Application of the Updated WCRF/AICR Cancer Prevention Score as an Outcome for Cancer Survivors Participating in a Tailored and Intensive Dietary and Physical Activity Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intensive Behavioral Intervention
2.3. Data Collection
2.3.1. Clinical Assessment Visits
2.3.2. Dietary Patterns
2.3.3. Physical Activity Patterns
2.3.4. Anthropometric and Clinical Measures
2.4. Calculation of WCRF/AICR Score
2.5. Adaptation of Scoring
2.6. Statistical Analysis
3. Results
3.1. Participants
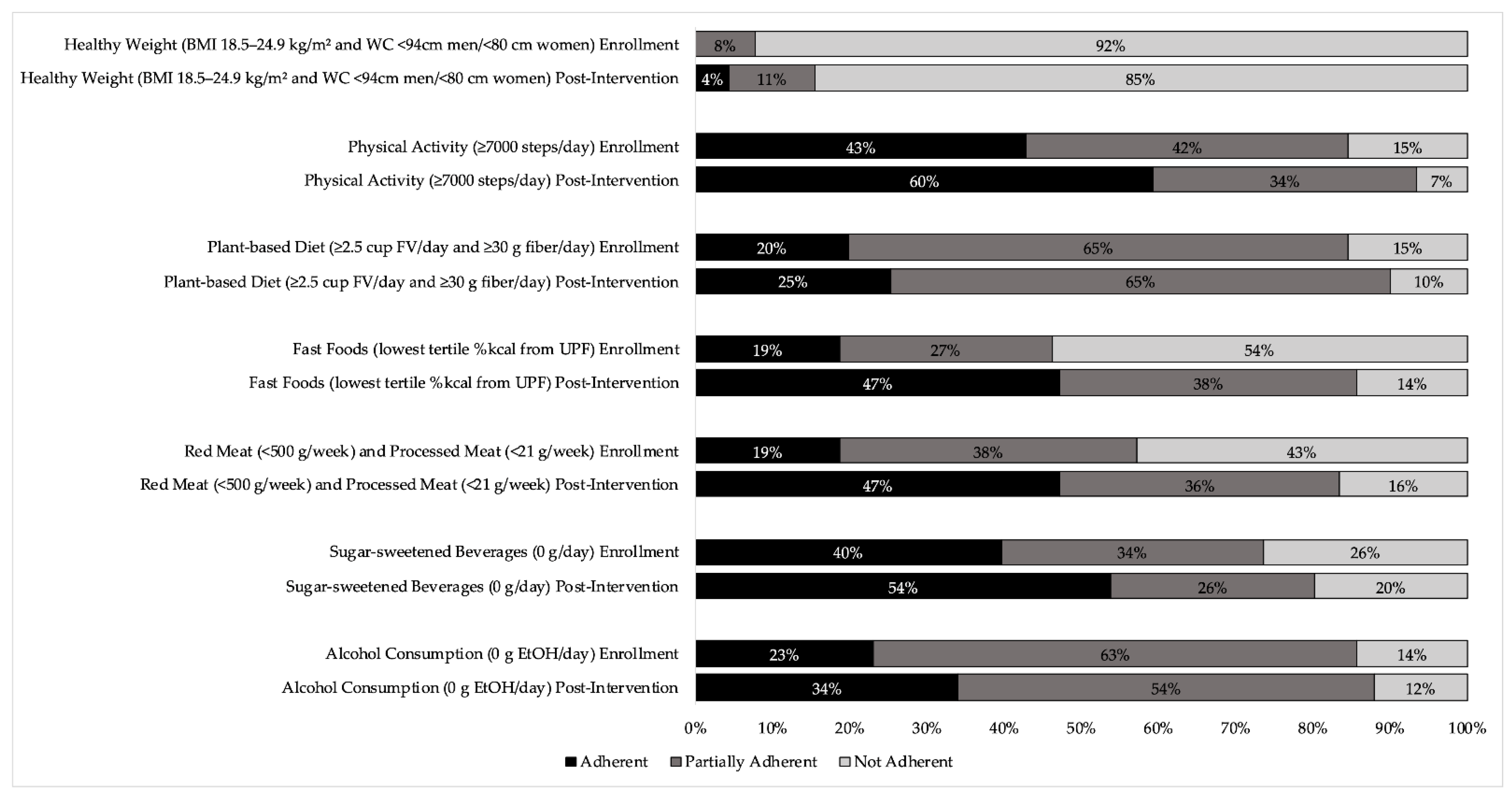
3.2. Changes in Lifestyle Behaviors
3.3. Changes in WCRF/AICR Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Moor, J.S.; Mariotto, A.B.; Parry, C.; Alfano, C.M.; Padgett, L.; Kent, E.E.; Forsythe, L.; Scoppa, S.; Hachey, M.; Rowland, J.H. Cancer Survivors in the United States: Prevalence across the Survivorship Trajectory and Implications for Care. Cancer Epidemiol. Biomark. Prev. 2013, 22, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pawlikowski, M.; Olivo-Marston, S.; Williams, K.P.; Bower, J.K.; Felix, A.S. Ten-Year Cardiovascular Risk among Cancer Survivors: The National Health and Nutrition Examination Survey. PLoS ONE 2021, 16, e0247919. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Deng, L.; Karr, M.A.; Wen, Y.; Wang, Q.; Perimbeti, S.; Shapiro, C.L.; Han, X. Chronic Comorbid Conditions among Adult Cancer Survivors in the United States: Results from the National Health Interview Survey, 2002–2018. Cancer 2022, 128, 828–838. [Google Scholar] [CrossRef]
- Stewart, B.W.; Bray, F.; Forman, D.; Ohgaki, H.; Straif, K.; Ullrich, A.; Wild, C.P. Cancer Prevention as Part of Precision Medicine: ‘Plenty to Be Done’. Carcinogenesis 2016, 37, 2–9. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity, and Cancer: A Global Perspective; Continuous Update Project Expert Report; 2018; Available online: https://www.wcrf.org/diet-activity-and-cancer/ (accessed on 12 October 2022).
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society Guideline for Diet and Physical Activity for Cancer Prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society Nutrition and Physical Activity Guideline for Cancer Survivors. CA Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). NCCN Guidelines for Patients: Survivorship Care for Healthy Living; NCCN: Plymouth Meeting, PA, USA, 2020; Available online: https://www.nccn.org/patients/guidelines/content/PDF/survivorship-hl-patient.pdf (accessed on 12 October 2022).
- American Society of Clinical Oncology (ASCO). Nutrition Recommendations during and after Treatment. Cancer.Net: Doctor Approved Patient Information from ASCO; ASCO: Alexandria, VA, USA, 2021; Available online: https://www.cancer.net/survivorship/healthy-living/nutrition-recommendations-during-and-after-treatment (accessed on 12 October 2022).
