A Scoping Review to Explore the Potential Benefits of Nutrition Interventions for Latino/a Adult Cancer Survivors in the US
Abstract
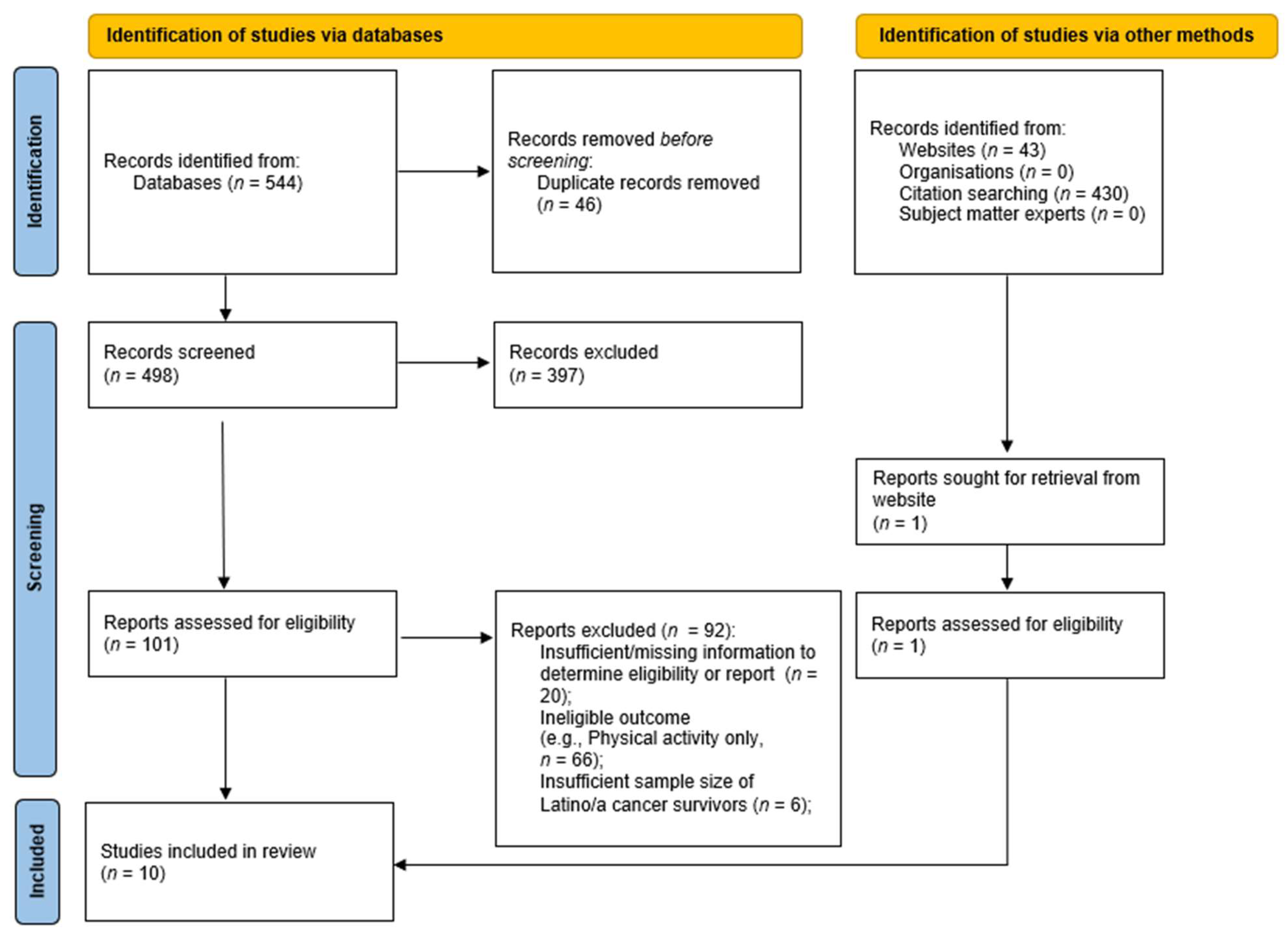
:1. Introduction
2. Methods
2.1. Research Team
2.2. Procedures
2.3. Data Extraction of Included Studies
2.4. Summarization and Synthesis of Research Findings
3. Results
3.1. Overview
3.2. Study Design
3.3. Intervention Foci
3.4. Participant Characteristics
3.5. Settings
3.6. Approaches and Theory
3.7. Intervention Components, Curricula, and Behavior Change Strategies
3.8. Delivery and Dose
3.9. Outcome Assessment
3.10. Intervention Effects
3.11. Retention and Engagement Strategies
3.12. Attrition and Attendance
4. Discussion
4.1. Key Messages
4.2. Limitations and Strengths
4.3. Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Census Bureau. Quick Facts: United States: Hispanic or Latino, Percent. Available online: https://www.census.gov/quickfacts/fact/table/US/RHI725221 (accessed on 28 September 2023).
- Krogstad, J.P.M.; Passel, J.S.; Moslimani, M.; Noe-Bustamante, L. Key Facts about U.S. Latinos for National Hispanic Heritage Month. Available online: https://www.pewresearch.org/short-reads/2023/09/22/key-facts-about-us-latinos-for-national-hispanic-heritage-month/ (accessed on 28 September 2023).
- Vespa, J.M.; Medina, L.; Armstrong, D.M. Demographic Turning Points for the United States: Population Projections for 2020 to 2060 Population Estimates and Projections. Current Population Report. 2020. Available online: https://www.census.gov/content/dam/Census/library/publications/2020/demo/p25-1144.pdf (accessed on 28 September 2023).
- Miller, K.D.; Ortiz, A.P.; Pinheiro, P.S.; Bandi, P.; Minihan, A.; Fuchs, H.E.; Martinez Tyson, D.; Tortolero-Luna, G.; Fedewa, S.A.; Jemal, A.M.; et al. Cancer statistics for the U.S. Hispanic/Latino population, 2021. CA Cancer J. Clin. 2021, 71, 466–487. [Google Scholar] [CrossRef]
- Shrider, E.A.; Creamer, J. Poverty in the United States: 2022 (P60-280). Current Population Reports. 2023. Available online: https://www.census.gov/content/dam/Census/library/publications/2023/demo/p60-280.pdf (accessed on 28 September 2023).
- Keisler-Starkey, K.; Bunch, L.N.; Lindstrom, R.A. Health Insurance Coverage in the United States: 2022 (P60-281). Current Population Reports. 2023. Available online: https://www.census.gov/library/publications/2023/demo/p60-281.html (accessed on 28 September 2023).
- American Cancer Society (ACS). Cancer Facts & Figures for Hispanic and Latino People. Available online: https://www.cancer.org/research/cancer-facts-statistics/hispanics-latinos-facts-figures.html (accessed on 28 September 2023).
- Montealegre, J.R.; Zhou, R.; Amirian, E.S.; Follen, M.; Scheurer, M.E. Nativity disparities in late-stage diagnosis and cause-specific survival among Hispanic women with invasive cervical cancer: An analysis of surveillance, epidemiology, and end results data. Cancer Causes Control 2013, 24, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Minority Health and Health Disparities (NIMHD). Cancer and Hispanic Americans. Available online: https://minorityhealth.hhs.gov/cancer-and-hispanic-americans (accessed on 28 September 2023).
- National Institute on Minority Health and Health Disparities (NIHMD). Minority Health and Health Disparities: Definitions and Parameters. Available online: https://www.nimhd.nih.gov/about/strategic-plan/nih-strategic-plan-definitions-and-parameters.html (accessed on 2 September 2023).
