronically measured by using a Tanita MC 780 SMA bio-electric impedance (BIA) device (Tanita Corporation, Tokyo, Japan) with three frequencies (5, 50 and 250 KHz). BIA is a non-invasive, reliable and safe clinical approach, which is well accepted by patients [48]. BIA showed excellent repeatability even in pregnancy [49] and is appropriate for revealing interactions between fat mass and adverse effects in mothers. During the postpartum period, the BIA method may be of added value in the prediction of future health care problems such as obesity and diabetes type 2 [48] and is suitable to detect changes in fat mass over time [50].
Waist circumference was assessed (rounded to 0.1 cm) by using a Seca 201 tape (Seca, Hamburg, Germany) and defined as the midway between the lowest rib and the hip bone [51]. All research staff received intensive training to ensure adequate and consistent measurements. The measurement was repeated 3 times, and the mean score was reported. In case of deviations (˃0.5 cm), the measurement was repeated until agreement was obtained.
BMI was calculated as weight (kg)/height × height (m2). Height was measured (rounded to 0.1cm) at the first study visit using a Seca 213 stadiometer (Seca, Hamburg, Germany), while women were standing upright with the head in the Frankfurt plane position [52].
The body measurements took place in the home of the participant, in hospital or elsewhere according to the preference of the participant.
2.5. Data Analyses
Statistical analyses were performed by using Statistical Package for the Social Science (SPSS) version 27.0 (IBM, Armonk, New York, NY, USA) and Statistical Analysis System (SAS) version 9.4 (Cary, New York, NY, USA).
The Kolmogorov–Smirnov test in addition to plots and histograms was used to assess the normality of distribution of continuous variables. All mental health variables showed a skewed distribution and were therefore analyzed by using non-parametric tests.
Descriptive characteristics were presented as mean and standard deviation or median and interquartile range for continuous variables and frequencies and percentages for categorical variables. To assess differences in participant characteristics between two groups, the unpaired t-test was used for continuous variables with a normal distribution and the Mann–Whitney U test for continuous variables with a skewed distribution. To assess differences in a continuous variable between 3 groups or more, the Kruskal–Wallis test was used. The likelihood ratio chi-square test, Fisher exact test (if expected cell count ≤10) or the linear-by-linear association chi-square was used to assess differences between categorical variables.
To assess the difference in mental health at 2 different time points in the same patients, the Mc-Nemar test was used for categorical variables and the Wilcoxon signed ranked test for continuous variables.
Participants were divided into low/high according to the pre-defined cut-off (Table 1), assessing whether or not participants changed from the cut-off group between 6 weeks after childbirth and the start of the next pregnancy (group 1: no symptoms of anxiety or depression, low SOC or low QoL at 6 weeks after childbirth or at the start of the next pregnancy; group 2: symptoms of anxiety or depression, low SOC or low QoL at 6 weeks after childbirth as well as at the start of the next pregnancy; group 3: no symptoms of anxiety or depression, low SOC or low QoL at 6 weeks after childbirth but symptoms of anxiety or depression, low SOC or low QoL at the start of the next pregnancy; group 4: symptoms of anxiety or depression, low SOC or low QoL at 6 weeks after childbirth but no symptoms of anxiety or depression, low SOC or low QoL at the start of the next pregnancy). If there was more than 1 measurement (mental health or body composition) within the year preconception, the measurement nearest to the next pregnancy was taken to conduct the analyses.
We performed regression analyses to assess whether mental health at the start and end of the interpregnancy interval were independent factors associated with BMI and body composition at the start of the next pregnancy. The outcome variables were postpartum weight retention, change in BMI at the start of each pregnancy and body composition at the start of the second pregnancy (BMI, fat percentage, waist circumference and visceral fat). We considered the mental health variables as categorical variables: high/low at the start, high/low at the end and the four possible combinations (high at start and end, high at start but low at end, etc.). We took into account as explanatory variables the following: pre-pregnancy BMI at the start of the previous pregnancy (continuous variable), level of education, exclusive breastfeeding at 6 months, interpregnancy interval (short (≤18 months) vs. normal (18–59 months)), sleep, history of depression, history of anxiety and mental health variables). Stepwise variable selection was performed to assess whether the mental health variables showed independent statistical significance to the outcome variables (BMI and body composition at the start of the next pregnancy).
3. Results
3.1. Participant Characteristics
Of the 1450 women randomized in the INTER-ACT trial, 276 women had a next pregnancy and complete data (Figure 1): mean age of 30 years (SD ± 3.6), 56% normal BMI at the start of the previous pregnancy and 42% had at least a master’s degree. Table S1 assesses the differences between these 276 patients and the patients who dropped out (Table S1).