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Kenkhuis, M.-F.; van der Linden, B.W.; Breedveld-Peters, J.J.L.; Koole, J.L.; van Roekel, E.H.; Breukink, S.O.; Mols, F.; Weijenberg, M.P.; Bours, M.J.L. Associations of the Dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Recommendations with Patient-Reported Outcomes in Colorectal Cancer Survivors 2–10 Years Post-Diagnosis: A Cross-Sectional Analysis. Br. J. Nutr. 2020, 125, 1188–1200. [Google Scholar] [CrossRef]
- Kenkhuis, M.-F.; Mols, F.; van Roekel, E.H.; Breedveld-Peters, J.J.L.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; van Duijnhoven, F.J.B.; Weijenberg, M.P.; Bours, M.J.L. Longitudinal Associations of Adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Lifestyle Recommendations with Quality of Life and Symptoms in Colorectal Cancer Survivors up to 24 Months Post-Treatment. Cancers 2022, 14, 417. [Google Scholar] [CrossRef]
- van Zutphen, M.; Boshuizen, H.C.; Kenkhuis, M.-F.; Wesselink, E.; Geijsen, A.J.M.R.; de Wilt, J.H.W.; van Halteren, H.K.; Spillenaar Bilgen, E.J.; Keulen, E.T.P.; Janssen-Heijnen, M.L.G.; et al. Lifestyle after Colorectal Cancer Diagnosis in Relation to Recurrence and All-Cause Mortality. Am. J. Clin. Nutr. 2021, 113, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Hastert, T.A.; Beresford, S.A.A.; Sheppard, L.; White, E. Adherence to the WCRF/AICR Cancer Prevention Recommendations and Cancer-Specific Mortality: Results from the Vitamins and Lifestyle (VITAL) Study. Cancer Causes Control 2014, 25, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Robien, K.; Lazovich, D. Adherence to the Wcrf/Aicr Guidelines for Cancer Prevention Is Associated with Lower Mortality among Older Female Cancer Survivors. Cancer Epidemiol. Biomark. Prev. 2013, 22, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Freisling, H.; Leitzmann, M.F.; Taljaard-Krugell, C.; Jacobs, I.; Kruger, H.S.; Smuts, C.M.; Pieters, M. Diet and Sedentary Behaviour in Relation to Cancer Survival. A Report from the National Health and Nutrition Examination Survey Linked to the U.S. Mortality Registry. Clin. Nutr. 2020, 39, 3489–3496. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Petimar, J.; Wang, M.; Tabung, F.K.; Song, M.; Liu, L.; Lee, D.H.; Giovannucci, E.L.; Zhang, X.; Smith-Warner, S.A. Adherence to the World Cancer Research Fund/American Institute for Cancer Research Cancer Prevention Recommendations and Colorectal Cancer Survival. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Tollosa, D.N.; Tavener, M.; Hure, A.; James, E.L. Adherence to Multiple Health Behaviours in Cancer Survivors: A Systematic Review and Meta-Analysis. J. Cancer Surviv. 2019, 13, 327–343. [Google Scholar] [CrossRef]
- Lee, E.; Zhu, J.; Velazquez, J.; Bernardo, R.; Garcia, J.; Rovito, M.; Hines, R.B. Evaluation of Diet Quality Among American Adult Cancer Survivors: Results From 2005–2016 National Health and Nutrition Examination Survey. J. Acad. Nutr. Diet. 2021, 121, 217–232. [Google Scholar] [CrossRef]