- Bailey, Z.D.; Fracassa, J.; Bassett, M.T. How structural racism works—Racist policies as a root cause of U.S. racial health inequities. N. Engl. J. Med. 2021, 384, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.I.; Ramirez, A.G.; San Miguel-Majors, S.L.; Castillo, L.; Fox, R.S.; Gallion, K.J.; Munoz, E.; Estabrook, R.; Perez, A.; Lad, T.; et al. Unmet supportive care needs in Hispanic/Latino cancer survivors: Prevalence and associations with patient-provider communication, satisfaction with cancer care, and symptom burden. Support. Care Cancer 2019, 27, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.G.; Muñoz, E.; Parma, D.L.; Michalek, J.E.; Holden, A.E.C.; Phillips, T.D.; Pollock, B.H. Lifestyle and clinical correlates of hepatocellular carcinoma in South Texas: A matched case-control study. Clin. Gastroenterol. Hepatol. 2017, 15, 1311–1312. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute (NCI), Division of Cancer Control & Population Sciences, Office of Cancer Survivorship. Statistics and Graphs. Available online: https://cancercontrol.cancer.gov/ocs/statistics (accessed on 17 November 2022).
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Rogers, L.Q.; Alfano, C.M.; Thomson, C.A.; Courneya, K.S.; Meyerhardt, J.A.; Stout, N.L.; Kvale, E.; Ganzer, H.; Ligibel, J.A. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors. CA Cancer J. Clin. 2015, 65, 167–189. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- American Cancer Society (ACS). American Cancer Society Guideline for Diet and Physical Activity. Available online: https://www.cancer.org/cancer/risk-prevention/diet-physical-activity/acs-guidelines-nutrition-physical-activity-cancer-prevention/guidelines.html (accessed on 28 September 2023).
- American Institute of Cancer Research (AICR). AICR Recommendations & Cancer Prevention American Institute of Cancer Research (AICR). 2023. Available online: https://healthy10challenge.org/cancer-prevention/ (accessed on 25 September 2023).
- Jankovic, N.; Geelen, A.; Winkels, R.M.; Mwungura, B.; Fedirko, V.; Jenab, M.; Illner, A.K.; Brenner, H.; Ordóñez-Mena, J.M.; Kiefte de Jong, J.C.; et al. Adherence to the WCRF/AICR dietary recommendations for cancer prevention and risk of cancer in elderly from Europe and the United States: A meta-analysis within the CHANCES Project. Cancer Epidemiol. Biomark. Prev. 2017, 26, 136–144. [Google Scholar] [CrossRef]
- Petimar, J.; Smith-Warner, S.A.; Rosner, B.; Chan, A.T.; Giovannucci, E.L.; Tabung, F.K. Adherence to the World Cancer Research Fund/American Institute for Cancer Research 2018 Recommendations for Cancer Prevention and risk of colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1469–1479. [Google Scholar] [CrossRef]
- Castro-Espin, C.; Agudo, A. The role of diet in prognosis among cancer survivors: A systematic review and meta-analysis of dietary patterns and diet interventions. Nutrients 2022, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Parsons, H.M.; Forte, M.L.; Abdi, H.I.; Brandt, S.; Claussen, A.M.; Wilt, T.J.; Klein, M.; Ester, E.; Landsteiner, A.; Shaukut, A.; et al. Nutrition as prevention for improved cancer health outcomes: A systematic literature review. JNCI Cancer Spectr. 2023, 7, pkad035. [Google Scholar] [CrossRef] [PubMed]
- Pichardo, M.S.; Sanft, T.; Ferrucci, L.M.; Romero-Ramos, Y.M.; Cartmel, B.; Harrigan, M.; Velazquez, A.I.; Fayanju, O.M.; Winer, E.P.; Irwin, M.L. Diet and physical activity interventions in Black and Latina women with breast cancer: A scoping review. Systematic Review. Front. Oncol. 2023, 13, 1079293. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 67–473. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Westphaln, K.K.; Regoeczi, W.; Masotya, M.; Vazquez-Westphaln, B.; Lounsbury, K.; McDavid, L.; Lee, H.; Johnson, J.; Ronis, S.D. From Arksey and O’Malley and Beyond: Customizations to enhance a team-based, mixed approach to scoping review methodology. MethodsX 2021, 8, 101375. [Google Scholar] [CrossRef]
- Ellis, K.R.; Raji, D.; Olaniran, M.; Alick, C.; Nichols, D.; Allicock, M. A systematic scoping review of post-treatment lifestyle interventions for adult cancer survivors and family members. J. Cancer Surviv. 2021, 16, 233–256. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. National Institutes of Health (NIH), National Library of Medicine (NLM). Available online: https://clinicaltrials.gov (accessed on 28 September 2023).
- NIH RePORTER. National Institutes of Health (NIH). Available online: https://reporter.nih.gov/ (accessed on 28 September 2023).
- Cochrane Central Register of Controlled Trials (CENTRAL). CochraneLibrary.com. Available online: https://www.cochranelibrary.com/central/about-central (accessed on 28 September 2023).
- Hiatt, R.A.; Clayton, M.F.; Collins, K.K.; Gold, H.T.; Laiyemo, A.O.; Truesdale, K.P.; Ritzwoller, D.P. The Pathways to Prevention Program: Nutrition as prevention for improved cancer outcomes. J. Natl. Cancer Inst. 2023, 115, 886–895. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- PRISMA-Statement.org. PRISMA Flow Diagram. PRISMA-Statement.org. Available online: http://www.prisma-statement.org/PRISMAStatement/FlowDiagram (accessed on 28 September 2023).