At baseline (6 weeks after childbirth), a difference in QoL was found between the intervention and control group (median score; 80 versus 81, p = 0.04). No statistically significant differences were found in pre-pregnancy weight (both previous pregnancy and next pregnancy), mental health at the start of the next pregnancy (sSTAI-6, EDS-3A, EPDS, GMDS, SOC-13 and QoL) or mental health at the end of the intervention (6 months postpartum) between the intervention and control group (Table S2). Therefore, no further analyses stratified by randomization arm were performed, and we assessed the total group of 276 participants.
3.2. Differences in Mental Health between 6 Weeks after Childbirth and Start of Next Pregnancy
The rate of women with symptoms of anxiety (sSTAI-6 ≥ 40) increased by 13% between 6 weeks after childbirth and the start of the next pregnancy (36% vs. 49%, p =≤ 0.001). Of the women who were not anxious (sSTAI < 40) at 6 weeks after childbirth, more than one-third (39%) developed anxiety (sSTAI ≥ 40) by the start of the next pregnancy. Of those who were anxious at 6 weeks after childbirth (sSTAI ≥ 40), 67% still experienced anxiety at the start of the next pregnancy (sSTAI ≥ 40) (p =≤ 0.001) (Figure 2).
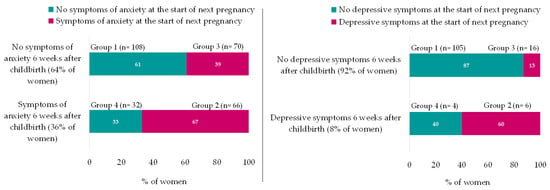
Figure 2.
Differences in sSTAI-6 and GMDS during the interpregnancy period. sSTAI-6 N = 276; GMDS N = 131; Symptoms of anxiety = sSTAI-6 ≥ 40; depressive symptoms = GMDS ≥ 13; and GMDS was recoded from 3 to 2 categories (<13 and ≥13) because of only 1 case in category 3. group 1 = No symptoms of anxiety at 6 weeks after childbirth and no symptoms of anxiety at the start of next pregnancy; group 2 = Symptoms of anxiety at 6 weeks after childbirth and still symptoms of anxiety at the start of next pregnancy; group 3 = No symptoms of anxiety at 6 weeks after childbirth but symptoms of anxiety at the start of next pregnancy; and group 4 = Symptoms of anxiety at 6 weeks after childbirth but no symptoms of anxiety at the start of next pregnancy.
Also, the rate of women who reported depressive symptoms at the start of the next pregnancy doubled compared to 6 weeks after childbirth (GMDS ≥ 13; 17% versus 8% respectively, p = 0.01).
Of the women who experienced depressive symptoms (GMDS ≥ 13) at 6 weeks after childbirth (n = 10), 60% of women still reported depressive symptoms (GMDS ≥ 13) at the start of the next pregnancy. In the group of women without depressive symptoms at 6 weeks after childbirth, 13% of women reported depressive symptoms at the start of the next pregnancy (p = 0.01) (Figure 2).
No statistically significant changes were shown for differences in EDS-3A, EPDS, SOC or QoL between 6 weeks after childbirth and the end of the next pregnancy (p = 0.37; 1; 0.29 and 0.91, respectively).
3.3. Mental-Health-Related Characteristics
3.3.1. Socio-Demographic Factors and Interpregnancy Interval
Women with a history of depressive feelings or a history of anxiety feelings reported more often depressive symptoms (EPDS ≥ 10, GMDS ≥ 13), anxiety (sSTAI-6 ≥ 40, EDS-3A ≥ 5) or a low SOC (SOC-13 ≤ 70) at the start of the next pregnancy. Also, women with obesity before the start of the previous pregnancy reported more common depressive symptoms (EPDS ≥ 10, GMDS ≥ 13) and anxiety (sSTAI-6 ≥ 40, EDS-3A ≥ 5) at the start of the next pregnancy compared to healthy weight or overweight women. Details are presented in Table S3.
There was no further statistically significant association between mental health at the start of the next pregnancy (sSTAI-6, EDS-3A, EPDS, GMDS, SOC, QoL or the interpregnancy differences in anxiety (STAI-6; group 1–4)) and parity, level of education, employment status, ethnicity, method of delivery and family composition (p = 0.35; 0.16; 0.33; 0.06; 0.97; and 0.94, respectively).
There was no association between the length of the interpregnancy interval and maternal mental health at 6 weeks after childbirth or at the start of the next pregnancy.
3.3.2. Sleep
Women with less than 6 h of sleep per night at the start of the next pregnancy more often reported a low SOC (SOC < 70; 5 h or less = 54%; 5–6 h = 59%) compared to women with more than 6 h of sleep per night (SOC < 70; 6–7 h = 35% and more than 7 h = 45%) (p = 0.05). Also, women more often reported levels of anxiety (sSTAI-6 ≥ 40) if they obtained less than 5 h of sleep per night at the start of the next pregnancy (77%), compared to women with 5–6 h (59%), 6–7 h (51%) or more than 7 h of sleep per night (41%) (p = 0.03) Figure 3.