- Arem, H.; Mama, S.K.; Duan, X.; Rowland, J.H.; Bellizzi, K.M.; Ehlers, D.K. Prevalence of Healthy Behaviors among Cancer Survivors in the United States: How Far Have We Come? Cancer Epidemiol. Biomark. Prev. 2020, 29, 1179–1187. [Google Scholar] [CrossRef]
- Stacey, F.G.; James, E.L.; Chapman, K.; Courneya, K.S.; Lubans, D.R. A Systematic Review and Meta-Analysis of Social Cognitive Theory-Based Physical Activity and/or Nutrition Behavior Change Interventions for Cancer Survivors. J. Cancer Surviv. 2015, 9, 305–338. [Google Scholar] [CrossRef]
- Amireault, S.; Fong, A.J.; Sabiston, C.M. Promoting Healthy Eating and Physical Activity Behaviors: A Systematic Review of Multiple Health Behavior Change Interventions Among Cancer Survivors. Am. J. Lifestyle Med. 2018, 12, 184–199. [Google Scholar] [CrossRef]
- Stull, V.B.; Snyder, D.C.; Demark-Wahnefried, W. Lifestyle Interventions in Cancer Survivors: Designing Programs That Meet the Needs of This Vulnerable and Growing Population. J. Nutr. 2007, 137, 243S–248S. [Google Scholar] [CrossRef] [PubMed]
- Spees, C.K.; Hill, E.B.; Grainger, E.M.; Buell, J.L.; White, S.E.; Kleinhenz, M.D.; Clinton, S.K. Feasibility, Preliminary Efficacy, and Lessons Learned from a Garden-Based Lifestyle Intervention for Cancer Survivors. Cancer Control 2016, 23, 302–310. [Google Scholar] [CrossRef]
- Christifano, D.N.; Fazzino, T.L.; Sullivan, D.K.; Befort, C.A. Diet Quality of Breast Cancer Survivors after a Six-Month Weight Management Intervention: Improvements and Association with Weight Loss. Nutr. Cancer 2016, 68, 1301–1308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lucas, A.R.; Focht, B.C.; Cohn, D.E.; Buckworth, J.; Klatt, M.D. A Mindfulness-Based Lifestyle Intervention for Obese, Inactive Endometrial Cancer Survivors: A Feasibility Study. Integr. Cancer Ther. 2016, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Shams-White, M.M.; Brockton, N.T.; Mitrou, P.; Romaguera, D.; Brown, S.; Bender, A.; Kahle, L.L.; Reedy, J. Operationalizing the 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Cancer Prevention Recommendations: A Standardized Scoring System. Nutrients 2019, 11, 1572. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Romaguera, D.; Mitrou, P.; Reedy, J.; Bender, A.; Brockton, N.T. Further Guidance in Implementing the Standardized 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Score. Cancer Epidemiol. Biomark. Prev. 2020, 29, 889–894. [Google Scholar] [CrossRef]
- Rocío, O.-R.; Macarena, L.-L.; Inmaculada, S.-B.; Antonio, J.-P.; Fernando, V.-A.; Marta, G.-C.; María-José, S.; José-Juan, J.-M. Compliance with the 2018 World Cancer Research Fund/American Institute for Cancer Research Cancer Prevention Recommendations and Prostate Cancer. Nutrients 2020, 12, 768. [Google Scholar] [CrossRef]
- Turati, F.; Dalmartello, M.; Bravi, F.; Serraino, D.; Augustin, L.; Giacosa, A.; Negri, E.; Levi, F.; La Vecchia, C. Adherence to the World Cancer Research Fund/American Institute for Cancer Research Recommendations and the Risk of Breast Cancer. Nutrients 2020, 12, 607. [Google Scholar] [CrossRef]