- Prado, A.M.; Young, S.; Kamaraju, S.; Henderson, M.; Sheean, P.; Stolley, M.R. Avanzando Juntas: Adapting an evidence-based weight loss program for Hispanic breast and gynecologic cancer survivors. Cancer Epidemiol. Biomark. Prev. 2020, 29, B010. [Google Scholar] [CrossRef]
- Conlon, B.A.; Kahan, M.; Martinez, M.; Isaac, K.; Rossi, A.; Skyhart, R.; Wylie-Rosett, J.; Moadel-Robblee, A. Development and evaluation of the curriculum for BOLD (Bronx Oncology Living Daily) healthy living: A diabetes prevention and control program for underserved cancer survivors. J. Cancer Educ. 2015, 30, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Aycinena, A.C.; Jennings, K.-A.; Gaffney, A.O.; Koch, P.A.; Contento, I.R.; Gonzalez, M.; Guidon, E.; Karmally, W.; Hershman, D.; Greenlee, H. ¡Cocinar Para Su Salud! Development of a culturally based nutrition education curriculum for Hispanic breast cancer survivors using a theory-driven procedural model. Health Educ. Behav. 2017, 44, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; Gaffney, A.O.; Aycinena, A.C.; Koch, P.; Contento, I.; Karmally, W.; Richardson, J.M.; Lim, E.; Tsai, W.Y.; Crew, K.; et al. ¡Cocinar Para Su Salud!: Randomized controlled trial of a culturally based dietary intervention among Hispanic breast cancer survivors. J. Acad. Nutr. Diet. 2015, 115, S42–S56.e3. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; Ogden Gaffney, A.; Aycinena, A.C.; Koch, P.; Contento, I.; Karmally, W.; Richardson, J.M.; Shi, Z.; Lim, E.; Tsai, W.-Y. Long-term diet and biomarker changes after a short-term intervention among Hispanic breast cancer survivors: The¡ Cocinar Para Su Salud! randomized controlled trial. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.A.; Crew, K.D.; Mata, J.M.; McKinley, P.S.; Rundle, A.G.; Zhang, W.; Liao, Y.; Tsai, W.Y.; Hershman, D.L. A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity 2013, 21, 65–76. [Google Scholar] [CrossRef]
- Thomson, C.A.; Crane, T.E.; Miller, A.; Garcia, D.O.; Basen-Engquist, K.; Alberts, D.S. A randomized trial of diet and physical activity in women treated for stage II–IV ovarian cancer: Rationale and design of the Lifestyle Intervention for Ovarian Cancer Enhanced Survival (LIVES): An NRG Oncology/Gynecologic Oncology Group (GOG-225) study. Contemp. Clin. Trials 2016, 9, 181–189. [Google Scholar] [CrossRef]
- Thomson, C.A.; Crane, T.E.; Miller, A.; Gold, M.A.; Powell, M.; Bixel, K.; Van Le, L.; DiSilvestro, P.; Ratner, E.; Lele, S. Lifestyle intervention in ovarian cancer enhanced survival (LIVES) study (NRG/GOG0225): Recruitment, retention and baseline characteristics of a randomized trial of diet and physical activity in ovarian cancer survivors. Gynecol. Oncol. 2023, 170, 11–18. [Google Scholar] [CrossRef]
- Contento, I.; Paul, R.; Marin-Chollom, A.M.; Ogden Gaffney, A.; Sepulveda, J.; Dominguez, N.; Gray, H.; Haase, A.M.; Hershman, D.L.; Koch, P.; et al. Developing a diet and physical activity intervention for Hispanic/Latina breast cancer survivors. Cancer Control 2022, 29, 10732748221133987. [Google Scholar] [CrossRef]
- Santiago-Torres, M.; Contento, I.; Koch, P.; Tsai, W.Y.; Brickman, A.M.; Gaffney, A.O.; Thomson, C.A.; Crane, T.E.; Dominguez, N.; Sepulveda, J.; et al. ¡Mi Vida Saludable! A randomized, controlled, 2 × 2 factorial trial of a diet and physical activity intervention among Latina breast cancer survivors: Study design and methods. Contemp. Clin. Trials 2021, 110, 106524. [Google Scholar] [CrossRef]
- Santiago-Torres, M.; Contento, I.; Koch, P.; Tsai, W.-Y.; Brickman, A.M.; Gaffney, A.O.; Thomson, C.A.; Crane, T.E.; Dominguez, N.; Sepulveda, J. Associations between acculturation and weight, diet quality, and physical activity among Latina breast cancer survivors: The ¡Mi Vida Saludable! Study. J. Acad. Nutr. Diet. 2022, 122, 1703–1716. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, J.; Oswald, L.B.; Baik, S.H.; Buitrago, D.; Iacobelli, F.; Phillips, S.M.; Perez-Tamayo, A.; Guitelman, J.; Penedo, F.J.; Yanez, B. My Health smartphone intervention decreases daily fat sources among Latina breast cancer survivors. J. Behav. Med. 2020, 43, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Crane, T.E.; Badger, T.A.; O’Connor, P.; Segrin, C.; Alvarez, A.; Freylersythe, S.J.; Penaloza, I.; Pace, T.W.; Sikorskii, A. Lifestyle intervention for Latina cancer survivors and caregivers: The Nuestra Salud randomized pilot trial. J. Cancer Surviv. 2021, 15, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.G.; Parma, D.L.; Muñoz, E.; Mendoza, K.D.; Harb, C.; Holden, A.E.; Wargovich, M. An anti-inflammatory dietary intervention to reduce breast cancer recurrence risk: Study design and baseline data. Contemp. Clin. Trials 2017, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, K.E.; Parma, D.L.; Muñoz, E.; Spaniol, M.; Wargovich, M.; Ramirez, A.G. Dietary intervention among breast cancer survivors increased adherence to a Mediterranean-style, anti-inflammatory dietary pattern: The Rx for Better Breast Health randomized controlled trial. Breast Cancer Res. Treat. 2019, 173, 145–154. [Google Scholar] [CrossRef]
- Paxton, R.J.; Jones, L.A.; Chang, S.; Hernandez, M.; Hajek, R.A.; Flatt, S.W.; Natarajan, L.; Pierce, J.P. Was race a factor in the outcomes of the Women’s Health Eating and Living Study? Cancer 2011, 117, 3805–3813. [Google Scholar] [CrossRef]
- Pierce, J.P.; Faerber, S.; Wright, F.A.; Rock, C.L.; Newman, V.; Flatt, S.W.; Kealey, S.; Jones, V.E.; Caan, B.J.; Gold, E.B.; et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: The Women’s Healthy Eating and Living (WHEL) Study. Control. Clin. Trials 2002, 3, 728–756. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.P.; Faerber, S.; Wright, F.A.; Newman, V.; Flatt, S.W.; Kealey, S.; Rock, C.L.; Hryniuk, W.; Greenberg, E.R. Feasibility of a randomized trial of a high-vegetable diet to prevent breast cancer recurrence. Nutr. Cancer 1997, 28, 282–288. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- American Cancer Society (ACS). Cancer Facts & Figures 2023. 2023. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf (accessed on 28 September 2023).
- National Cancer Institute (NCI). Find a Cancer Center. Available online: https://www.cancer.gov/research/infrastructure/cancer-centers/find#California (accessed on 28 September 2023).
- Samuel, C.A.; Mbah, O.M.; Elkins, W.; Pinheiro, L.C.; Szymeczek, M.A.; Padilla, N.; Walker, J.S.; Corbie-Smith, G. Calidad de Vida: A systematic review of quality of life in Latino cancer survivors in the USA. Qual. Life Res. 2020, 29, 2615–2630. [Google Scholar] [CrossRef]
- Cousins, J.H.; Rubovits, D.S.; Dunn, J.K.; Reeves, R.S.; Ramirez, A.G.; Foreyt, J.P. Family versus individually oriented intervention for weight loss in Mexican American women. Public Health Rep. 1992, 107, 549. [Google Scholar] [PubMed]
- Foreyt, J.P.; Ramirez, A.G.; Cousins, J.H. Cuidando El Corazon—A weight-reduction intervention for Mexican Americans. Am. J. Clin. Nutr. 1991, 53, 1639S–1641S. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbon, M.L.; Gapstur, S.M.; Knight, S.J. Mujeres Felices por Ser Saludables: A breast cancer risk reduction program for Latino women. Prev. Med. 2003, 36, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, S.F.; Giacinto, R.E.; Medeiros, E.A.; Brongiel, I.; Cardona, O.; Perez, P.; Talavera, G.A. Academic-community partnership to develop a patient-centered breast cancer risk reduction program for Latina primary care patients. J. Racial Ethn. Health Disparities 2016, 3, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Skiba, M.B.; Lopez-Pentecost, M.; Werts, S.J.; Ingram, M.; Vogel, R.M.; Enriquez, T.; Garcia, L.; Thomson, C.A. Health promotion among Mexican-origin survivors of breast cancer and caregivers living in the United States-Mexico border region: Qualitative analysis from the Vida Plena Study. JMIR Cancer 2022, 8, e33083. [Google Scholar] [CrossRef]
- Werts, S.J.; Lopez-Pentecost, M.; Skiba, M.B.; Vogel, R.; Enriquez, T.; Garcia, L.; Ingram, M.; Thomson, C. Conducting photovoice with binational cancer survivors to identify health behavior change intervention preferences. Prog. Community Health Partnersh. Res. Educ. Action, 2022; awaiting publication. [Google Scholar]
- ClinicalTrials.org. SMLI With Hispanic Cancer Survivors and Caregivers. ClinicalTrials.org. Available online: https://clinicaltrials.gov/study/NCT05364372 (accessed on 28 September 2023).