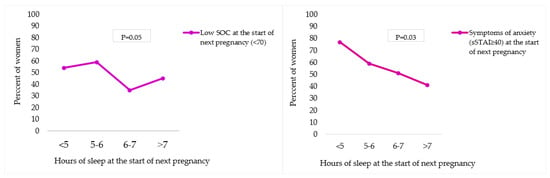
Figure 3.
Association between sleep duration and SOC/anxiety at the start of next pregnancy. sSTAI-6 = Spielberger State-Trait Anxiety Inventory 6-item. Significance level was calculated using the Pearson Chi-Square test.
3.4. Association with Pre-Pregnancy BMI and Body Composition
Regression analyses showed that when taking into account pre-pregnancy BMI of the previous pregnancy, level of education and breastfeeding, only sense of coherence at the start of the next pregnancy was independently associated with women’s BMI and fat percentage at the start of the next pregnancy and BMI change between two pregnancies (Table 2).

Table 2.
Association between mental health (SOC, sSTAI-6, EDS-3A, EPDS, GMDS and QoL) and pre-pregnancy BMI and body composition (regression models after stepwise variable selection).
4. Discussion
We showed a significant increase in anxiety (+13%, p =≤ 0.001) and depressive symptoms (+9%, p = 0.01) throughout the interpregnancy period. Remarkably, of the women who were not anxious at 6 weeks after the previous childbirth, 39% evolved to experience levels of anxiety at the start of the next pregnancy (p =≤ 0.001). Women with a history of depression, a history of anxiety or a pre-pregnancy BMI ≥ 30 were more vulnerable to report symptoms of anxiety and depression at the start of the next pregnancy (Table S3). Also, women with less than 6 h of sleep per night were more likely to report low SOC (p = 0.05) or symptoms of anxiety (p = 0.03) at the start of the next pregnancy, compared to women with more than 6 h of sleep per night. Furthermore, from our multivariate regression analysis, we showed that sense of coherence was independently associated with women’s BMI and fat percentage at the start of the next pregnancy (Table 2).
In contrast to the STAI-6 (symptoms of anxiety), sense of coherence is a personality trait that indicates the ability to understand, manage and give meaning to situations. It increases resistance to stress and promotes the person’s development [53]. The sSTAI-6, on the other hand, represents a feeling linked to a specific situation at a specific moment in time [37]. Our results showed a significant increase in sSTAI-6 between 6 weeks after childbirth and the start of the next pregnancy (+13%, p =≤0.001), suggesting that specific events related to a new pregnancy or due to postpartum events can cause changes in anxiety, while the effect on weight and fat percentage at the start of the next pregnancy appears to be significantly related to a person’s ability to cope with stressful daily events. As pre-pregnancy weight is an important risk factor for several health problems during and after pregnancy, it seems important to consider preconception interventions that focus on coping strategies that empower women to cope with stressful daily situations.
We also found that symptoms of anxiety and low SOC at the start of the next pregnancy were significantly more common in women with less than 6 h of sleep per night. However, studies investigating sleep during the preconception period and its impact on mother and child outcomes are rather scarce [54,55]. Moreover, preconception care guidelines unfortunately do not take into account interventions focusing on sleep within preconception care [56,57]. Future randomized controlled trials need to focus on the improvement of sleep quantity during the preconception period and study the impact on women’s mental health as a possible relevant and important mediator for maternal weight management. Since both anxiety and sense of coherence appear to be associated with sleep duration, preconception interventions should not only target the causes of anxiety during preconception but should also target coping strategies to enhance women’s ability to cope with stressful situations, as the latter was a significant predictor for women’s preconception BMI and fat percentage.
A remarkably high prevalence (49%) of women who experienced higher levels of anxiety (sSTAI-6 ≥ 40) at the start of the next pregnancy was shown. Most importantly, two-thirds of these women had already high levels of anxiety at 6 weeks after childbirth (i.e., during the postpartum period of the previous pregnancy), without improvement at the next pregnancy. Similarly, one-third of women who were not anxious at 6 weeks after childbirth evolved to experience levels of anxiety at the start of the next pregnancy. A possible reason for the high prevalence of levels of anxiety and depression could be the fact that we only included women with excessive GWG in a previous pregnancy, of which more than 50% were women with overweight/obesity. From recent systematic reviews, it is shown that a high maternal BMI is associated with higher levels of anxiety and depression [7,11,58]. Research showed that unfavorable mental health at the start of the next pregnancy is associated with adverse outcomes such as excessive GWG [14], maternal mental health problems after childbirth [59], low birth weight [60] and a disturbed mother–child bonding [61]. In addition, our results showed that mental health problems at the start of the next pregnancy were more common in women who had obesity, in women with less sleep and in women with a history of anxiety or depression. A standard mental health screening throughout the interpregnancy period for this population at risk could be recommended. If symptoms of depression, anxiety, low SOC or low QoL are present, timely mental health support with long-term follow-up throughout the interpregnancy period could be indicated. This may lead to benefits for mother and child and significantly less health costs in the longer term. Intervention studies on the effect of standard mental health screening during the interpregnancy/preconception period would be appropriate, as, to the best of our knowledge, these do not yet exist.