- Kaluza, J.; Harris, H.R.; Håkansson, N.; Wolk, A. Adherence to the WCRF/AICR 2018 Recommendations for Cancer Prevention and Risk of Cancer: Prospective Cohort Studies of Men and Women. Br. J. Cancer 2020, 122, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Rodríguez, R.; Toledo, E.; Martinez-Gonzalez, M.A.; Aguilera-Buenosvinos, I.; Romanos-Nanclares, A.; Jiménez-Moleón, J.J. Adherence to the 2018 World Cancer Research Fund/American Institute for Cancer Research Recommendations and Breast Cancer in the SUN Project. Nutrients 2020, 12, 2076. [Google Scholar] [CrossRef]
- Barrubés, L.; Babio, N.; Hernández-Alonso, P.; Toledo, E.; Ramírez Sabio, J.B.; Estruch, R.; Ros, E.; Fitó, M.; Alonso-Gómez, A.M.; Fiol, M.; et al. Association between the 2018 WCRF/AICR and the Low-Risk Lifestyle Scores with Colorectal Cancer Risk in the Predimed Study. J. Clin. Med. 2020, 9, 1215. [Google Scholar] [CrossRef] [PubMed]
- van Zutphen, M.; Boshuizen, H.C.; Kok, D.E.; van Baar, H.; Geijsen, A.J.M.R.; Wesselink, E.; Winkels, R.M.; van Halteren, H.K.; de Wilt, J.H.W.; Kampman, E.; et al. Colorectal Cancer Survivors Only Marginally Change Their Overall Lifestyle in the First 2 Years Following Diagnosis. J. Cancer Surviv. 2019, 13, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Spees, C.K.; Braun, A.C.; Hill, E.B.; Grainger, E.M.; Portner, J.; Young, G.S.; Kleinhenz, M.D.; Chitchumroonchokchai, C.; Clinton, S.K. Impact of a Tailored Nutrition and Lifestyle Intervention for Overweight Cancer Survivors on Dietary Patterns, Physical Activity, Quality of Life, and Cardiometabolic Profiles. J. Oncol. 2019, 2019, 1503195. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans 2015–2020; U.S. Department of Agriculture: Washington, DC, USA, 2015.
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2018.
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Kristal, A.R.; Kolar, A.S.; Fisher, J.L.; Plascak, J.J.; Stumbo, P.J.; Weiss, R.; Paskett, E.D. Evaluation of Web-Based, Self-Administered, Graphical Food Frequency Questionnaire. J. Acad. Nutr. Diet. 2014, 114, 613–621. [Google Scholar] [CrossRef]
- Nutrition Coordinating Center Nutrition Data System for Research: NDSR 2017. Available online: http://www.ncc.umn.edu/products/ndsr-user-manual/ (accessed on 12 October 2022).
- Bassett, D.R.; Wyatt, H.R.; Thompson, H.; Peters, J.C.; Hill, J.O. Pedometer-Measured Physical Activity and Health Behaviors in United States Adults. Med. Sci. Sport. Exerc. 2010, 42, 1819–1825. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.; Moubarac, J.-C.; Jaime, P.; Martins, A.P.; Canella, D.; Louzada, M.; Parra, D. NOVA. The Star Shines Bright. World Nutr. 2016, 7, 28–38. [Google Scholar]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Costa Louzada, M.d.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System. Rome FAO 2019, 49. Available online: https://www.fao.org/3/ca5644en/ca5644en.pdf (accessed on 12 October 2022).
