The Association between Circulating Lipids and Female Infertility Risk: A Univariable and Multivariable Mendelian Randomization Analysis
Abstract
1. Introduction
2. Materials and Methods
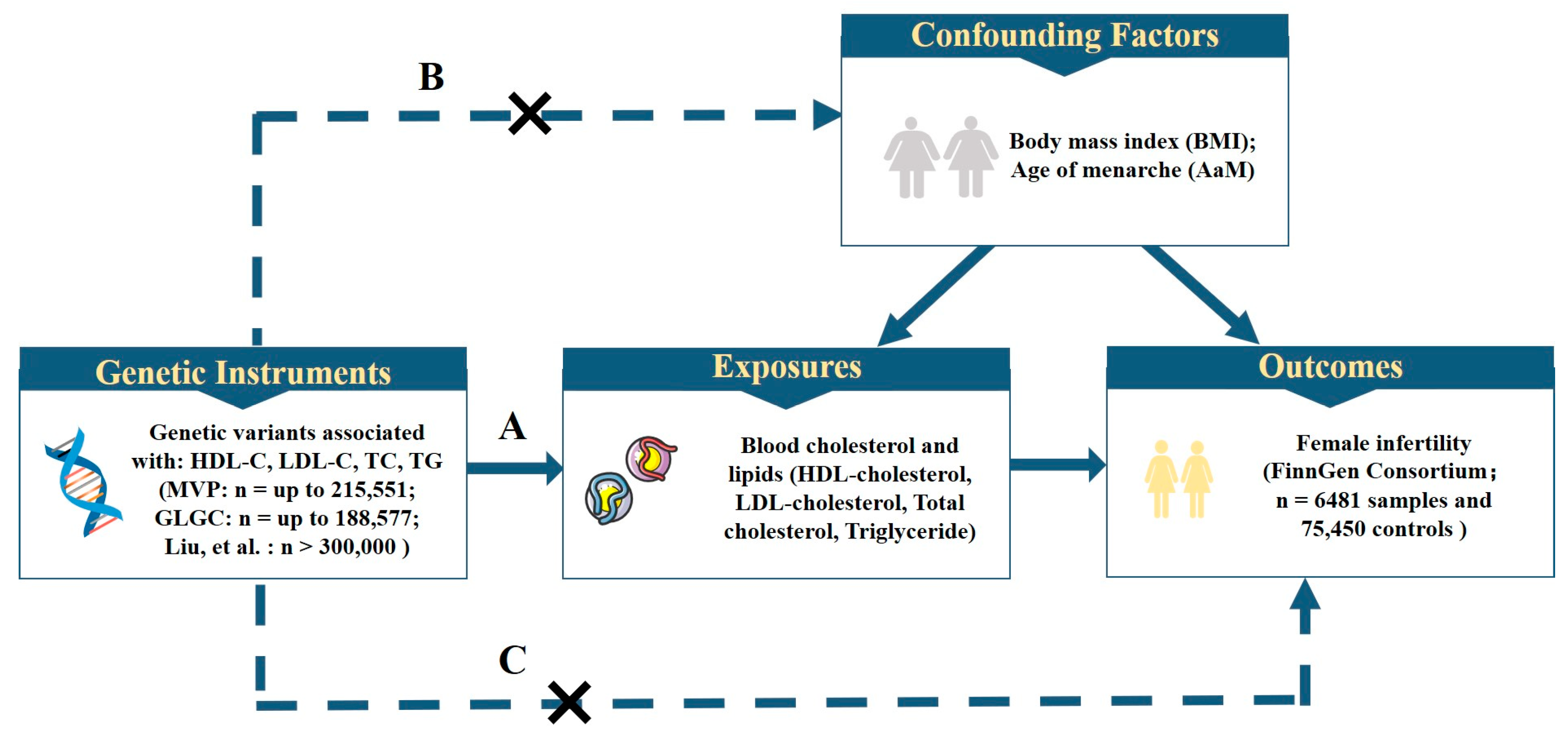
2.1. Study Design and Data Sources
2.2. Genetic Instrument Selection
2.3. Statistical Analysis
3. Results
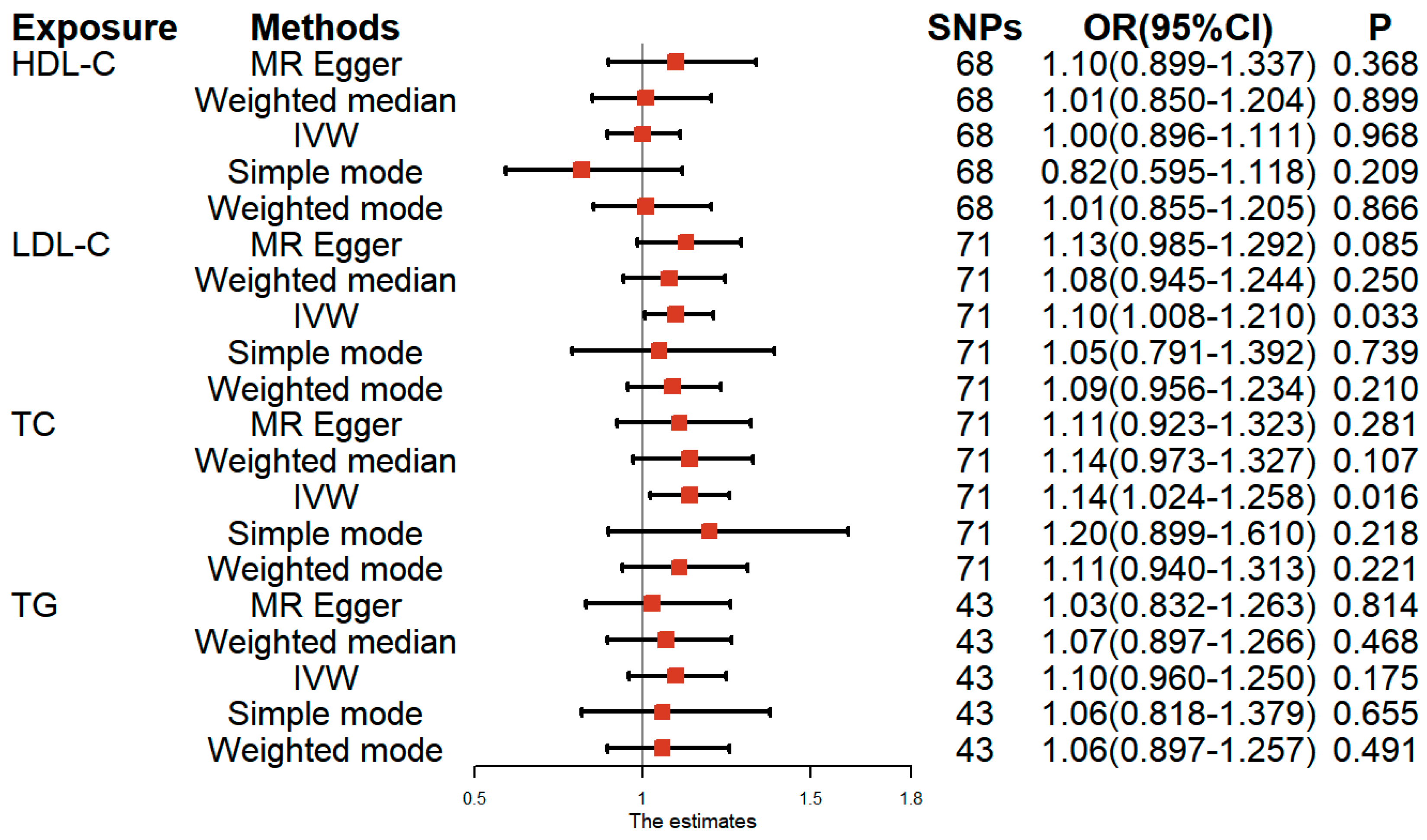
3.1. Univariable MR Analysis of Lipid-Related Traits and Female Infertility
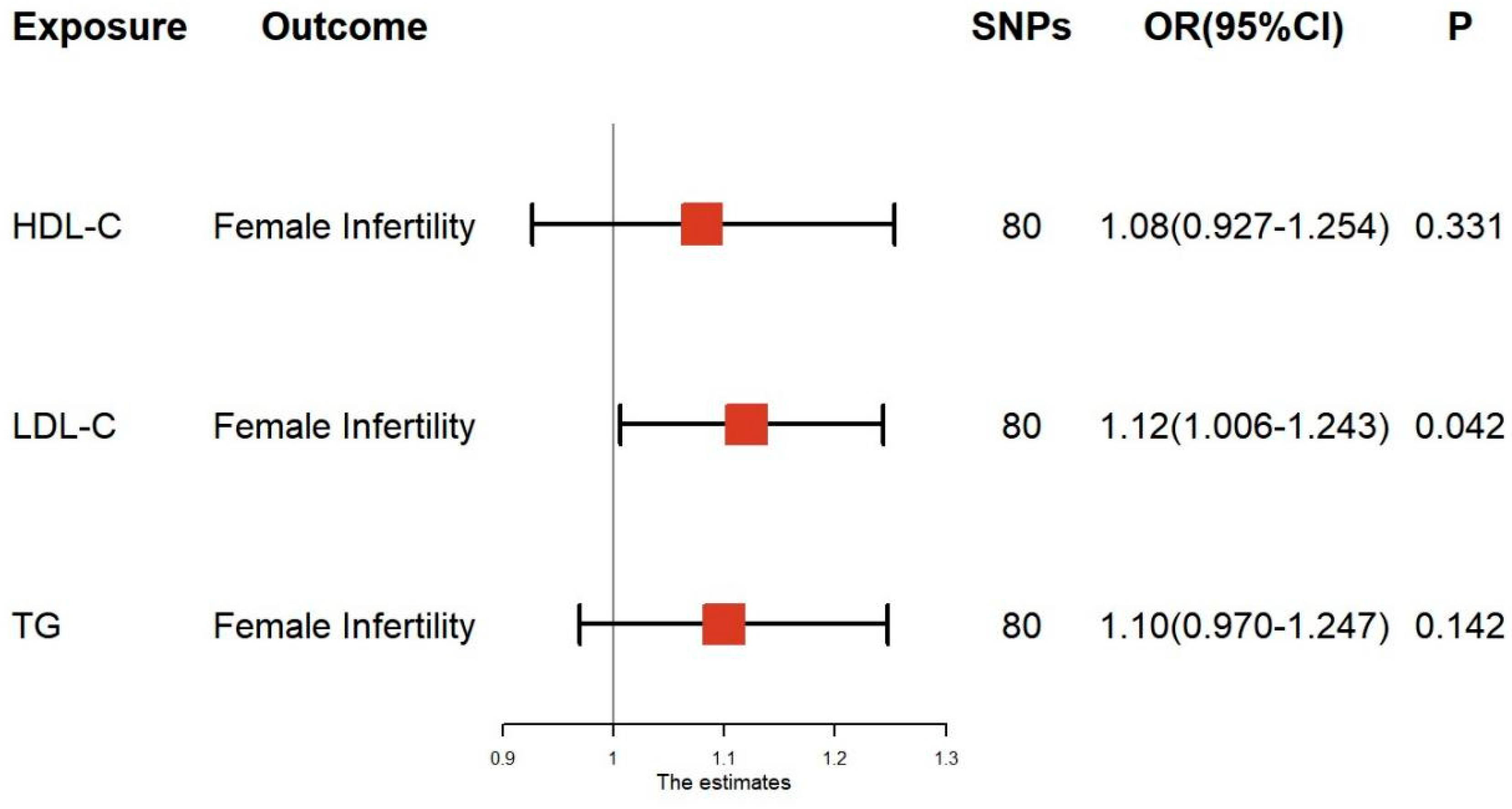
3.2. Multivariable MR Analysis of Lipid-Related Traits and Female Infertility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). International Classification of Diseases; 11th Revision (ICD-11); WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Ombelet, W.; Cooke, I.; Dyer, S.; Serour, G.; Devroey, P. Infertility and the provision of infertility medical services in developing countries. Hum. Reprod. Update 2008, 14, 605–621. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, D.; Wu, H.; Li, R.; Xu, S.; Kang, Y.; Cao, Y.; Chen, X.; Zhu, Y.; Xu, S.; et al. Epidemiology of infertility in China: A population-based study. BJOG 2018, 125, 432–441. [Google Scholar] [CrossRef]
- Liang, S.; Chen, Y.; Wang, Q.; Chen, H.; Cui, C.; Xu, X.; Zhang, Q.; Zhang, C. Prevalence and associated factors of infertility among 20-49 year old women in Henan Province, China. Reprod. Health 2021, 18, 254. [Google Scholar] [CrossRef]
- Broughton, D.E.; Moley, K.H. Obesity and female infertility: Potential mediators of obesity’s impact. Fertil. Steril. 2017, 107, 840–847. [Google Scholar] [CrossRef]
- Finelli, R.; Mottola, F.; Agarwal, A. Impact of Alcohol Consumption on Male Fertility Potential: A Narrative Review. Int. J. Environ. Res. Public. Health 2021, 19, 328. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, A.K.; Hatch, E.E.; Rothman, K.J.; Mikkelsen, E.M.; Aschengrau, A.; Wise, L.A. Prospective study of cigarette smoking and fecundability. Hum. Reprod. 