Women who dropped out of the study were more likely to be living with overweight or obesity before the previous pregnancy and reported worse mental health scores at 6 weeks after childbirth (Table S1). We assume that due to the high drop-out rate of vulnerable women, our results cannot be generalized to the total population of women in the interpregnancy period. Further research into improving the involvement of vulnerable women during the interpregnancy period could be of added value in the improvement of preconception care pathways.
A statistically significant increase in levels of depression during the interpregnancy period was shown using the GMDS questionnaire. However, this finding was not supported by the EPDS questionnaire, which is a validated and reliable scale to measure depressive symptoms in women after childbirth [32]. Further research on the validity of the GMDS for detecting levels of depression in women after childbirth is recommended.
The strength of our study was the use of data from a large longitudinal prospective randomized controlled trial. Furthermore, we analyzed four different mental health outcomes. Including SOC and QoL in addition to depression and anxiety provided new insights and added value to the knowledge of the overall concept of mental health [62]. A further strength is the use of body composition measurement in addition to weight, as visceral fat and fat and muscle mass are strong markers for women’s global metabolic health [63].
Our study also has some limitations. Firstly, our study included only women with previous excessive GWG, which is, in general, prevalent in 40–50% of women [22,64]. Excessive GWG is one of the most important risk factors for PPWR [17]. This may have an impact on the presented rate of women with PPWR during the preconception period. Secondly, the current analyses focused on women who reported a subsequent pregnancy and completed mental health measurements at baseline (6 weeks after childbirth) and at the start of the next pregnancy. Therefore, selection bias must be taken into account when interpreting and generalizing our study results to the entire population of mothers. A third limitation was that the BIA measurements could not be performed according to the standard guidelines [48]. Since our studied population consisted of postpartum women, it was not recommended that breastfeeding mothers fast for 4 h, nor was physical activity assessed in the 12 h prior to the BIA measurement, so our results could not be controlled for this. A fourth limitation was that the GMDS was added later in the study. This resulted in small groups (group 4: n = 4; group 2: n = 6) when we studied differences in GMDS during the interpregnancy period (Figure 3). Therefore, further analyses of differences in levels of depression (GMDS) on related characteristics, weight and body composition were not conducted, except for the regression analyses. A last limitation was the use of self-reported mental health questionnaires and self-reported pre-pregnancy weight. Shame and minimalization in new mothers may have led to underreporting of mental health problems during the interpregnancy period. Self-reported pre-pregnancy weight is prone to errors [65] and, in some cases, underreported, compared to objective measures by professionals [66].
5. Conclusions
Our data show a significant increase in anxiety and depressive symptoms between the start and the end of the interpregnancy period. Of the women who were not anxious at the start, 39% experienced anxiety at the end of interpregnancy. Sense of coherence at the start of the next pregnancy was independently associated with women’s pre-pregnancy BMI and fat percentage. Our results indicate that the interpregnancy period appears to offer an opportunity to develop innovative preventative interventions. We believe that the development of preconception lifestyle interventions that focus on both weight reduction and support in understanding, managing and giving meaning to stressful events (sense of coherence) may be of added value to optimize women’s preconception health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15143152/s1, Table S1: Participant characteristics; Table S2: Participant characteristics and the difference between intervention and control group; Table S3: Prevalence of symptoms of anxiety and depression, low SOC and low QoL at the end of interpregnancy in relation to participant characteristics.
Author Contributions
Conceptualization, H.V.U., A.B., L.A. and R.D.; methodology, H.V.U., A.B., L.A. and R.D.; software, L.A.; validation, H.V.U., A.B., L.A. and R.D.; formal analysis, H.V.U. and L.A.; investigation, H.V.U. and L.A.; resources, A.B. and R.D.; data curation, L.A.; writing—original draft preparation, H.V.U.; writing—review and editing, L.A., A.B., R.D., Y.J., C.V.H. and A.S.; visualization, H.V.U. and A.B.; supervision, A.B.; project administration, L.A., A.B. and R.D.; funding acquisition, A.B. and R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Flemish fund for Scientific research (FWO), grant numbers 1803311N and TN005116N, and the Rotary Foundation, Limburg (Houthalen), Belgium.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved on 9 March 2017 by the Institutional Review Board (or Ethics Committee) of UZ Leuven (protocol code B322201730956/S59889).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The study protocol is publicly available. Data requests may be submitted to the Principal Investigator accompanied by a proposal with a planned objective for use of data.