- Tudor-Locke, C.; Leonardi, C.; Johnson, W.D.; Katzmarzyk, P.T.; Church, T.S. Accelerometer Steps/Day Translation of Moderate-to-Vigorous Activity. Prev. Med. 2011, 53, 31–33. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Craig, C.L.; Aoyagi, Y.; Bell, R.C.; Croteau, K.A.; De Bourdeaudhuij, I.; Ewald, B.; Gardner, A.W.; Hatano, Y.; Lutes, L.D.; et al. How Many Steps/Day Are Enough? For Older Adults and Special Populations. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 80. [Google Scholar] [CrossRef]
- Agudo, A. Measuring Intake of Fruit and Vegetables; Background paper for joint FAO/WHO workshop on fruits and vegetables for health; World Health Organization: Kobe, Japan, 2004. [Google Scholar]
- Terranova, C.O.; Winkler, E.A.H.; Healy, G.N.; Demark-Wahnefried, W.; Eakin, E.G.; Reeves, M.M. Dietary and Physical Activity Changes and Adherence to WCRF/AICR Cancer Prevention Recommendations Following a Remotely Delivered Weight Loss Intervention for Female Breast Cancer Survivors: The Living Well after Breast Cancer Randomized Controlled Trial. J. Acad. Nutr. Diet. 2022, 122, 1644–1664.e7. [Google Scholar] [CrossRef] [PubMed]
- Solans, M.; Chan, D.S.M.; Mitrou, P.; Norat, T.; Romaguera, D. A Systematic Review and Meta-Analysis of the 2007 WCRF/AICR Score in Relation to Cancer-Related Health Outcomes. Ann. Oncol. 2020, 31, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Good, M.; Braun, A.C.; Taylor, C.A.; Spees, C.K. US Adults Fall Short of the Dietary Guidelines for Cancer Prevention Regardless of BMI Category. J. Acad. Nutr. Diet. 2021, 122, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. International Agency for Research on Cancer Monograph Working Group Carcinogenicity of Consumption of Red and Processed Meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Iqbal, R.; Dehghan, M.; Mente, A.; Rangarajan, S.; Wielgosz, A.; Avezum, A.; Seron, P.; AlHabib, K.F.; Lopez-Jaramillo, P.; Swaminathan, S.; et al. Associations of Unprocessed and Processed Meat Intake with Mortality and Cardiovascular Disease in 21 Countries [Prospective Urban Rural Epidemiology (PURE) Study]: A Prospective Cohort Study. Am. J. Clin. Nutr. 2021, 114, 1049–1058. [Google Scholar] [CrossRef]
- Frank, S.M.; Jaacks, L.M.; Batis, C.; Vanderlee, L.; Taillie, L.S. Patterns of Red and Processed Meat Consumption across North America: A Nationally Representative Cross-Sectional Comparison of Dietary Recalls from Canada, Mexico, and the United States. Int. J. Environ. Res. Public Health 2021, 18, 357. [Google Scholar] [CrossRef]
- Poore, J.; Nemecek, T. Reducing Food’s Environmental Impacts through Producers and Consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Dietary Guidelines Advisory Committee. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2020.
- Baraldi, L.G.; Martinez Steele, E.; Canella, D.S.; Monteiro, C.A. Consumption of Ultra-Processed Foods and Associated Sociodemographic Factors in the USA between 2007 and 2012: Evidence from a Nationally Representative Cross-Sectional Study. BMJ Open 2018, 8, e020574. [Google Scholar] [CrossRef]
- Juul, F.; Parekh, N.; Martinez-Steele, E.; Monteiro, C.A.; Chang, V.W. Ultra-Processed Food Consumption among US Adults from 2001 to 2018. Am. J. Clin. Nutr. 2022, 115, 211–221. [Google Scholar] [CrossRef]
- Pagliai, G.; Dinu, M.; Madarena, M.; Bonaccio, M.; Iacoviello, L.; Sofi, F. Consumption of Ultra-Processed Foods and Health Status: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2021, 125, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Heshmati, J.; Shahinfar, H.; Tripathi, N.; Daneshzad, E. Ultra-Processed Food and the Risk of Overweight and Obesity: A Systematic Review and Meta-Analysis of Observational Studies. Int. J. Obes. 2020, 44, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Simões, B.D.S.; Litvak, J.; Martinez-Steele, E.; Deierlein, A.; Vadiveloo, M.; Parekh, N. Processing Level and Diet Quality of the US Grocery Cart: Is There an Association? Public Health Nutr. 2019, 22, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Andrianasolo, R.M.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultra-Processed Food Intake and Risk of Cardiovascular Disease: Prospective Cohort Study (NutriNet-Santé). BMJ 2019, 365, l1451. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.M.; Terranova, C.O.; Eakin, E.G.; Demark-Wahnefried, W. Weight Loss Intervention Trials in Women with Breast Cancer: A Systematic Review. Obes. Rev. 2014, 15, 749–768. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Reeves, M.M. Weight Loss Randomized Intervention Trials in Female Cancer Survivors. J. Clin. Oncol. 2016, 34, 4238–4248. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.; Li, X.; He, X.; Yang, Y.; Zhu, S. Emerging Trends of Technology-Based Dietary Assessment: A Perspective Study. Eur. J. Clin. Nutr. 2021, 75, 582–587. [Google Scholar] [CrossRef]