2019, 34, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. JAMA 2021, 326, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Ngai, F.W.; Lam, W. Perception of family sense of coherence among Chinese couples with infertility. J. Clin. Nurs. 2021, 30, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Ngai, F.W.; Loke, A.Y. Relationships between infertility-related stress, family sense of coherence and quality of life of couples with infertility. Hum. Fertil. 2022, 25, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Gwynne, J.T.; Strauss, J.F., 3rd. The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr. Rev. 1982, 3, 299–329. [Google Scholar] [CrossRef]
- Bukhari, S.A.; Zafar, K.; Rajoka, M.; Ibrahim, Z.; Javed, S.; Sadiq, R. Oxidative stress-induced DNA damage and homocysteine accumulation may beinvolved in ovarian cancer progression in both young and old patients. Turk. J. Med. Sci. 2016, 46, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Mumford, S.L.; Browne, R.W.; Barr, D.B.; Chen, Z.; Louis, G.M. Lipid concentrations and couple fecundity: The LIFE study. J. Clin. Endocrinol. Metab. 2014, 99, 2786–2794. [Google Scholar] [CrossRef] [PubMed]
- Pugh, S.J.; Schisterman, E.F.; Browne, R.W.; Lynch, A.M.; Mumford, S.L.; Perkins, N.J.; Silver, R.; Sjaarda, L.; Stanford, J.B.; Wactawski-Wende, J.; et al. Preconception maternal lipoprotein levels in relation to fecundability. Hum. Reprod. 2017, 32, 1055–1063. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.J.; Wei, Y.Y.; Zhang, R.Y.; Chen, F. Application of mendelian randomization methods in causal inference of observational study. Zhonghua Yu Fang Yi Xue Za Zhi 2019, 53, 619–624. [Google Scholar] [CrossRef]