Acknowledgments
We are very grateful to all the participants of the INTER-ACT trial. We would also like to extend our great thanks to the INTER-ACT research group for their contribution to the data collection. We thank the University Hospital Antwerp, Gasthuiszusters Antwerp, St-Franciscus Hospital Heusden-Zolder, Hospital Oost-Limburg, Jessa Hospital Hasselt, University Hospital Leuven, Wit-Gele Kruis Limburg, Kind & Gezin Limburg, Antwerp, study nurses and INTER-ACT coaches. We also thank Kate Maslin for her assistance in reviewing the English version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. WHO Guide for Integration of Perinatal Mental Health in Maternal and Child Health Services; World Health Organization (WHO): Geneva, Switzerland, 2022; p. 66. [Google Scholar]
- Geier, M.L.; Hills, N.; Gonzales, M.; Tum, K.; Finley, P.R. Detection and treatment rates for perinatal depression in a state Medicaid population. CNS Spectr. 2015, 20, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Yeaton-Massey, A.; Herrero, T. Recognizing maternal mental health disorders: Beyond postpartum depression. Curr. Opin. Obstet. Gynecol. 2019, 31, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Cox, E.Q.; Sowa, N.A.; Meltzer-Brody, S.E.; Gaynes, B.N. The Perinatal Depression Treatment Cascade: Baby Steps toward Improving Outcomes. J. Clin. Psychiatry 2016, 77, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Callanan, F.; Tuohy, T.; Bright, A.M.; Grealish, A. The effectiveness of psychological interventions for pregnant women with anxiety in the antenatal period: A systematic review. Midwifery 2022, 104, 103169. [Google Scholar] [CrossRef]
- Maternal Mental Health Alliance. Perinatal Mental Health. Available online: https://maternalmentalhealthalliance.org/about/perinatal-mental-health/ (accessed on 20 June 2023).
- Zanardo, V.; Giliberti, L.; Giliberti, E.; Grassi, A.; Perin, V.; Parotto, M.; Soldera, G.; Straface, G. The role of gestational weight gain disorders in symptoms of maternal postpartum depression. Int. J. Gynaecol. Obstet. 2021, 153, 234–238. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Bs, S.M.; Chai, H.; Lewis, J.B.; Levine, J.; Tobin, J.N.; Ickovics, J.R. Postpartum Depressive Symptoms: Gestational Weight Gain as a Risk Factor for Adolescents Who Are Overweight or Obese. J. Midwifery Women’s Health 2018, 63, 178–184. [Google Scholar] [CrossRef]
- Dachew, B.A.; Ayano, G.; Betts, K.; Alati, R. The impact of pre-pregnancy BMI on maternal depressive and anxiety symptoms during pregnancy and the postpartum period: A systematic review and meta-analysis. J. Affect. Disord. 2021, 281, 321–330. [Google Scholar] [CrossRef]
- Zhao, X.H.; Zhang, Z.H. Risk factors for postpartum depression: An evidence-based systematic review of systematic reviews and meta-analyses. Asian J. Psychiatry 2020, 53, 102353. [Google Scholar]
- Bliddal, M.; Pottegård, A.; Kirkegaard, H.; Olsen, J.; Jørgensen, J.S.; Sørensen, T.I.; Wu, C.; A Nohr, E. Mental disorders in motherhood according to prepregnancy BMI and pregnancy-related weight changes—A Danish cohort study. J. Affect. Disord. 2015, 183, 322–329. [Google Scholar]
- Bazzazian, S.; Riazi, H.; Vafa, M.; Mahmoodi, Z.; Nasiri, M.; Mokhtaryan-Gilani, T.; Ozgoli, G. The relationship between depression, stress, anxiety, and postpartum weight retention: A systematic review. J. Educ. Health Promot. 2021, 10, 230. [Google Scholar]
- Bogaerts, A.F.; Van den Bergh, B.R.; Witters, I.; Devlieger, R. Anxiety during early pregnancy predicts postpartum weight retention in obese mothers. Obesity 2013, 21, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Farias, D.R.; Carrilho, T.R.B.; Freitas-Costa, N.C.; Batalha, M.A.; Gonzalez, M.; Kac, G. Maternal mental health and gestational weight gain in a Brazilian Cohort. Sci. Rep. 2021, 11, 10787. [Google Scholar] [CrossRef] [PubMed]
- Ladyman, C.; Sweeney, B.; Sharkey, K.; Bei, B.; Wright, T.; Mooney, H.; Huthwaite, M.; Cunningham, C.; Firestone, R.; Signal, T.L. A scoping review of non-pharmacological perinatal interventions impacting maternal sleep and maternal mental health. BMC Pregnancy Childbirth 2022, 22, 659. [Google Scholar] [CrossRef] [PubMed]
- The International Weight Management in Pregnancy (i-WIP) Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: Meta-analysis of individual participant data from randomised trials. BMJ 2017, 358, j3119. [Google Scholar]