- ClinicalTrials.org. “COACH” Study: Individualized COaching in Young Adult Cancer Survivors to Encourage Healthy Behaviors. ClinicalTrials.org. Available online: https://clinicaltrials.gov/study/NCT05434702 (accessed on 28 September 2023).
- ClinicalTrials.org. Remotely Delivered, Culturally Tailored Weight Loss Interventions among Latina Breast Cancer Survivors. ClinicalTrials.org. Available online: https://clinicaltrials.gov/study/NCT05930483 (accessed on 28 September 2023).
- ClinicalTrials.org. Trial of Exercise and Lifestyle in Women with Ovarian Cancer (TEAL). Available online: https://clinicaltrials.gov/study/NCT05761561 (accessed on 28 September 2023).
- Hooper, M.W.; Pérez-Stable, E.J. Health equity is not possible without addressing disparities. Health Psychol. 2023, 42, 625–627. [Google Scholar] [CrossRef]
- Johnson, C.M.; Sharkey, J.R.; Umstattd Meyer, M.R.; Gómez, L.; Allicock, M.A.; Prochnow, T.; Beltrán, E.; Martinez, L. Designing for multilevel behavior change: A father-focused nutrition and physical activity program for Mexican-heritage families in South Texas border communities. Int. J. Environ. Res. Public Health 2021, 18, 10117. [Google Scholar] [CrossRef]
- Balcazar, H.G.; Byrd, T.L.; Ortiz, M.; Tondapu, S.R.; Chavez, M. A randomized community intervention to improve hypertension control among Mexican Americans: Using the promotoras de salud community outreach model. J. Health Care Poor Underserved 2009, 20, 1079–1094. [Google Scholar] [CrossRef]
- Nguyen, M.; Chaudhry, S.I.; Desai, M.M.; Dzirasa, K.; Cavazos, J.E.; Boatright, D. Gender, racial, and ethnic and inequities in receipt of multiple National Institutes of Health research project grants. JAMA Netw. Open 2023, 6, e230855. [Google Scholar] [CrossRef]
- Ramirez, A.G.; Chalela, P. Equitable representation of Latinos in clinical research is needed to achieve health equity in cancer care. JCO Oncol. Pract. 2022, 18, e797–e804. [Google Scholar] [CrossRef]
- Oyer, R.A.; Hurley, P.; Boehmer, L.; Hurley, P.; Boehmer, L.; Bruinooge, S.S.; Levit, K.; Barrett, N.; Benson, A.; Bernick, L.A.; et al. Increasing racial and ethnic diversity in cancer clinical trials: An American Society of Clinical Oncology and Association of Community Cancer Centers joint research statement. J. Clin. Oncol. 2022, 40, 2163–2171. [Google Scholar] [CrossRef]
- Yancey, A.K.; Ortega, A.N.; Kumanyika, S.K. Effective recruitment and retention of minority research participants. Annu. Rev. Public Health 2006, 27, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Allicock, M.A.; Sharkey, J.R.; Umstattd Meyer, M.R.; Gómez, L.; Prochnow, T.; Laviolette, C.; Beltrán, E.; Garza, L.M. Promotoras de salud in a father-focused nutrition and physical activity program for border communities: Approaches and lessons learned from collaboration. Int. J. Environ. Res. Public Health 2022, 19, 11660. [Google Scholar] [CrossRef] [PubMed]
- Raber, M.; Jackson, A.; Basen-Engquist, K.; Bradley, C.; Chambers, S.; Gany, F.M.; Halbert, C.H.; Lindau, S.T.; Pérez-Escamilla, R.; Seligman, H. Food insecurity among people with cancer: Nutritional needs as an essential component of care. J. Natl. Cancer Inst. 2022, 114, 1577–1583. [Google Scholar] [CrossRef]
- Alfano, C.M.; Bluethmann, S.M.; Tesauro, G.; Perna, F.; Agurs-Collins, T.; Elena, J.W.; Ross, S.A.; O’Connell, M.; Bowles, H.R.; Greenberg, D.; et al. NCI funding trends and priorities in physical activity and energy balance research among cancer survivors. J. Natl. Cancer Inst. 2016, 108, djv285. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.W.; Oakley-Girvan, I. Achieving value in mobile health applications for cancer survivors. J. Cancer Surviv. 2017, 11, 498–504. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.org. eHealth Diet and Physical Activity Program for the Improvement of Health in Rural Latino Cancer Survivors (MiVSEEV). Available online: https://clinicaltrials.gov/study/NCT04081298 (accessed on 28 September 2023).
- Paxton, R.J.; Hajek, R.; Newcomb, P.; Dobhal, M.; Borra, S.; Taylor, W.C.; Parra-Medina, D.; Chang, S.; Courneya, K.S.; Block, G.; et al. A lifestyle intervention via email in minority breast cancer survivors: Randomized parallel-group feasibility study. JMIR Cancer 2017, 3, e13. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.K.; Pierce, J.P.; Mohler, J.; Paskett, E.; Jung, S.H.; Morris, M.J.; Small, E.; Hahn, O.; Humphrey, P.; Taylor, J.; et al. Men’s Eating and Living (MEAL) study (CALGB 70807 [Alliance]): Recruitment feasibility and baseline demographics of a randomized trial of diet in men on active surveillance for prostate cancer. BJU Int. 2018, 121, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.K.; Zahrieh, D.; Mohler, J.L.; Paskett, E.; Hansel, D.E.; Kibel, A.S.; Liu, H.; Seisler, D.K.; Natarajan, L.; White, M.; et al. Effect of a behavioral intervention to increase vegetable consumption on cancer progression among men with early-stage prostate cancer: The MEAL randomized clinical trial. JAMA 2020, 323, 140–148. [Google Scholar] [CrossRef]
- Zong, J.A. Mosaic Not a Monolith: A Profile of the U.S. Latino Population, 2000-2020. UCLA Latino Policy & Politics Institute. Available online: https://latino.ucla.edu/research/latino-population-2000-2020/ (accessed on 28 September 2023).
- World Cancer Research Fund (WCRF); American Institute for Cancer Research (AICR). Diet, Nutrition, Physical Activity and Cancer: A Global Perspective: A Summary of the Third Expert Report. Continuous Update Project Expert Report. 2018. Available online: https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf (accessed on 28 September 2023).
- Mortimer, J.E.; Seewaldt, V. Who will benefit from metformin? JAMA Oncol. 2022, 8, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.C.; Dietze, E.C.; Jovanovic-Talisman, T.; McCune, J.S.; Seewaldt, V.L. Metformin and chemoprevention: Potential for heart-healthy targeting of biologically aggressive breast cancer. Front. Public Health 2020, 8, 509714. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.P.; Natarajan, L.; Caan, B.J.; Parker, B.A.; Greenberg, E.R.; Flatt, S.W.; Rock, C.L.; Kealey, S.; Al-Delaimy, W.K.; Bardwell, W.A. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 2007, 298, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Marrero, A.; Mattei, J. Reclaiming traditional, plant-based, climate-resilient food systems in small islands. Lancet Planet. Health 2022, 6, e171–e179. [Google Scholar] [CrossRef] [PubMed]
- Mattei, J.; Alfonso, C. Strategies for healthy eating promotion and behavioral change perceived as effective by nutrition professionals: A mixed-methods study. Front. Nutr. 2020, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Mattei, J.; McClain, A.C.; Falcón, L.M.; Noel, S.E.; Tucker, K.L. Dietary acculturation among Puerto Rican adults varies by acculturation construct and dietary measure. J. Nutr. 2018, 148, 1804–1813. [Google Scholar] [CrossRef]
- National Heart Lung, and Blood Institute (NHLBI); National Institutes of Health (NIH). Advancing Health Equity Through Culture-Centered Dietary Interventions to Address Chronic Diseases. Available online: https://www.nhlbi.nih.gov/events/2023/advancing-health-equity-through-culture-centered-dietary-interventions (accessed on 28 September 2023).