- Pickrell, J.K.; Berisa, T.; Liu, J.Z.; Segurel, L.; Tung, J.Y.; Hinds, D.A. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 2016, 48, 709–717. [Google Scholar] [CrossRef]
- Klarin, D.; Damrauer, S.M.; Cho, K.; Sun, Y.V.; Teslovich, T.M.; Honerlaw, J.; Gagnon, D.R.; DuVall, S.L.; Li, J.; Peloso, G.M.; et al. Genetics of blood lipids among ~300,000 multi-ethnic participants of the Million Veteran Program. Nat. Genet. 2018, 50, 1514–1523. [Google Scholar] [CrossRef]
- Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; Buchkovich, M.L.; Mora, S.; et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 2013, 45, 1274–1283. [Google Scholar] [CrossRef]
- Hunter-Zinck, H.; Shi, Y.; Li, M.; Gorman, B.R.; Ji, S.G.; Sun, N.; Webster, T.; Liem, A.; Hsieh, P.; Devineni, P.; et al. Genotyping Array Design and Data Quality Control in the Million Veteran Program. Am. J. Hum. Genet. 2020, 106, 535–548. [Google Scholar] [CrossRef]
- Liu, D.J.; Peloso, G.M.; Yu, H.; Butterworth, A.S.; Wang, X.; Mahajan, A.; Saleheen, D.; Emdin, C.; Alam, D.; Alves, A.C.; et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat. Genet. 2017, 49, 1758–1766. [Google Scholar] [CrossRef]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389, Erratum in Eur. J. Epidemiol. 2017, 32, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Sanderson, E.; Spiller, W.; Bowden, J. Testing and correcting for weak and pleiotropic instruments in two-sample multivariable Mendelian randomization. Stat. Med. 2021, 40, 5434–5452. [Google Scholar] [CrossRef]
- Bowden, J.; Holmes, M.V. Meta-analysis and Mendelian randomization: A review. Res. Synth. Methods 2019, 10, 486–496. [Google Scholar] [CrossRef]
- Xu, W.; You, Y.; Yu, T.; Li, J. Insights into Modifiable Risk Factors of Infertility: A Mendelian Randomization Study. Nutrients 2022, 14, 4042. [Google Scholar] [CrossRef]