- Eldridge, A.L.; Piernas, C.; Illner, A.-K.; Gibney, M.J.; Gurinović, M.A.; de Vries, J.H.M.; Cade, J.E. Evaluation of New Technology-Based Tools for Dietary Intake Assessment—An ILSI Europe Dietary Intake and Exposure Task Force Evaluation. Nutrients 2018, 11, 55. [Google Scholar] [CrossRef]
- Naska, A.; Lagiou, A.; Lagiou, P. Dietary Assessment Methods in Epidemiological Research: Current State of the Art and Future Prospects. F1000Res 2017, 6, 926. [Google Scholar] [CrossRef]
- Thompson, F.E.; Kirkpatrick, S.I.; Subar, A.F.; Reedy, J.; Schap, T.E.; Wilson, M.M.; Krebs-Smith, S.M. The National Cancer Institute’s Dietary Assessment Primer: A Resource for Diet Research. J. Acad. Nutr. Diet. 2015, 115, 1986–1995. [Google Scholar] [CrossRef]
- Subar, A.F.; Freedman, L.S.; Tooze, J.A.; Kirkpatrick, S.I.; Boushey, C.; Neuhouser, M.L.; Thompson, F.E.; Potischman, N.; Guenther, P.M.; Tarasuk, V.; et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. J. Nutr. 2015, 145, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Dragsted, L.O.; Gao, Q.; Scalbert, A.; Vergères, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Afman, L.A.; Wishart, D.S.; Andres Lacueva, C.; et al. Validation of Biomarkers of Food Intake—Critical Assessment of Candidate Biomarkers. Genes Nutr. 2018, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Jahns, L.; Johnson, L.K.; Mayne, S.T.; Cartmel, B.; Picklo, M.J.; Ermakov, I.V.; Gellermann, W.; Whigham, L.D. Skin and Plasma Carotenoid Response to a Provided Intervention Diet High in Vegetables and Fruit: Uptake and Depletion Kinetics. Am. J. Clin. Nutr. 2014, 100, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.; Nash, S.H.; Kristal, A.R.; Hopkins, S.; Boyer, B.B.; O’Brien, D.M. The Carbon Isotope Ratio of Alanine in Red Blood Cells Is a New Candidate Biomarker of Sugar-Sweetened Beverage Intake. J. Nutr. 2013, 143, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, H.; McNulty, B.A.; Nugent, A.P.; Walton, J.; Flynn, A.; Gibney, M.J.; Brennan, L. A Metabolomics Approach to the Identification of Biomarkers of Sugar-Sweetened Beverage Intake. Am. J. Clin. Nutr. 2015, 101, 471–477. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Madrid-Gambin, F.; Garcia-Aloy, M.; Andres-Lacueva, C.; Logue, C.; Gallagher, A.M.; Mack, C.; Kulling, S.E.; Gao, Q.; Praticò, G.; et al. Biomarkers of Intake for Coffee, Tea, and Sweetened Beverages. Genes Nutr. 2018, 13, 15. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, P.; Sha, W.; Sang, S. Urinary Biomarkers of Whole Grain Wheat Intake Identified by Non-Targeted and Targeted Metabolomics Approaches. Sci. Rep. 2016, 6, 36278. [Google Scholar] [CrossRef]
- Ross, A.B.; Kamal-Eldin, A.; Åman, P. Dietary Alkylresorcinols: Absorption, Bioactivities, and Possible Use as Biomarkers of Whole-Grain Wheat–and Rye–Rich Foods. Nutr. Rev. 2004, 62, 81–95. [Google Scholar] [CrossRef]
- Jawhara, M.; Sørensen, S.B.; Heitmann, B.L.; Andersen, V. Biomarkers of Whole-Grain and Cereal-Fiber Intake in Human Studies: A Systematic Review of the Available Evidence and Perspectives. Nutrients 2019, 11, 2994. [Google Scholar] [CrossRef]
- Vassbakk-Brovold, K.; Kersten, C.; Fegran, L.; Mjåland, O.; Mjåland, S.; Seiler, S.; Berntsen, S. Cancer Patients Participating in a Lifestyle Intervention during Chemotherapy Greatly Over-Report Their Physical Activity Level: A Validation Study. BMC Sport. Sci. Med. Rehabil. 2016, 8, 10. [Google Scholar] [CrossRef]
- Smith, L.; Lee, J.A.; Mun, J.; Pakpahan, R.; Imm, K.R.; Izadi, S.; Kibel, A.S.; Colditz, G.A.; Grubb, R.L.; Wolin, K.Y.; et al. Levels and Patterns of Self-Reported and Objectively-Measured Free-Living Physical Activity among Prostate Cancer Survivors: A Prospective Cohort Study. Cancer 2019, 125, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Gresham, G.; Schrack, J.; Gresham, L.M.; Shinde, A.M.; Hendifar, A.E.; Tuli, R.; Rimel, B.J.; Figlin, R.; Meinert, C.L.; Piantadosi, S. Wearable Activity Monitors in Oncology Trials: Current Use of an Emerging Technology. Contemp. Clin. Trials 2018, 64, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Hadgraft, N.T.; Moore, M.M.; Rosenberg, D.E.; Lynch, C.; Reeves, M.M.; Lynch, B.M. A Qualitative Evaluation of Breast Cancer Survivors’ Acceptance of and Preferences for Consumer Wearable Technology Activity Trackers. Support Care Cancer 2017, 25, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Pew Research Center. About One-in-Five Americans Use a Smart Watch or Fitness Tracker; Pew Research Center: Washington, DC, USA, 2020; Available online: https://www.pewresearch.org/fact-tank/2020/01/09/about-one-in-five-americans-use-a-smart-watch-or-fitness-tracker/ (accessed on 12 October 2022).