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef]
- Shi, L.W.; Wu, Y.L.; Hu, J.J.; Yang, P.F.; Sun, W.P.; Gao, J.; Wang, K.; Peng, Y.; Wu, J.J.; Zhong, G.C. Dietary acid load and the risk of pancreatic cancer: A prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1009–1019. [Google Scholar] [CrossRef]
- Ward, C.; Meehan, J.; Gray, M.E.; Murray, A.F.; Argyle, D.J.; Kunkler, I.H.; Langdon, S.P. The impact of tumour pH on cancer progression: Strategies for clinical intervention. Explor. Target. Antitumor Ther. 2020, 1, 71–100. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Müller, A.; Zimmermann-Klemd, A.M.; Lederer, A.K.; Hannibal, L.; Kowarschik, S.; Huber, R.; Storz, M.A. A vegan diet is associated with a significant reduction in dietary acid load: Post hoc analysis of a randomized controlled trial in healthy individuals. Int. J. Environ. Res. Public Health 2021, 18, 9998. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Manz, F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am. J. Clin. Nutr. 1994, 59, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Storz, M.A. Health Inequities in the USA: A role for dietary acid load? Results from the National Health and Nutrition Examination Surveys. J. Racial Ethn. Health Disparities 2022, 10, 2851–2860. [Google Scholar] [CrossRef] [PubMed]
- Tessou, K.D.; Lemus, H.; Hsu, F.C.; Pierce, J.; Hong, S.; Brown, L.; Wu, T. Independent and joint impacts of acid-producing diets and depression on physical health among breast cancer survivors. Nutrients 2021, 13, 2422. [Google Scholar] [CrossRef]
- Wu, T.; Seaver, P.; Lemus, H.; Hollenbach, K.; Wang, E.; Pierce, J.P. Associations between dietary acid load and biomarkers of inflammation and hyperglycemia in breast cancer survivors. Nutrients 2019, 11, 1913. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). Grants and Funding. Inclusion of Women and Minorities as Participants in Research Involving Human Subjects. Available online: https://grants.nih.gov/policy/inclusion/women-and-minorities.htm (accessed on 28 September 2023).
- U.S. Census Bureau. Quick Facts: United States: California. Available online: https://www.census.gov/quickfacts/fact/table/CA/PST045222 (accessed on 11 November 2023).

| Authors (Year) | Name of Intervention (Study Dates; NCT Number) | Focus | % Subgroup in Total Sample (n); Total Sample (n) | % Female | % Cancer Type | Cancer Stage and Active Treatment | Location | Intervention Site | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Stolley (2020) [36] | Avanzando Juntas/Moving Forward (2019–2022) a NCT04321135 b | Culturally adapted and community-based weight loss (nutrition and PA) | 100% Hispanic or Latina survivors (n = 32) c | 100% female | 100% breast cancer and Gynecological cancer | Stage 0–III breast and gynecological cancer; at least 3 months post-treatment | Milwaukee, WI | Community site |
| 2 | Conlon et al. (2015) [37] | Bronx Oncology Living Daily (BOLD) Healthy Living (2011–2012) No NCT | Community-based diabetes prevention and control (nutrition and PA) | 26.5% Hispanic/Latino survivors and co-survivors (n = 22); total sample (n = 83) survivors and co-survivors | 95.2% female | 75.7% breast cancer; 6.1% gynecological cancer; 6.1% lung; 30.1% Other cancer type | No additional criteria | Bronx Co., NY | Community site |
| 3 | Greenlee et al. (2015) [38,39,40] | Cocinar para Su Salud (Cook for Your Health/Life) (2011–2012) NCT01414062 b | Culturally based and community-based lifestyle (nutrition only) | 100% Hispanic survivors (n = 70) All Spanish speakers | 100% female | 100% breast cancer | Stage 0–III breast cancer; at least 3 months post-treatment | New York City, NY | University site (e.g., teaching facility at Columbia University) |
| 4 | Greenlee et al. (2013) [41] | La Vida Activa/An Active Life (2011–2012) NCT00811824 b | Weight loss (nutrition and PA) | 79% Hispanic survivors (n = 33); total sample (n = 42) | 100% female | 100% breast cancer | Stage 0–IIIa breast cancer; at least 6 months post-treatment | New York City, NY | Community site d |
| 5 | Thomson et al. (2023) [42,43] | LIVES (Lifestyle Intervention for Ovarian Cancer Enhanced Survival) (2012–2018) NCT00719303 b | Cancer-free progression (nutrition and PA) | 5.5% Hispanic survivors (n = 63); total sample (n = 1205) | 100% female | 100% ovarian cancer | Complete clinical remission of ovarian cancer (stage II–IV); Gynecological Oncology Group Performance Grade of 0–2; between 6 weeks and 6.5 months post-treatment | Multisite; recruited participants from NRG sites across the U.S. and Canada | Remote only by telephone and internet (with electronic health and intervention platform, eHIP) |
| 6 | Greenlee et al. (2022) [44,45,46] | Mi Vida Saludable/My Healthy Life (2016–2020) NCT02780271 b | Culturally tailored and community-based lifestyle (nutrition and PA) | 100% Hispanic or Latina survivors (n = 167) | 100% female | 100% breast cancer | Stage 0–III breast cancer; at least 3 months post-treatment | Columbia University Medical Center New York, NY | Hybrid; clinical site (e.g., Columbia University Medical Center (CUMC)) and remote by phone/internet (with electronic health and intervention platform, eHIP) |
| 7 | Yanez, et al. (2020) [47] | My Health (2015–2019) NCT03645005 b | Culturally based and community-based lifestyle (nutrition and PA) | 100% Latina survivors (n = 80) | 100% female | 100% breast cancer | Stage 0–III breast cancer; within 2–24 months of completing treatment. | Chicago, IL | Remote only by app/internet |
| 8 | Crane et al. (2020) [48] | Nuestra Salud/For Your Health (2018–2019) a NCT04314479 b | Lifestyle and symptom management (nutrition and PA) | 100% Latina survivors (n = 37) and caregivers without cancer; total sample (n = 71) | 100% female | 81% breast cancer (n = 30) Included cancer survivors of head or neck, liver, colon, kidney, and lymphoma | No eligibility related to cancer stage. Completed primary treatment for solid tumor cancers and included participants undergoing active treatment (n = 6 cancer survivors) | U.S.–Mexico border Southern AZ | Remote only by telephone and internet (with electronic health and intervention platform, eHIP) |
| 9 | Ramirez et al. (2017 [49]); Zuniga et al. (2019) [50] | Prescription (Rx) for Better Breast Health (2013–2017) a NCT02279303 b | Patient-navigated, anti-inflammatory, culinary-based (nutrition only) | 51.2% Hispanic survivors (n = 79); total sample (n = 153) All English speakers | 100% female | 100% breast cancer | Early stage (0–III); At least 2 months post-treatment | San Antonio, TX | University site (e.g., Institute for Health Promotion Research at UT Health San Antonio) and remote by telephone |
| 10 | Pierce et al. (2002) [51]; Paxton et al. (2011) [52] | Women’s Health Eating and Living Study (WHEL) (1995–2000) No NCT b | Cancer-free progression (nutrition only) | 5.3% Hispanic survivors (n = 165); total sample (n = 3088) All English speakers | 100% female | 100% breast cancer | Stage I–IIIA breast cancer within 4 years of study entry. Completed treatment with no evidence of recurrent disease | Multisite. Recruited participants from CA, AZ, OR, and TX | Community/clinical site (details NR) and remote by telephone |