- Johnson, K.E.; Siewert, K.M.; Klarin, D.; Damrauer, S.M.; Chang, K.M.; Tsao, P.S.; Assimes, T.L.; Maxwell, K.N.; Voight, B.F. The relationship between circulating lipids and breast cancer risk: A Mendelian randomization study. PLoS Med. 2020, 17, e1003302. [Google Scholar] [CrossRef]
- Jansen, H.; Lieb, W.; Schunkert, H. Mendelian Randomization for the Identification of Causal Pathways in Atherosclerotic Vascular Disease. Cardiovasc. Drugs Ther. 2016, 30, 41–49. [Google Scholar] [CrossRef]
- Cai, W.Y.; Luo, X.; Ma, H.L.; Shao, X.G.; Wu, X.K. Association between preconception serum lipid concentrations and treatment outcomes in women with PCOS who underwent ovulation induction. Reprod. Biomed. Online 2022, 45, 805–814. [Google Scholar] [CrossRef]
- Dallel, S.; Tauveron, I.; Brugnon, F.; Baron, S.; Lobaccaro, J.M.A.; Maqdasy, S. Liver X Receptors: A Possible Link between Lipid Disorders and Female Infertility. Int. J. Mol. Sci. 2018, 19, 2177. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, R.; Nouri, M.; Hamdi, K.; Shaaker, M.; Mehdizadeh, A.; Darabi, M. Fatty acids of follicular fluid phospholipids and triglycerides display distinct association with IVF outcomes. Reprod. Biomed. Online 2021, 42, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Marín Bivens, C.L.; Lindenthal, B.; O’Brien, M.J.; Wigglesworth, K.; Blume, T.; Grøndahl, C.; Eppig, J.J. A synthetic analogue of meiosis-activating sterol (FF-MAS) is a potent agonist promoting meiotic maturation and preimplantation development of mouse oocytes maturing in vitro. Hum. Reprod. 2004, 19, 2340–2344. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Qu, J.; Tian, M.; Yang, R.; Song, X.; Li, R.; Yan, J.; Qiao, J. Lipid Metabolic Process Involved in Oocyte Maturation During Folliculogenesis. Front. Cell Dev. Biol. 2022, 10, 806890. [Google Scholar] [CrossRef]
- Stouffer, R.L.; Xu, F.; Duffy, D.M. Molecular control of ovulation and luteinization in the primate follicle. Front. Biosci. 2007, 12, 297–307. [Google Scholar] [CrossRef]