- Ferrara, A.; Hedderson, M.M.; Brown, S.D.; Ehrlich, S.F.; Tsai, A.-L.; Feng, J.; Galarce, M.; Marcovina, S.; Catalano, P.; Quesenberry, C.P. A telehealth lifestyle intervention to reduce excess gestational weight gain in pregnant women with overweight or obesity (GLOW): A randomised, parallel-group, controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 490–500. [Google Scholar] [CrossRef]
- Leonard, S.A.; Rasmussen, K.M.; King, J.C.; Abrams, B. Trajectories of maternal weight from before pregnancy through postpartum and associations with childhood obesity. Am. J. Clin. Nutr. 2017, 106, 1295–1301. [Google Scholar] [CrossRef]
- Lally, P.; van Jaarsveld, C.H.; Potts, H.W.; Wardle, J. How are habits formed: Modelling habit formation in the real world. Eur. J. Soc. Psychol. 2010, 40, 998–1009. [Google Scholar] [CrossRef]
- Bogaerts, A.F.; Devlieger, R.; Nuyts, E.; Witters, I.; Gyselaers, W.; Van den Bergh, B.R. Effects of lifestyle intervention in obese pregnant women on gestational weight gain and mental health: A randomized controlled trial. Int. J. Obes. 2013, 37, 814–821. [Google Scholar] [CrossRef]
- Sanaati, F.; Charandabi, S.M.; Eslamlo, H.F.; Mirghafourvand, M. A randomized controlled trial on the effect of lifestyle education for Iranian women and their husbands on post-partum anxiety and depression. Health Educ. Res. 2018, 33, 416–428. [Google Scholar] [CrossRef]
- Dodd, J.M.; Deussen, A.R.; Louise, J. A Randomised Trial to Optimise Gestational Weight Gain and Improve Maternal and Infant Health Outcomes through Antenatal Dietary, Lifestyle and Exercise Advice: The OPTIMISE Randomised Trial. Nutrients 2019, 11, 2911. [Google Scholar] [CrossRef]
- Altazan, A.D.; Redman, L.M.; Burton, J.H.; Beyl, R.A.; Cain, L.E.; Sutton, E.F.; Martin, C.K. Mood and quality of life changes in pregnancy and postpartum and the effect of a behavioral intervention targeting excess gestational weight gain in women with overweight and obesity: A parallel-arm randomized controlled pilot trial. BMC Pregnancy Childbirth. 2019, 19, 50. [Google Scholar] [CrossRef]
- Chojenta, C.; William, J.; Martin, M.A.; Byles, J.; Loxton, D. The impact of a history of poor mental health on health care costs in the perinatal period. Arch. Women’s Ment. Health 2019, 22, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Van Uytsel, H.; Ameye, L.; Devlieger, R.; Bijlholt, M.; Jacquemyn, Y.; Catry, V.; Schreurs, A.; Bogaerts, A. Mental health after childbirth and the impact on postpartum weight retention and body composition. Data from the INTER-ACT randomized controlled trial. Clin. Obes. 2022, 12, e12550. [Google Scholar] [CrossRef] [PubMed]
- Van Uytsel, H.; Bijlholt, M.; Devlieger, R.; Ameye, L.; Jochems, L.; van Holsbeke, C.; Schreurs, A.; Catry, V.; Bogaerts, A. Effect of the e-health supported INTER-ACT lifestyle intervention on postpartum weight retention and body composition, and associations with lifestyle behavior: A randomized controlled trial. Prev. Med. 2022, 164, 107321. [Google Scholar] [CrossRef] [PubMed]
- Bijlholt, M.; Ameye, L.; Van Uytsel, H.; Devlieger, R.; Bogaerts, A. The INTER-ACT E-Health Supported Lifestyle Intervention Improves Postpartum Food Intake and Eating Behavior, but Not Physical Activity and Sedentary Behavior-A Randomized Controlled Trial. Nutrients 2021, 13, 1287. [Google Scholar] [CrossRef] [PubMed]
- Bijlholt, M.; Ameye, L.; van Uytsel, H.; Devlieger, R.; Bogaerts, A. Evolution of Postpartum Weight and Body Composition after Excessive Gestational Weight Gain: The Role of Lifestyle Behaviors-Data from the INTER-ACT Control Group. Int. J. Environ. Res. Public Health 2021, 18, 6344. [Google Scholar] [CrossRef] [PubMed]
- Bijlholt, M.; Maslin, K.; Ameye, L.; Shawe, J.; Bogaerts, A.; Devlieger, R. Phase Angle and Bio-Impedance Values during the First Year after Delivery in Women with Previous Excessive Gestational Weight Gain: Innovative Data from the Belgian INTER-ACT Study. Int. J. Environ. Res. Public Health 2021, 18, 7482. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, A.; Ameye, L.; Bijlholt, M.; Amuli, K.; Heynickx, D.; Devlieger, R. INTER-ACT: Prevention of pregnancy complications through an e-health driven interpregnancy lifestyle intervention—Study protocol of a multicentre randomised controlled trial. BMC Pregnancy Childbirth 2017, 17, 154. [Google Scholar] [CrossRef]
- Institute of Medicine, National Research Council Committee to Reexamine IOMPWG. The National Academies Collection: Reports funded by National Institutes of Health. In Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Falana, S.D.; Carrington, J.M. Postpartum Depression: Are You Listening? Nurs. Clin. N. Am. 2019, 54, 561–567. [Google Scholar] [CrossRef]