| Intervention Name | Theory | Intervention Components | Delivery | Interventionist | Primary Outcome | Secondary Outcome for Well-Being and Quality of Life (QOL) | Estimated Dose Delivered | |
|---|---|---|---|---|---|---|---|---|
| 1 | Avanzando Juntas/Moving Forward [36] | NR ACS guidelines for nutrition and PA included in informational binder. | Culturally adapted and community-based nutrition education class with weekly text messages for reinforcement. a | F2F (group classes) and remote/virtual via text messaging (individual). a Language: bilingual. a | NR | Dietary Intake: fruits and vegetables, red meat, and processed meat; overall dietary quality assessed with HEI (self-administered 24 h dietary recall with ASA24). a | HRQOL (Patient-Reported Outcomes Measurement Information System, PROMIS) | 180 min (3 h) sessions × 16 sessions over 16 weeks = 2880 min (48 h). Note: Weekly text messages not counted in dose (2–3 per week) a Dose: 48 h |
| 2 | Bronx Oncology Living Daily (BOLD) Healthy Living [37] | Social–ecological framework RE-AIM Framework b ADA, ACS, and AICR guidelines used in curriculum | Community-based nutrition education class and group exercise. | F2F group class. Language: bilingual. | Health-care professionals, including RDNs, EP, and certified trainers. | Body composition: Height, weight, WC (direct observation). | HRQOL (2-items from the SF-36), including perceived health status and perceived pain. | Dose: NR |
| 3 | Cocinar Para Su Salud/Cook for Your Health/Life [38,39,40] | DESIGN Framework. c Transtheoretical Model (TTM, stages-of-change construct). Social Cognitive Theory (SCT). AICR and ACS guidelines used for curriculum. | Culturally tailored nutrition education class, cooking class, and food shopping field trips with monthly telephone calls from study coordinator (RD) for retention. | F2F group class. Language: Spanish only. | A bilingual, Latino/a RD and bilingual Latino/a chef. | Dietary intake: Fruits and vegetables (24-h dietary recalls) and percentage of energy from total fat; Biomarker of dietary intake of FV (blood carotenoid concentration). | None | 1.5-to-3.5 h sessions × 9 sessions (24 h total for session) over 12 weeks Note: Telephone calls not counted in dose. Author-reported total dose. Dose: 24 h |
| 4 | La Vida Activa/An Active Life [41] | NR | Curves Weight Management Program with use of Curves fitness centers and a Curves diet plan, with Curve curriculum with books, DVDs, and instructor’s manual plus nutrition courses for study participants and weekly motivational telephone calls from instructor. | F2F group class. Language: bilingual. | Nutrition courses led by bilingual instructor and exercise classes led by Curves staff and trainers. | Body composition: weight change (weight loss). | None | 1 h nutrition sessions × 6 sessions over 6 weeks and additional contacts for exercise. Note: Exercise classes and telephone calls not counted in dose because number and duration varied. Dose: 6 h |
| 5 | LIVES (Lifestyle Intervention for Ovarian Cancer Enhanced Survival) [42,43] | SCT. Theory of Planned Behavior. ACS guidelines used for nutrition goals and AICR and ACS guidelines for PA goals. | Tele-coaching, based on MI, with text messages and emails for reinforcement. | Remote/virtual via smartphone (calls and texts) and internet-based (email). Language: bilingual. | Health coaches (undergraduate students majoring in nutrition science or dietetics and trained in evidence and behavior theory-based strategies, including MI, for 6 weeks) | Progression-free survival or death from any cause (measured: the number of months between study enrollment and documentation of disease progression). | QOL (RAND-36) | 33 coaching sessions × NR minutes/session over 24 months with a phased approach. Note: Text messages not counted in dose. Dose: NR |
| 6 | Mi Vida Saludable/My Healthy Life [44,45,46] | DESIGN Framework. c TTM. SCT. AICR and ACS guidelines used for curriculum. | Culturally tailored nutrition education classes, hands-on skills-building exercises, field trips, written materials about ACS and AICR guidelines, FitBit for self-monitoring of PA, text messages, emails, access to interactive project website, with monthly telephone calls for retention. | F2F or remote/virtual via smartphone (texts) and internet. The ehealth components included 2–3 text messages per week and 2 e-newsletters per month sent via eHIP and linked to the project’s website for 11 months. Language: bilingual. | Intervention staff include a trained chef, a nutrition and physical activity educator, and a dance-class instructor. | Dietary intake: intake of FVs and total energy density (interviewer-administered 24-h dietary recalls). Biomarker of dietary intake of FV (blood carotenoid concentration). | PROMIS Scale v1.2—global health and PROMIS-43 profile v2 for QOL, including physical, mental, and social health. | Note: Study included different arms with varied contacts. Minimum dose for F2F: 16 h in the first month. Minimum dose for ehealth: NR. Dose: NR |
| 7 | My Health [47] | Quality-of-Life Cancer Survivorship Framework. | My Health app plus tele-coaching calls with tele-coach trained in MI (3 calls before weeks 1, 2, and 6, and for low-app-usage participants, additional calls at weeks 3, 4, and 5). Culturally appropriate materials. | Remote/virtual via app on smartphone or internet platform. Language: bilingual. | Bilingual health coaches trained by a licensed clinical psychologist in MI, problem solving, and goal setting. | Dietary intake: intake of fruits and vegetables and fewer daily fat sources (23-Item Brief Dietary Assessment Tool for Hispanics). | None | 2 h of app use per week over 6 weeks = 12 h Note: Three to six tele-coaching calls not counted in dose. Dose: 12 h |
| 8 | Nuestra Salud/For Your Health [48] | SCT and integrated symptom management and lifestyle intervention (SMLI). ACS guidelines used for nutrition and PA goals. | Telephone coaching sessions, based on MI, (each session 20–30 min) and text messages delivered via eHIP and FitBit for self-monitoring of PA. | Remote/virtual via telephone calls and text messages. Language: bilingual. | Bilingual and bicultural health coaches (≥4 years of experience) | Dietary intake: Usual diet over past 30 days (19-items from NCI DSQ). PA: frequency and duration over the last 7 days (9-items from the WHI PAQ). | None. (Study used PROMIS to assess self-efficacy for symptom management.) | 12 coaching sessions × 25 min/session over 12 weeks = 300 min Dose: 5 h |
| 9 | Prescription (Rx) for Better Breast Health [49,50] | SCT and Bandura stages of change construct. USDA dietary intake guidelines for five major food groups used for nutrition goals. | Nutrition and cooking anti-inflammation workshops (“dietary workshops), MI (individually tailored) tele-coaching sessions, and tailored newsletters based on stages of change for reinforcements. | F2F (group classes) and remote/virtual via tele-counseling (individual). a Language: English only. | Workshop facilitator: NR. A chef skilled in anti-inflammatory food preparation led culinary cooking demonstrations. Coaches were patient navigators and trained in MI by certified member of the MI Network of Trainers. | Inflammation: CRP and IL-10. | Physical, social/family, emotional, and functional well-being (subscales from the Breast Cancer Functional Assessment of Cancer Therapy Scale (FACT-B). | 6-monthly workshops × NR minutes/workshop + monthly MI coaching sessions × minutes/session within 4 weeks after each workshop for first 6 months and every month over 12 months Dose: NR |