- Willnow, T.E.; Hammes, A.; Eaton, S. Lipoproteins and their receptors in embryonic development: More than cholesterol clearance. Development 2007, 134, 3239–3249. [Google Scholar] [CrossRef]
- Yesilaltay, A.; Dokshin, G.A.; Busso, D.; Wang, L.; Galiani, D.; Chavarria, T.; Vasile, E.; Quilaqueo, L.; Orellana, J.A.; Walzer, D.; et al. Excess cholesterol induces mouse egg activation and may cause female infertility. Proc. Natl. Acad. Sci. USA 2014, 111, E4972–E4980. [Google Scholar] [CrossRef]
- Jeon, H.; Blacklow, S.C. Structure and physiologic function of the low-density lipoprotein receptor. Annu. Rev. Biochem. 2005, 74, 535–562. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, L.; Cheng, D.; Liu, T.; An, L.; Li, W.P.; Zhang, C. Low-density lipoprotein receptor affects the fertility of female mice. Reprod. Fertil. Dev. 2015, 27, 1222–1232. [Google Scholar] [CrossRef]
- Fujimoto, V.Y.; Kane, J.P.; Ishida, B.Y.; Bloom, M.S.; Browne, R.W. High-density lipoprotein metabolism and the human embryo. Hum. Reprod. Update 2010, 16, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Yesilaltay, A.; Morales, M.G.; Amigo, L.; Zanlungo, S.; Rigotti, A.; Karackattu, S.L.; Donahee, M.H.; Kozarsky, K.F.; Krieger, M. Effects of hepatic expression of the high-density lipoprotein receptor SR-BI on lipoprotein metabolism and female fertility. Endocrinology 2006, 147, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.; Quiroz, A.; Santander, N.; Morselli, E.; Busso, D. Implications of High-Density Cholesterol Metabolism for Oocyte Biology and Female Fertility. Front. Cell Dev. Biol. 2022, 10, 941539. [Google Scholar] [CrossRef] [PubMed]

| Exposure/Outcome | Trait | Consortium/Cohort Study | Ethnicity | Sample Sizes/Participants | Pubmed ID or Web Source |
|---|---|---|---|---|---|