- Greaves, C.J.; Sheppard, K.E.; Abraham, C.; Hardeman, W.; Roden, M.; Evans, P.H.; Schwarz, P. The IMAGE Study Group Systematic Review of Reviews of Intervention Components Associated with Increased Effectiveness in Dietary and Physical Activity Interventions. BMC Public Health 2011, 11, 119. [Google Scholar] [CrossRef]
- Teasdale, N.; Elhussein, A.; Butcher, F.; Piernas, C.; Cowburn, G.; Hartmann-Boyce, J.; Saksena, R.; Scarborough, P. Systematic Review and Meta-Analysis of Remotely Delivered Interventions Using Self-Monitoring or Tailored Feedback to Change Dietary Behavior. Am. J. Clin. Nutr. 2018, 107, 247–256. [Google Scholar] [CrossRef]
- Compernolle, S.; DeSmet, A.; Poppe, L.; Crombez, G.; De Bourdeaudhuij, I.; Cardon, G.; van der Ploeg, H.P.; Van Dyck, D. Effectiveness of Interventions Using Self-Monitoring to Reduce Sedentary Behavior in Adults: A Systematic Review and Meta-Analysis. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 63. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef]
| 2018 WCRF/AICR Recommendations | Operationalization of Recommendations [28] | Adaptation | Points |
|---|---|---|---|
| BMI (kg/m2) | None | |
| 18.5–24.9 | 0.5 | ||
| 25–29.9 | 0.25 | ||
| <18.5 or ≥30 | 0 | ||
| Waist circumference (cm) | None | ||
| Men: <94 | 0.5 | ||
| Women: <80 | |||
| Men: 94–<102 | 0.25 | ||
| Women: 80–<88 | |||
| Men: ≥102 | 0 | ||
| Women: ≥88 | |||
| Total moderate-vigorous physical activity (minutes/week) | Total steps (steps/day) | |
| ≥150 | ≥7000 | 1 | |
| 75–<150 | 3500–6999 | 0.5 | |
| <75 | <3500 | 0 | |
| Fruits and vegetables (g/day) | Fruits and vegetables (cup eq/day) | |
| ≥400 | ≥2.5 | 0.5 | |
| 200–<400 | 1.25–2.5 | 0.25 | |
| <200 | <1.25 | 0 | |
| Total fiber (g/day) | None | ||
| ≥30 | 0.5 | ||
| 15–<30 | 0.25 | ||
| <15 | 0 | ||
| Percent of total kcal from ultra-processed foods (UPFs) a | None | |
| Tertile 1 | 1 | ||
| Tertile 2 | 0.5 | ||
| Tertile 3 | 0 | ||
| Total red meat (g/week) and processed meat (g/week) | None | |
| Red meat <500 and processed meat <21 | 1 | ||
| Red meat <500 and processed meat 21–<100 | 0.5 | ||
| Red meat >500 or processed meat ≥100 | 0 | ||
| Total sugar-sweetened drinks (g/day) | None | |
| 0 | 1 | ||
| >0–≤250 | 0.5 | ||
| >250 | 0 | ||
| Total ethanol (g/day) | None | |
| 0 | 1 | ||
| Men: >0–≤28 | 0.5 | ||
| Women: >0–≤14 | |||
| Men: >28 | 0 | ||
| Women: >14 | |||
| Total WCRF/AICR Score Range | 0–7 | ||
| Participant Characteristic | n (%) | |
|---|---|---|
| Age, years (mean ± SD) | 53.0 ± 11.5 | |
| Sex | Female | 82 (90.1) |
| Male | 9 (9.9) | |
| Race/Ethnicity | White/Caucasian | 82 (90.1) |
| Black/African American | 7 (7.7) | |
| Asian | 2 (2.2) | |
| Marital Status | Married | 54 (59.3) |
| Never Married | 19 (20.9) | |
| Divorced | 12 (13.2) | |
| Other a | 6 (6.6) | |
| Education | Grade 12 Equivalent | 7 (7.7) |
| College 1 to 3 years | 11 (12.1) | |
| College 4 years or more | 38 (41.8) | |
| Professional or Graduate | 35 (38.5) | |