| 10 | Women’s Healthy Eating and Living Study (WHEL) [51,52] | SCT | Cooking classes, tele-counseling, and monthly newsletters. | F2F (Group classes) and Remote/virtual via tele-counseling (individual) a Language: English only. | Counselors (for tele-counseling) trained in MI. | Cancer recurrence, new primary breast cancer, and death from any cause (interviewer-administered survey and confirmation of recurrence using medical records). | QOL (RAND-36 scale included in the Thoughts and Feelings Questionnaire). | Note: A stepped intervention with three phases. The number of tele-counseling sessions varied. Dose: NR |
| Intervention Name | Study design (Duration and Timing of Assessment) | Primary Outcome | Secondary Outcome for Well-Being and Quality of Life (QOL) | Additional Secondary Outcomes for Weight or Physical Activity (PA) | Main Effects | Secondary Effects | |
|---|---|---|---|---|---|---|---|
| 1 | Avanzando Juntas/Moving Forward [36] | Pilot RCT (4 months) Baseline, post-test (4 months) No follow-up. | Dietary intake: fruits and vegetables, red meat and processed meat, and overall dietary quality assessed with HEI (self-administered 24-h dietary recall with ASA24). a | HRQOL (Patient-Reported Outcomes Measurement Information System, PROMIS). | Body composition: ratio of lean mass to fat mass (direct observation: measured by Bio-electrical Impedance Analysis Scale). PA: moderate-to-vigorous PA (accelerometry via ActiGraph monitors) and change in resistance training (measured: 30 s Chair Stand). | No: Effects on dietary quality, measured with HEI total or HEI component scores, though within-group increases for total vegetables, greens and beans, whole fruit, total protein, and sodium for intervention group; between-group differences favored total vegetables, greens and beans, and total protein. NS for between-group differences. b | NR c |
| 2 | Bronx Oncology Living Daily (BOLD) Healthy Living [37] | Pilot RCT (4 weeks–12 weeks). Baseline and 4 and 12 weeks. No follow-up. | Body composition: height, weight, and WC (direct observation). | HRQOL (2 items from the SF-36), including perceived health status and perceived pain. | None | Yes: At 12 weeks, no effects on BMI, but effects for decrease in WC for participants in 12-week vs. 4-week program (41.8 vs. 40.8 inches, p = 0.03). d | Yes: At 4 weeks and 12 weeks, effects on perceived health status (p = 0.001) and borderline effects on perceived pain (p = 0.05). d |
| 3 | Cocinar Para Su Salud/Cook for Your Health/Life [38,39,40] | Pilot RCT (12 weeks). Baseline and 3, 6, and 12 months. Included follow-up at 6 and 12 months post-baseline. | Dietary intake: fruits and vegetables (24-h dietary recalls) and percentage of energy from total fat. | None | Body composition: height, weight, WC, and HC (direct observation). | Yes: At 3 months, effect on all FVs (+1.1 vs. −0.3, p = 0.05) and targeted FVs (p = 0.004) for intervention vs. control; effect on % calories from total fat (−7.1% vs. −1.6%, p = 0.01) for intervention vs. control. At 6 months, effect on all FVs and targeted FVs maintained; effect on % calories from total fat: NS. | Yes. At 6 months, effect on WC between groups (−1.6 cm vs. +1.7 cm, p = 0.05). Effect on weight outcomes: NS. |
| 4 | La Vida Activa/An Active Life [41] | Pilot RCT (6 months). Baseline and every 3 months through 12 months. Included follow-up at 12 months. | Body composition: weight change (weight loss). | None | Body composition: DEXA, height, weight, HC, and WC. PA: survey for type, frequency, duration, and intensity (Kaiser Physical Activity Survey) and cardiopulmonary exercise stress test (VO2max). Dietary intake (110-item Block questionnaire used in NHANES with food items for Hispanic populations). | Yes: At month 6, women in the intervention group lost an average 3.3% (±3.5%) of body weight (range: 1.7% gain to 10.6% loss), as compared with 1.8% (±2.9%) weight loss in the control group (p = 0.04). At month 12, on average women in the IA regained some but not all of the weight lost during the first 6 months (p = 0.02). d | Yes: At 6 months, WC decreased, marginally statistically significant (p = 0.07). d |
| 5 | LIVES: Lifestyle Intervention for Ovarian Cancer Enhanced Survival [42,43] | Pilot RCT (6 months). Baseline and every 3 months from baseline through 24 months (2 years). Included follow-up at 24 months. | Progression-free survival or death from any cause (measured: the number of months between study enrollment and documentation of disease progression). | QOL (measured: the RAND-36 e scale with 36 items and bowel health (Gastrointestinal Symptom Scale). | Dietary intake: usual diet (Arizona FFQ). Body composition: BMI (direct observation). PA: moderate-to-vigorous PA (MET-h/week), sedentary time (h/week), and steps/day (accelerometry and self-reported in Arizona PAQ). | NR c,d | NR c,d |
| 6 | Mi Vida Saludable/My Healthy Life [44,45,46] | RCT (12 months) Baseline and 6 and 12 months. No follow-up. | Dietary intake: intake of FVs and total energy density (interviewer-administered 24-h dietary recalls). Biomarker of dietary intake of FVs (blood carotenoid concentration). | PROMIS Scale v1.2—global health and PROMIS-43 profile v2 for QOL, including physical, mental, and social health. | PA: moderate-to-vigorous PA (FitBit) and survey, 7-day Physical Activity Recall (7DPAR). Body composition: height, weight, WC, and HC (direct observation). | NR c,f | NR c,f |
| 7 | My Health [47] | Pilot RCT (6 weeks) Baseline and 6 weeks. Included follow-up two weeks after immediate post-test. | Dietary intake: intake of fruits and vegetables and fewer daily fat sources (23-item Brief Dietary Assessment Tool for Hispanics). | None | PA: moderate-to-vigorous PA (FitBit) and survey, 7-item International Physical Activity Questionnaire Short Form (IPAQ-SF). | Yes: At 6 weeks and 2 weeks later, significant interaction of time and condition on daily fat sources in intervention (My Health) relative to control (My Guide) with p = 0.015 and = 0.009, respectively. Average daily fat sources did not significantly differ between groups. No effects on daily servings of FVs. | None |
| 8 | Nuestra Salud/For Your Health [48] | Pilot RCT (12 weeks) Baseline and 12 weeks No follow-up. | Dietary intake: Usual diet over past 30 days (19-items from NCI DSQ). PA: frequency and duration over the last 7 days (9-items from the WHI PAQ). | None. (Study used PROMIS to assess self-efficacy for symptom management.) | None. (PA was primary outcome.) | Yes: At post-intervention, cancer survivors had medium-to-large effects for goals related to FV servings (d = 0.55), vegetables (d = 0.72), sugar intake (d = 0.51); medium effects (clinically significant) for increases in total minutes of PA per week (d = 0.42) and grams of fiber intake (d = 0.40). | Yes: At post-intervention, cancer survivors had medium-to-large effects for improved symptom severity (d = 0.74). |