- National Collaborating Centre for Mental Health. Antenatal and Postnatal Mental Health: The NICE Guideline on Clinical Management and Service Guidance; British Psychological Society: Leicester, UK, 2018. [Google Scholar]
- Psouni, E.; Agebjörn, J.; Linder, H. Symptoms of depression in Swedish fathers in the postnatal period and development of a screening tool. Scand. J. Psychol. 2017, 58, 485–496. [Google Scholar]
- Madsen, S.A.; Juhl, T. Paternal depression in the postnatal period assessed with traditional and male depression scales. J. Men’s Health Gend. 2007, 4, 26–31. [Google Scholar] [CrossRef]
- Girardi, P.; Pompili, M.; Innamorati, M.; Serafini, G.; Berrettoni, C.; Angeletti, G.; Koukopoulos, A.; Tatarelli, R.; Lester, D.; Roselli, D.; et al. Temperament, post-partum depression, hopelessness, and suicide risk among women soon after delivering. Women Health 2011, 51, 511–524. [Google Scholar] [CrossRef] [PubMed]
- van der Bij, A.K.; de Weerd, S.; Cikot, R.J.; Steegers, E.A.; Braspenning, J.C. Validation of the dutch short form of the state scale of the Spielberger State-Trait Anxiety Inventory: Considerations for usage in screening outcomes. Community Genet. 2003, 6, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Tendais, I.; Costa, R.; Conde, A.; Figueiredo, B. Screening for depression and anxiety disorders from pregnancy to postpartum with the EPDS and STAI. Span. J. Psychol. 2014, 17, E7. [Google Scholar] [CrossRef]
- Kruyen, P.M.; Emons, W.H.; Sijtsma, K. Shortening the S-STAI: Consequences for research and clinical practice. J. Psychosom. Res. 2013, 75, 167–172. [Google Scholar] [CrossRef]
- Furmli, H.; Seeto, R.A.; Hewko, S.L.; Dalfen, A.; Jones, C.A.; Murphy, K.E.; Bocking, A. Maternal Mental Health in Assisted and Natural Conception: A Prospective Cohort Study. J. Obstet. Gynaecol. Can. 2019, 41, 1608–1615. [Google Scholar] [CrossRef]
- Van Damme, R.; Van Parys, A.S.; Vogels, C.; Roelens, K.; Lemmens, G. Screening en Detectie van Perinatale Mentale Stoornissen: Richtlijn Als Leidraad Voor Het Ontwikkelen van Een Zorgpad; Universitair Ziekenhuis Gent: Brussel, Belgium; Universiteit Gent: Ghent, Belgium, 2018. [Google Scholar]
- Antonovsky, A. Unraveling the Mystery of Health: How People Manage Stress and Stay Well; Jossey-Bass: San Francisco, CA, USA, 1987. [Google Scholar]
- Marchand, M. (Shortened) Sense of Coherence. 2014. Available online: http://docplayer.nl/31755966-Shortened-sense-of-coherence.html (accessed on 20 June 2023).
- Austin, M.P.; Hadzi-Pavlovic, D.; Saint, K.; Parker, G. Antenatal screening for the prediction of postnatal depression: Validation of a psychosocial Pregnancy Risk Questionnaire. Acta Psychiatr. Scand. 2005, 112, 310–317. [Google Scholar] [CrossRef]
- Reilly, N.; Harris, S.; Loxton, D.; Chojenta, C.; Forder, P.; Austin, M.P. The impact of routine assessment of past or current mental health on help-seeking in the perinatal period. Women Birth 2014, 27, e20–e27. [Google Scholar] [CrossRef]
- Guelinckx, I.; Devlieger, R.; Bogaerts, A.; Pauwels, S.; Vansant, G. The effect of pre-pregnancy BMI on intention, initiation and duration of breast-feeding. Public Health Nutr. 2012, 15, 840–848. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Obuchowska, A.; Standyło, A.; Kimber-Trojnar, Ż.; Leszczyńska-Gorzelak, B. The Possibility of Using Bioelectrical Impedance Analysis in Pregnant and Postpartum Women. Diagnostics 2021, 11, 1370. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Susic, D.; O’sullivan, A.J.; Henry, A. Reproducibility of Bioelectrical Impedance Analysis in Pregnancy and the Association of Body Composition with the Risk of Gestational Diabetes: A Substudy of MUMS Cohort. J. Obes. 2020, 2020, 3128767. [Google Scholar] [CrossRef] [PubMed]
- Ellegård, L.; Bertz, F.; Winkvist, A.; Bosaeus, I.; Brekke, H.K. Body composition in overweight and obese women postpartum: Bioimpedance methods validated by dual energy X-ray absorptiometry and doubly labeled water. Eur. J. Clin. Nutr. 2016, 70, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Vatier, C.; Poitou, C.; Clément, K. Evaluation of Visceral Fat in Massive Obesity. In Nutrition in the Prevention and Treatment of Abdominal Obesity; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–77. [Google Scholar]
- National HES Manual. Belgium. 2011. Available online: https://ehes.info/manuals/national_manuals/national_manual_Belgium_EN.pdf (accessed on 20 June 2023).