| Genetic instruments for lipid-related traits in univariable MR analysis | HDL-C | MVP/GLGC | European | 210,967/188,577 | PubMed ID: 30275531/PubMed ID: 24097068 |
| LDL-C | MVP/GLGC | European | 215,196/188,577 | ||
| TC | MVP/GLGC | European | 215,551/188,577 | ||
| TG | MVP/GLGC | European | 211,491/188,577 | ||
| Genetic instruments for lipid-related traits in multivariable MR analysis | HDL-C/LDL-C/TC/TG | GLGC | European | >300,000 | PubMed ID: 29083408 |
| Genetic instruments for female infertility | Female infertility | FinnGen Consortium | European | 6481 samples and 75,450 controls | (https://r6.finngen.fi/, accessed on 3 February 2023) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Hong, X.; Wu, J.; Zhao, F.; Wang, W.; Huang, L.; Li, J.; Wang, B. The Association between Circulating Lipids and Female Infertility Risk: A Univariable and Multivariable Mendelian Randomization Analysis. Nutrients 2023, 15, 3130. https://doi.org/10.3390/nu15143130
Zhu X, Hong X, Wu J, Zhao F, Wang W, Huang L, Li J, Wang B. The Association between Circulating Lipids and Female Infertility Risk: A Univariable and Multivariable Mendelian Randomization Analysis. Nutrients. 2023; 15(14):3130. https://doi.org/10.3390/nu15143130
Chicago/Turabian StyleZhu, Xiaoqi, Xiang Hong, Jingying Wu, Fanqi Zhao, Wei Wang, Lingling Huang, Jiuming Li, and Bei Wang. 2023. "The Association between Circulating Lipids and Female Infertility Risk: A Univariable and Multivariable Mendelian Randomization Analysis" Nutrients 15, no. 14: 3130. https://doi.org/10.3390/nu15143130
APA StyleZhu, X., Hong, X., Wu, J., Zhao, F., Wang, W., Huang, L., Li, J., & Wang, B. (2023). The Association between Circulating Lipids and Female Infertility Risk: A Univariable and Multivariable Mendelian Randomization Analysis. Nutrients, 15(14), 3130. https://doi.org/10.3390/nu15143130