| 9 | Prescription (Rx) for Better Breast Health [49,50] | Pilot RCT (6 months) Baseline and 6 and 12 months Included follow-up at 12 months (1 year). | Inflammation: CRP and IL-10. | Physical, social/family, emotional, and functional well-being (subscales from the Breast Cancer Functional Assessment of Cancer Therapy Scale (FACT-B). | Body composition: body mass index. Dietary intake: Mediterranean Diet Score (14-item from Diet Assessment Tool), total energy intake, % calories from macronutrients, and FV intake. PA: monitoring of PA and inactivity (measured via 14-item IPAQ Short Form, the International PA Questionnaire). | NR c,d | Yes: At 6 months: effects on secondary outcomes change in Mediterranean diet score, +1.6 change in Mediterranean diet score in intervention group vs. +0.2 change in control group (p < 0.001). Effects on total energy intake (−195.5 vs. + 34.8, p = 0.045). No effects on other dietary outcomes, including FVs and fiber. d |
| 10 | Women’s Health Eating and Living (WHEL) Study [51,52] | RCT (12 months) Baseline and 12 months Included follow-up annually at year 2, 3, 4, and 6. | Cancer recurrence, new primary breast cancer, and death from any cause (interviewer-administered survey and confirmation of recurrence using medical records). | QOL (RAND-36 QOL scale included in the Thoughts and Feelings Questionnaire). | Body composition: height, weight, HC, and WC (direct observation). Dietary intake: usual diet over the past 3 months (Arizona FFQ). Intake over the past 3 weeks (3 × 24-h dietary recalls for different subsamples). Biomarker of dietary intake of FVs (blood plasma carotenoid concentration) for subsample. | No: At final time point year (average 7.3 years), no effects on cancer recurrence or mortality by race/ethnicity for Hispanic versus White survivors. | Yes: At 1 year and 4 years: effects on secondary outcomes for dietary intake of fiber, FV, and percent energy from fat for Hispanic survivors. Effects on QOL or body composition or weight outcomes: NR c |
| Intervention Name | Assessment | Timing | Biomarkers for Dietary Intake of Fruits and Vegetables | Biomarkers of Inflammation, Metabolism, Lipids, or Cancer Recurrence | Effects |
|---|---|---|---|---|---|
| Avanzando Juntas/Moving Forward [36] | Blood sample. | Collected at baseline, post-test (4 months). No follow-up. | N/A | Inflammatory and metabolic biomarkers: cholesterol, lipid, HbA1c adiponectin, leptin, C-peptide, hs-CRP, and insulin resistance measured with glucose, glycogen, and insulin. a | NR b |
| Bronx Oncology Living Daily (BOLD) Healthy Living [37] | N/A | N/A | N/A | N/A | N/A |
| Cocinar para Su Salud/Cook for Your Health/Life [38,39,40] | Blood sample (fasting). DNA sample. | Collected at baseline and 3, 6, and 12 months. | Serum carotenoids: total carotenoids, lutein, α-carotene, beta-carotene, beta-cryptoxanthin, and retinol. | Inflammatory biomarkers: IL-1α, IL-6, IL-8, IL-10, hs-CRP, GM-CSF, and TNF-α. | Increased total carotenoids and serum lutein in the intervention group. Non-significant decreases in inflammatory biomarkers: IL-1α, IL-6, IL-10, TNF-α, and hs-CRPs in the intervention group at 6 months. Borderline increased global DNA methylation in the intervention group. |
| La Vida Activa/An Active Life c [41] | Blood sample (fasting morning blood draw). | Collected at baseline and 3, 6, 9, and 12 months. | N/A | Inflammatory and metabolic biomarkers: serum cholesterol (total, high-density lipoprotein, indirect low-density lipoprotein), triglycerides, glucose, hs-CRP, insulin, adiponectin, IGF-I, total IGF binding protein-1, IGF binding protein-3, and HOMA-IR. | No effects on metabolic biomarkers using intent-to-treat analysis. Fat loss ≥ 2% decreased insulin, glucose, and HOMA-IR at 6 months. Weight loss ≥ 5% increased IGF-1 BP1 and decreased glucose at 6 and 12 months. |
| LIVES (Lifestyle Intervention for Ovarian Cancer Enhanced Survival) c [42,43] | Blood sample. DNA sample. Tissue collection of tumor tissue for pathology report. | Collected at baseline and 6, 12, and 24 months. | Plasma carotenoids. | Metabolic, lipid, and mechanistic biomarkers modified by diet and physical activity: insulin glucose, lipids, and telomere length. Prognostic biomarkers: IL-6, and omentin. | NR b |
| Mi Vida Saludable/My Healthy Life [44,45,46] | Blood sample (optional, fasting blood draw). | Collected at baseline and 6 and 12 months. | Plasma carotenoid and tocopherol concentrations. | Inflammatory biomarkers: hs-CRP, IL-6, TNF-α), oxidative stress (e.g., isoprostane). DNA methylation. Metabolic biomarkers: insulin, glucose, insulin growth factor (IGF), and lipid panel. | NR b |
| My Health [47] | N/A | N/A | N/A | N/A | N/A |
| Nuestra Salud/For Your Health [48] | N/A | N/A | N/A | N/A | N/A |
| Prescription (Rx) for Better Breast Health c [49,50] | Blood sample (12-h fasting). | Collected at baseline and 6 and 12 months. | None | Pro- and anti-inflammatory biomarkers: IL-3, IL-6, IL-8, and IL-10; CRP; and TNFα. Circulating ASCs. Lipid biomarkers: total cholesterol, triglycerides, LDL, and HDL. Metabolic biomarker: HbA1c. | NR b |
| Women’s Health Eating and Living Study (WHEL) [51,52] | Blood draw (fasting). Interviews with review of medical records; tissue collection of tumor tissue for pathology report. | Baseline, year 1, and at end of year 4 (racial/ethnic subgroup analysis). | Plasma carotenoids (β-carotene, α-carotene, lutein/zeaxanthin, lycopene, and β-cryptoxanthin). | None | No effects on cancer recurrence or mortality rates by racial/ethnic group. NR: Effects on carotenoids as biomarker. b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, C.M.; Stubblefield, E.; Godinich, B.M.; Walker, M.; Salcedo Price, R.; Allicock, M.A. A Scoping Review to Explore the Potential Benefits of Nutrition Interventions for Latino/a Adult Cancer Survivors in the US. Nutrients 2023, 15, 4963. https://doi.org/10.3390/nu15234963
Johnson CM, Stubblefield E, Godinich BM, Walker M, Salcedo Price R, Allicock MA. A Scoping Review to Explore the Potential Benefits of Nutrition Interventions for Latino/a Adult Cancer Survivors in the US. Nutrients. 2023; 15(23):4963. https://doi.org/10.3390/nu15234963
Chicago/Turabian StyleJohnson, Cassandra M., Emily Stubblefield, Brandon M. Godinich, Miranda Walker, Ramona Salcedo Price, and Marlyn A. Allicock. 2023. "A Scoping Review to Explore the Potential Benefits of Nutrition Interventions for Latino/a Adult Cancer Survivors in the US" Nutrients 15, no. 23: 4963. https://doi.org/10.3390/nu15234963
APA StyleJohnson, C. M., Stubblefield, E., Godinich, B. M., Walker, M., Salcedo Price, R., & Allicock, M. A. (2023). A Scoping Review to Explore the Potential Benefits of Nutrition Interventions for Latino/a Adult Cancer Survivors in the US. Nutrients, 15(23), 4963. https://doi.org/10.3390/nu15234963