- Mittelmark, M.B.; Bauer, G.F.; Vaandrager, L.; Pelikan, J.M.; Sagy, S.; Eriksson, M.; Lindström, B.; Meier Magistretti, C. (Eds.) The Handbook of Salutogenesis; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Toivonen, K.I.; Oinonen, K.A.; Duchene, K.M. Preconception health behaviours: A scoping review. Prev. Med. 2017, 96, 1–15. [Google Scholar] [CrossRef]
- Opray, N.; Grivell, R.; Deussen, A.R.; Dodd, J. Directed preconception health programs and interventions for improving pregnancy outcomes for women who are overweight or obese. Cochrane Database Syst. Rev. 2015, 7, CD010932. [Google Scholar] [CrossRef]
- World Health Organization. Preconception Care Regional Expert Group Consultation; World Health Organization: New Delhi, India, 2013. [Google Scholar]
- Shawe, J.; Delbaere, I.; Ekstrand, M.; Hegaard, H.K.; Larsson, M.; Mastroiacovo, P.; Stern, J.; Steegers, E.; Stephenson, J.; Tydén, T. Preconception care policy, guidelines, recommendations and services across six European countries: Belgium (Flanders), Denmark, Italy, the Netherlands, Sweden and the United Kingdom. Eur. J. Contracept. Reprod. Health Care 2015, 20, 77–87. [Google Scholar] [CrossRef]
- Dayan, F.; Javadifar, N.; Tadayon, M.; Malehi, A.S.; Sani, H.K. The Relationship between Gestational Weight Gain and Postpartum Depression in Normal and Overweight Pregnant Women. J. Pregnancy 2018, 2018, 9315320. [Google Scholar] [CrossRef]
- Witt, W.P.; Wisk, L.E.; Cheng, E.R.; Hampton, J.M.; Creswell, P.D.; Hagen, E.W.; Spear, H.A.; Maddox, T.; DeLeire, T. Poor prepregnancy and antepartum mental health predicts postpartum mental health problems among US women: A nationally representative population-based study. Womens Health Issues 2011, 21, 304–313. [Google Scholar] [CrossRef]
- Witt, W.P.; Wisk, L.E.; Cheng, E.R.; Hampton, J.M.; Hagen, E.W. Preconception mental health predicts pregnancy complications and adverse birth outcomes: A national population-based study. Matern. Child Health J. 2012, 16, 1525–1541. [Google Scholar] [CrossRef]
- Olsson, C.A.; Spry, E.A.; Alway, Y.; Moreno-Betancur, M.; Youssef, G.; Greenwood, C.; Letcher, P.; Macdonald, J.A.; McIntosh, J.; Hutchinson, D.; et al. Preconception depression and anxiety symptoms and maternal-infant bonding: A 20-year intergenerational cohort study. Arch. Women’s Ment. Health 2021, 24, 513–523. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; de Pablo, G.S.; De Micheli, A.; Nieman, D.H.; Correll, C.U.; Kessing, L.V.; Pfennig, A.; Bechdolf, A.; Borgwardt, S.; Arango, C.; et al. What is good mental health? A scoping review. Eur. Neuropsychopharmacol. 2020, 31, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Aydin, B.; Yalçin, S.S. Changes in maternal anthropometric measurements in the first postpartum month and associated factors. Am. J. Hum. Biol. 2022, 34, e23580. [Google Scholar] [CrossRef] [PubMed]
- Deputy, N.P.; Sharma, A.J.; Kim, S.Y.; Hinkle, S. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet. Gynecol. 2015, 125, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Abrams, B.; Sridhar, S.; Xu, F.; Hedderson, M. Validity of Self-Reported Pre-Pregnancy Weight and Body Mass Index Classification in an Integrated Health Care Delivery System. Paediatr. Perinat. Epidemiol. 2016, 30, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.M.; Oken, E.; Rifas-Shiman, S.L.; Téllez-Rojo, M.; Just, A.; Svensson, K.; Deierlein, A.L.; Chandler-Laney, P.C.; Miller, R.C.; McNamara, C.; et al. Do Women Know Their Prepregnancy Weight? Obesity 2019, 27, 1161–1167. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).