| Employment | Employed or Self-employed | 68 (74.7) |
| Retired | 21 (23.1) | |
| Other b | 2 (2.2) | |
| Household Income | USD 10,000–24,999 | 6 (6.6) |
| USD 25,000–49,000 | 9 (9.9) | |
| USD 50,000–74,999 | 5 (5.5) | |
| ≥USD 75,000 | 28 (30.8) | |
| Do not know/Prefer not to answer | 43 (47.3) | |
| Primary Cancer | Breast | 50 (54.9) |
| Leukemia/Lymphoma | 10 (11.0) | |
| Ovarian/Uterine/Cervical/Endometrial | 9 (9.9) | |
| Thyroid | 6 (6.6) | |
| Prostate | 5 (5.5) | |
| Oral/Head/Neck | 4 (4.4) | |
| Colorectal | 2 (2.2) | |
| Other c | 5 (5.5) | |
| WCRF/AICR Component | (Mean ± SD) | Difference (95% CI) | p-Value | |
|---|---|---|---|---|
| Enrollment | Post-Intervention | |||
| Healthy weight | ||||
| BMI (kg/m2) | 32.7 ± 4.6 | 31.5 ± 4.8 | −1.1 (−1.4, −0.8) | <0.001 |
| Waist circumference (cm) | 105.4 ± 11.9 | 103.0 ± 13.0 | −2.4 (−3.6, −1.1) | <0.001 |
| Physical activity (steps/day) a | 6958 ± 3258 | 8066 ± 3215 | 1107 (522, 1693) | <0.001 |
| Plant-based diet | ||||
| Fruits and non-starchy vegetables (cup eq/day) b | 3.7 ± 1.8 | 5.1 ± 2.3 | 1.39 (1.27, 1.52) | <0.001 |
| Fiber (g/day) b | 23.7 ± 11.6 | 25.6 ± 10.1 | 1.11 (1.03, 1.19) | 0.007 |
| Fast foods (% kcal from UPF/day) | 45.1 ± 14.0 | 34.6 ± 11.4 | −10.5 (−13.5, −7.5) | <0.001 |
| Red meat (g/week) b | 258 ± 206 | 162 ± 142 | 0.62 (0.48, 0.81) | <0.001 |
| Processed meat (g/week) b | 107 ± 130 | 36 ± 43 | 0.30 (0.21, 0.42) | <0.001 |
| Sugar-sweetened beverages (g/day) c | 228.8 ± 398.5 | 161.9 ± 345.0 | 0.0 (−82.7, 0.0) | 0.008 |
| Alcohol (g/day) c | 6.7 ± 12.4 | 5.4 ± 11.0 | 0.0 (−1.5, 0.0) | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, E.B.; Grainger, E.M.; Young, G.S.; Clinton, S.K.; Spees, C.K. Application of the Updated WCRF/AICR Cancer Prevention Score as an Outcome for Cancer Survivors Participating in a Tailored and Intensive Dietary and Physical Activity Intervention. Nutrients 2022, 14, 4751. https://doi.org/10.3390/nu14224751
Hill EB, Grainger EM, Young GS, Clinton SK, Spees CK. Application of the Updated WCRF/AICR Cancer Prevention Score as an Outcome for Cancer Survivors Participating in a Tailored and Intensive Dietary and Physical Activity Intervention. Nutrients. 2022; 14(22):4751. https://doi.org/10.3390/nu14224751
Chicago/Turabian StyleHill, Emily B., Elizabeth M. Grainger, Gregory S. Young, Steven K. Clinton, and Colleen K. Spees. 2022. "Application of the Updated WCRF/AICR Cancer Prevention Score as an Outcome for Cancer Survivors Participating in a Tailored and Intensive Dietary and Physical Activity Intervention" Nutrients 14, no. 22: 4751. https://doi.org/10.3390/nu14224751
APA StyleHill, E. B., Grainger, E. M., Young, G. S., Clinton, S. K., & Spees, C. K. (2022). Application of the Updated WCRF/AICR Cancer Prevention Score as an Outcome for Cancer Survivors Participating in a Tailored and Intensive Dietary and Physical Activity Intervention. Nutrients, 14(22), 4751. https://doi.org/10.3390/nu14224751







